Abstract
Estimating juvenile salmon habitat carrying capacities is a critical need for restoration planning. We assimilated more than 4500 unique estimates of published juvenile densities (e.g., fish/m2) in estuarine and floodplain habitats. These density data were categorized by species and life stage, habitat type, seasonal period, and geographic region to develop frequency statistics (e.g., 25th and 75th percentiles, or quartiles). These frequency statistics were then used in a habitat expansion approach to estimate carrying capacities based on habitat extent. We demonstrate the habitat expansion approach by applying the quantiles of observed juvenile Chinook salmon (Oncorhynchus tshawytscha) and coho salmon (O. kisutch) densities (fish/ha) to spatial data describing current, historical or potential, and predicted (based on seal level rise) habitat extents for 16 coastal Oregon estuaries to estimate carrying capacities. Current carrying capacities based on 75th percentile springtime (Apr–Jun) densities ranged from 2902 to 33,817 fish/delta for Chinook salmon and 2507 to 20,206 fish/delta for coho salmon. Historic carrying capacities during the peak rearing period (spring) ranged from 3869 to 71,844 fish/delta for Chinook salmon and 3201 to 38,337 fish/delta for coho salmon, representing a 3 to 72% loss in Chinook salmon capacity and 2 to 67% loss in coho salmon capacity. Estimated carrying capacities were predicted to decline by 2 to 54% with 1.4 m of sea level rise in systems that are projected to lose vegetated tidal wetland habitat, while a 1 to 320% increase in capacity was predicted for systems that are predicted to increase in area with sea level rise. Finally, we demonstrate how the carrying capacity estimates can be used to estimate changes in juvenile Chinook and coho salmon capacity following restoration, which can be used to both design and evaluate restoration projects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries and floodplains are critical habitats for many marine and freshwaters fishes. These habitats are particularly important to Pacific salmon (Oncorhynchus spp.) populations because they support juvenile life stages and ecosystem processes that are critical to complex salmon life cycles (Simenstad et al. 1982; Beamer et al. 2005; Hall et al. 2018a). Unfortunately, these low-gradient habitats are often the most impacted by human development and losses or degradation of these habitats has been linked to population declines of Pacific salmon throughout the Pacific Northwestern USA (Simenstad et al. 1982; Simenstad and Cordell 2000; Beamer et al. 2005; Bottom et al. 2005; Lotze et al. 2006; Beechie et al. 2013; Greene et al. 2015; Hall et al. 2018a). Construction of levees and dikes; filling, drainage, and conversion of land for agricultural, industrial, and urban uses; and simplification of channel networks have disrupted lateral connectivity with estuarine floodplains and reduced accessible habitat for salmon (Pess et al. 2005; Morley et al. 2012; Finn et al. 2021). Levees and dikes, as well as other flood control infrastructures, may also constrain estuaries in the face of potential sea level rise (SLR) with climate change (Brophy et al. 2019). Because of the extensive loss of estuarine and floodplain habitats, they have been the focus of many restoration efforts for salmon recovery (Roni et al. 2019).
Like most of the U.S. Pacific Coast, estuarine habitats in coastal Oregon have been significantly impacted by anthropogenic development since European settlement, with an estimated 59% loss of estuarine emergent marsh and a more than 95% loss of estuarine scrub-shrub and forested tidal wetland habitats (Brophy et al. 2019). Currently, the Oregon Coast coho salmon (O. kisutch) evolutionary significant unit (ESU) is listed as threatened under the Endangered Species Act (ESA) (NMFS 2016; article 16 U.S.C. §§ 1531–1544). Petitions to list Oregon Coast Chinook salmon (O. tshawytscha) ESUs were considered but determined unwarranted (National Marine Fisheries Service (NMFS) 2021). Given the importance of estuarine habitats to Chinook and coho salmon juvenile life stages (Simenstad et al. 1982; Beamer et al. 2005; Magnusson and Hilborn 2003; Koski 2009), restoration of these habitats is a priority for recovery of coastal Oregon salmon populations (NMFS 2016). Understanding the potential benefits of estuary restoration for fish populations is critical for planning (e.g., setting recovery targets), prioritizing restoration projects, monitoring, and physical and biological evaluation (e.g., progress toward recovery goals).
There are a handful of approaches for estimating the amount of habitat that needs to be restored to achieve salmon recovery goals, the potential benefits of a proposed restoration project or specific restoration design alternatives, or the potential effects of climate change on salmon recovery (Roni et al. 2018; Pess and Jordan 2019). These include various types of modeling efforts, long-term monitoring and evaluation of treatment and control sites, habitat equivalency analysis, or a meta-analysis of habitat use or restoration effectiveness in similar habitats (Roni et al. 2010, 2018; Beechie et al. 2015; Ehinger et al. 2015; Baker et al. 2020; Pess and Jordan 2019; See et al. 2021). All these approaches use estimates of changes in habitat and fish densities to model or estimate survival, current abundance, or carrying capacity. Carrying capacity (hereafter, “capacity”)—the maximum number of fish a given area of habitat can sustainably support—is determined by features of the habitat (e.g., food or cover availability, competition), which can therefore act as limiting factors on fish abundance. Understanding changes in capacity is particularly useful for assessing progress toward population recovery and restoration planning.
Recent efforts have attempted to estimate capacity of juvenile spring Chinook salmon during summer in the interior Columbia Basin and capacity and growth for fall Chinook salmon in the Nisqually River Delta (Bond et al. 2019; See et al. 2021; Davis et al. 2022). There have also been a handful of efforts to estimate juvenile coho and Chinook salmon habitat capacity along the Oregon Coast. For example, life cycle models were developed for Oregon Coast coho salmon in freshwater (e.g., Reeves et al. 1989; Nickelson and Lawson 1998), though the models did not provide detailed information on estuarine capacity. Nickelson (2012) estimated the current and historic capacity and restoration potential of the Coquille River but focused on one species and life stage (i.e., yearling coho salmon), and did not differentiate between key habitat strata (i.e., tidally influenced versus freshwater wetland habitats). However, little information exists on juvenile coho and Chinook salmon capacity in estuarine or floodplain habitats in Oregon, which is a critical need for restoration planning and evaluation. Therefore, we used published juvenile Chinook and coho salmon densities in estuaries and floodplains from across their North American range in a habitat expansion approach to estimate habitat-specific carrying capacities in Oregon coastal estuaries and floodplains.
The habitat expansion approach to estimate capacity has been used in numerous studies to estimate potential benefits from restoration actions, the amount of restoration needed to accomplish recovery or project goals, or evaluate restoration needed to mitigate losses (e.g., Roni et al. 2010, 2018; Beechie et al. 2015; Ehinger et al. 2015; Bond et al. 2019; Baker et al. 2020; Pess and Jordan 2019; See et al. 2021). The habitat expansion approach is not without limitations, but it does have several advantages over other approaches. Life cycle or capacity models can include a variety of impacts and management scenarios to estimate changes related to restoration scenarios (e.g., Jorgensen et al. 2021). However, these models often make many assumptions, typically require extensive input data, may be costly to develop and parameterize, can be difficult to validate (and understand), are open to extensive criticism, and often include some level of habitat expansion in the underlying calculation of capacities. In contrast, the habitat expansion approach is based on empirical data from published studies, uses transparent and easy to explain methods, and provides a robust and defensible approach (Roni et al. 2010; Beechie et al. 2015). The habitat expansion approach provides a consistent snapshot of capacity estimates that can be used to compare habitat extent or restoration scenarios across broad geographic scales (Bond et al. 2019).
To support use of the habitat expansion approach, we synthesized existing data on juvenile Chinook and coho salmon densities in estuaries, large rivers, and floodplains from across their North American range using a meta-analysis approach. We then applied this approach to coastal Oregon estuaries where detailed spatial data have been developed for historic, current, and future estuarine habitat extents (e.g., Brophy and Ewald 2017; Beechie et al. 2018; Brophy et al. 2019; PMEP 2019). The fish density data we compiled for this study can be used to support such estimates, and the habitat expansion approach can be applied to large river and floodplain habitats once similar spatial data are developed for coastal Oregon systems. In this study, we used the compiled density data and habitat expansion approach to estimate capacities for current, potential or historical, and predicted habitat extents for 16 coastal estuaries in Oregon to answer the following questions:
-
How does juvenile salmon density differ among various habitat types?
-
What is the current juvenile Chinook and coho salmon capacity of coastal Oregon estuaries and how does this compare to historic or potential estuarine habitat extent?
-
How will projected sea level rise (SLR) affect estuarine habitat extent and capacity estimates?
-
What are the implications for restoration planning?
Methods
Our overall approach involved three steps (Fig. 1): (1) extracting salmon density data from the literature, (2) stratifying and summarizing densities, and (3) using the stratified and summarized densities in a habitat expansion approach to estimate capacities for 16 coastal Oregon estuaries (Fig. 2). Steps 1 and 2 are described in the “Salmon Density” section below, while step 3 is described in the “Current, Historic, and Future Oregon Coast Estuarine Habitat Extent” and “Juvenile Salmon Capacity Estimates” sections that follow. We also estimated juvenile capacities for historic and future habitat extents to determine potential changes in capacity as a result of restoration and to provide context for restoration potential.
Infographic showing habitat expansion process from (1) reviewing literature and extracting fish density data; (2) stratifying and summarizing fish density data by seasonal periods, habitat type, species, and life history; and (3) habitat expansion method to estimate capacities for systems, habitats, species, and seasons by applying 75th percentile densities (Q3 Density) to optimal habitat areas derived from spatial data
Salmon Density
To develop a database of salmon densities for use in the habitat expansion approach, we first compiled and reviewed literature to extract fish density data (Fig. 1). We expanded on previously completed literature reviews on restoration effectiveness and juvenile salmon densities (Roni et al. 2002, 2008, 2014; Hillman et al. 2016; Hall and Roni 2018; Burgess et al. 2019), which were compiled using a variety of database searches (Google Scholar, Web of Science, and other search engines). These previous efforts screened more than 1200 papers and data from 125 sources with relevant information on juvenile salmon densities in different habitat types and included published and unpublished data from peer reviewed sources and reports. We used Google Scholar to identify potential sources based on the first ten pages of records returned from the search (completed in January 2021) and included the following terms in our search: habitat OR restoration OR rehabilitation OR improvement OR enhancement AND nearshore OR estuary OR floodplain OR mainstem OR juvenile OR salmon OR density. From these searches, an additional 36 sources were screened, and 11 had relevant juvenile salmon density data, which resulted in a final total of 136 data sources.
Screening of papers and data for the database focused only on studies that reported discrete juvenile salmon density data in fish/m2 or were reported in such a way that values could be converted to fish/m2 (e.g., abundance was reported along with net sizes or area sampled). Tabular data were extracted as reported, while graphical data were extracted using digitizing tools to ensure it was consistent and calibrated to the axis (e.g., rather than visually estimating values from graphs). We excluded long duration (e.g., months) time integrated data sources (e.g., smolt trap data), or data that were reported as peak values only. We noted gear types in our database, but compiled data regardless of gear type because detailed data on differences in gear efficiency within and among studies were not available. Extracted density data were stratified and summarized by several categorical factors that included geographic context, species information, seasonal periods, and habitat types (Fig. 1).
For geographic context, we classified data by the following geographic regions: Puget Sound (all Puget Sound watersheds), Pacific Northwest (coastal Washington and Oregon), British Columbia, Alaska, and California. System (e.g., Columbia River or Skagit River) and sub-location (e.g., site or tributary) information were also recorded, where possible, to support future filtering or evaluation of the data to specific areas. However, the system and site level information were not used in the analyses for this study.
We compiled data on a variety of salmon species, but we focused our literature review and limited our analysis to juvenile coho and Chinook salmon. We only included density data for unmarked (non-hatchery) or natural origin juveniles in our analysis, and we assumed data were for natural origin fish if mark type (marked or hatchery and unmarked or natural origin) was not reported. Reported densities may not include hatchery origin information for several reasons including lack of hatchery supplementation programs in the basin, internal marking strategies (e.g., thermal otolith or coded wire tag marking), or lack of marking programs for releases. In some cases, the presence of hatchery origin fish was noted but catch data were not reported in a way that natural origin densities could be extracted. Any mixed catch data were excluded, unless a dominant species or mark type that exceeded 90% of the catch was reported, in which case the densities were extracted and assigned to the dominant species or mark type without adjustment. No run type classifications such as spring, summer, or fall were considered but rather we extracted data by life stage and classified them as either subyearling or yearlings. Data were not considered if a subyearling or yearling life stage could not be determined. Due to variations in reporting, we considered data as smolts, yearlings, age 1 + , river types, or fish with fork lengths exceeding 100 mm as yearlings. Data were classified as subyearling when life stages or life histories were reported as fry, parr, fingerling, age 0 + , or ocean type, or when fork lengths were less than 100 mm. If the study mixed density data of multiple life stages, we only considered data when a dominant life stage exceeded 90% of the mixed catch.
To categorize by season, we used reported season or meteorological season if dates or months were reported, and aggregated juvenile fish density data into seasonal groups based on typical juvenile salmon migration and river flow patterns as follows (Apgar et al. 2020):
-
Winter—occurring from December through March and representing winter flow conditions, incubation of eggs in gravels, and the beginning of emergence and downstream migration pulse for juvenile salmon
-
Spring—occurring from April through June and representing spring melt pulse flow conditions and the typical peak downstream migration pulse of juvenile salmon
-
Summer—occurring from July through September and representing a low flow condition with little juvenile salmon downstream migration
-
Fall—occurring from October through November representing an increasing flow condition with little juvenile salmon downstream migration
Genetic differences between populations, geographic gradients, and interannual variations in environmental conditions influence the timing of adult migrations and spawning as well as the timing and duration of incubation, rearing, and juvenile outmigration (Apgar et al. 2020). Therefore, we used these seasonal groups to describe general phenology and not phenology specific to populations. The Kolmogorov–Smirnov test was used to evaluate differences in the distribution of densities among geographic regions. Densities were compiled as seasonal means, with data being aggregated by individual site or aggregations of sites by habitat type and year, depending on the level of reporting detail, to maximize sample sizes.
Extracted data were classified and aggregated by key habitat strata and substrata, which included estuary, large river, and floodplain. These strata included substrata and unit type classifications that were determined based on reported detail and definitions for key habitat types provided in Table 1. We opportunistically compiled data from nearshore and tributary habitats to support future analyses, but we do not report on these data in this study. We report on density data compiled for large river and floodplain habitats to support future analyses and for context, but only use data from estuaries in this study.
Ultimately, we identified 9636 records of juvenile salmon density measurements for a range of regions, habitat types, and species. Most data were from the Puget Sound (n = 6700) and coastal Oregon and Washington (n = 3185), with some data from Idaho (n = 809), British Columbia (n = 549), California (n = 55), Alaska (n = 144), and others (n = 29, including Russia or unknown). These data were from observations predominately in estuarine habitats (n = 3602), large river habitats (n = 3052), and floodplain habitats (n = 546), with the rest (n = 4271) from other habitat types (e.g., nearshore or tributary). Most data compiled were for Chinook and coho salmon (n = 4570), but we also identified density data for steelhead (O. mykiss, n = 1940), cutthroat trout (O. clarkii, n = 1115), chum salmon (O. keta, n = 598), pink salmon (O. gorbuscha, n = 452), sockeye salmon (O. nerka, n = 220), bull trout (Salvelinus confluentus, n = 441), and other species (n = 2135). For the habitat expansion analysis, we filtered the data to non-zero measurements of subyearling Chinook salmon (n = 973), subyearling coho salmon (n = 550), yearling Chinook salmon (n = 83), and yearling coho salmon (n = 176) in estuarine, large river, and floodplain habitats from all geographic regions to generate frequency statistics for density data. By filtering the data to non-zero measurements, the frequency statistics are intended to represent the distribution of densities where fish were observed. This represented data from 72 sources, with references provided in the Supplemental Data materials (S1. Reference list for fish density data).
Current, Historic, and Future Oregon Coast Estuarine Habitat Extent
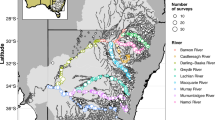
Spatial information on habitat extents with sufficient detail on habitat types is needed to support a habitat expansion analysis (Fig. 1). To quantify estuarine habitat extents, we relied on spatial data from several sources and did not digitize any features as part of this analysis. We focused on 16 coastal Oregon estuary and river systems (Fig. 2), which ranged in size from approximately 3900 to 1.2 million hectares in drainage basin area, with variable geomorphic and valley forms (Brophy et al. 2019). We focused on this subset of systems because they had sufficient spatial data to support estimation of current, historic, and potential habitat areas. All spatial data layers were reprojected to the same coordinate reference system (Oregon Statewide 2992) to generate lengths and areas for features and habitat types. All spatial data processing (e.g., reprojections, clipping, reclassifications, and geometry calculations) was completed in QGIS (QGIS Development Team 2009).
The current extent of vegetated tidal wetland habitat was also derived from the Pacific Marine and Estuarine Fish Habitat Partnership’s (PMEP) Indirect Assessment of West Coast USA Tidal Wetland Loss data (Brophy et al. 2019) for the subset of systems with data coverage (11 out of 16). Current extents for these systems were derived by combining polygon areas classified as retained and restored in Brophy et al. (2019). For the Necanicum River, Rogue River, and Sixes River, we estimated current wetland extent from a linear regression between wetland area in PMEP’s West Coast USA Estuarine Biotic Habitat (PMEP 2019) and the current extent from Brophy et al. (2019). Vegetated marsh area from PMEP (2019) was highly correlated with current extent from Brophy et al. (2019) (linear regression: slope = 0.92, R2 = 0.95). Coos Bay was excluded from the regression because large amounts of fringe wetlands along distributary channels were included in PMEP (2019) for Coos Bay, which resulted in higher estimates of current wetland extent. The linear regression estimate was used for consistency with current extent estimates derived from Brophy et al. (2019). However, vegetated wetland extents for two small systems (Chetco River and Elk River) from PMEP (2019) were used directly rather than regression estimates given that the current estimates exceeded historical extents.
Marsh type can influence habitat use and therefore density, and juvenile salmon primarily use distributary and blind tidal channel features (hereafter referred to as tidal channels) in estuaries (Beamer et al. 2005; Greene et al. 2021). Therefore, we also quantified the amount of marsh and channel type habitat, consistent with the habitat strata used in our synthesis of fish density data. We derived marsh type information from PMEP’s West Coast USA Estuarine Biotic Habitat (PMEP 2019) to support capacity estimates based on the relative amount of marsh types among systems. The current extent of estuarine emergent marsh (EEM), estuarine scrub-shrub (ESS), and forested riverine tidal (FRT) wetland habitat extents (which were used as habitat strata in our synthesis of fish density data) were based on the emergent tidal marsh, tidal forest/woodland, and tidal scrub-shrub Coastal and Marine Ecological Classification Standard Biotic Component habitat classifications, respectively (PMEP 2019). For channel types, we used spatial data from the Salmon Habitat Status and Trends Monitoring Program (SHSTMP), which provided higher resolution mapping of channel features (Beechie et al. 2018) compared to other spatial data used in this analysis (Brophy et al. 2019; PMEP 2019). Mapped features in the SHSTMP datasets included polygons for distributary channels and large tidal channels (> 2–3 m wide) mapped to vegetated edges of channels and polylines for small tidal channels (< 2–3 m wide), but these data did not provide depth information for channel features. All features were digitized at 1:1000 scale using true-color Google satellite and aerial imagery collected from May 5, 2013, to August 17, 2016 (Beechie et al. 2018). We spatially joined PMEP’s West Coast USA Estuarine Biotic Habitat polygons to SHSTMP channel feature edges to quantify channel habitat within different marsh types (EEM, ESS, and FRT). Due to spatial alignment and marsh type resolution, portions of channels were not allocated to marsh type with the spatial joins, and we assigned unallocated lengths to marsh type based on allocated marsh type ratios. However, the high-resolution SHSTMP channel data were only available for six of the Coastal Oregon systems (Coos Bay, Necanicum River, Nehalem River, Nestucca Bay, Siletz Bay, and Tillamook Bay), limiting our ability to estimate current habitat extents by channel and marsh type, but this was addressed using regression analyses (described in the “Juvenile Salmon Capacity Estimates” section).
Historical wetland extents were determined using a combination of PMEP’s Indirect Assessment of West Coast USA Tidal Wetland Loss (Brophy et al. 2019) and potential vegetated wetland extent from Brophy and Ewald (2017). Wetland loss data from PMEP (Brophy et al. 2019) were only available for a subset of systems (Alsea Bay, Coos Bay, Coquille River, Nehalem River, Nestucca Bay, Salmon River, Siletz Bay, Siuslaw River, Tillamook Bay, Umpqua River, and Yaquina Bay). Historical area was derived by combining the polygon areas classified as retained or restored. Potential vegetated wetland extent from Brophy and Ewald (2017) were available for all 16 estuaries considered in this analysis, but potential extents were 13% lower, on average, than historical area estimates from the more recent analyses from Brophy et al. (2019). For estuaries with data coverage from both Brophy et al. (2019) and Brophy and Ewald (2017), historical estuary extent (Brophy et al. 2019) was highly correlated with potential vegetated wetland extent (Brophy and Ewald 2017) (linear regression: slope = 1.16, R2 = 0.99). Therefore, we used the regression to estimate historical extent for estuaries that lacked PMEP data (Brophy et al. 2019) coverage (Chetco River, Elk River, Necanicum River, Rogue River, and Sixes River).
Predicted future vegetated tidal wetland extents for a range of SLR scenarios were available for all 16 systems (Brophy and Ewald 2017). These scenarios represent the extent of habitat within an appropriate elevation range that would sustain vegetated marshes, but the extent does not account for connectivity (e.g., levees and dikes) and therefore represents the maximum potential vegetated tidal wetland extent for each scenario (Brophy and Ewald 2017). In addition, the scenarios do not include vertical land motion for deep surface processes given that vertical land motion rate estimates (about 2–3 mm/year) were more than an order of magnitude less than uncertainty in SLR estimates (about 25–130 cm) and digital elevation models (10–20 cm) (Brophy and Ewald 2017). Potential vegetated tidal wetland extents were available for seven SLR scenarios modeled at 0.0 m (0.0 ft), 0.23 m (0.8 ft), 0.48 m (1.6 ft), 0.75 m (2.5 ft), 1.42 m (4.7 ft), 2.5 m (8.2 ft), and 3.5 m (11.5 ft) above current sea level, and these represent upper ranges or intermediate to high estimates for years 2030, 2050, 2070, 2100, 2130, and 2160 respectively (Brophy and Ewald 2017). We focus on the 0.0–1.42 m SLR scenarios (including the 0.0 m, 0.23 m, 0.48 m, 0.75 m, and 1.42 m scenarios), or current conditions to 2070, for our analysis. Predicted habitat extents with SLR were not estimated by channel and marsh type, and regressions were used to address this, as described in the capacity section below.
Juvenile Salmon Capacity Estimates
Juvenile salmon capacities were estimated using a habitat expansion approach, which included applying density scalars from compiled fish density data to habitat area estimates from spatial data (Fig. 1). Juvenile salmon typically prefer slow-water channel edge habitats with optimal velocities, depth, and cover over mid-channel habitats (Beamer et al. 2005; Nickelson 2012; Beechie et al. 2017; Lowery et al. 2017). Therefore, optimal channel area scalars were used to estimate habitat capacities for estuarine habitats based on usable habitat area, rather than total channel area, to reduce overestimation of capacities. We estimated usable habitat area for distributary channels using a fixed width of 5 m for both banks (delineated by polygon edges), which falls within the reported optimal edge habitat width for distributary channels that are 50–100 m wide based on water velocities and depth (Beamer et al. 2005). For large tidal channels, we used edges or polygon features and a fixed width of 1 m from both banks to estimate usable habitat area, which represents a maximum of a 2 m wide extent of usable habitat within large tidal channel features and the minimum width for which large tidal channel polygons were digitized (Beechie et al. 2018). For small tidal channels, we assumed a fixed width of 1 m for polyline features and the entire channel area represents usable fish habitat and all small tidal channel features are less than 2–3 m wide (Beechie et al. 2018). However, the scalars do not account for actual variations in depth and velocity and are intended as a proxy for usable area.
For our purposes, we defined capacity as the upper range of fish densities that can be supported based on available habitat quantity. We assumed that individual measurements of densities can be a poor proxy for habitat capacities because instantaneous occupancy of habitats can exceed the actual capacity of the habitats (See et al. 2021; Greene et al. 2021). Therefore, maximum observed densities were not used to estimate capacities. Rather, we assumed higher values within a range of observed densities are a more suitable proxy for habitat capacities, given that many factors can influence observed densities (e.g., habitat quality, habitat connectivity, population status, and other environmental conditions). In addition, we filtered the compiled juvenile salmon densities to all non-zero measurements of juvenile Chinook and coho salmon and derived frequency statistics to describe the range of densities where fish were observed by season, habitat type, and life history (subyearling and yearling). Therefore, the frequency statistics represent a range of densities where fishes were present in habitat types and reduce zero inflation bias. We assumed the 25th percentile of observed densities generally represents lower functioning (e.g., degraded, recently restored, or lower quality habitats in an early stage of a recovery trajectory) or under-seeded habitats (e.g., low population abundance). Conversely, we assumed the 75th percentile of observed densities where fish are encountered represents a proxy for capacity in more functional (e.g., higher quality habitats or desired future conditions for restoration sites) or fully seeded habitats (similar to See et al. 2021). Where sample sizes did not support calculation of 25th or 75th percentiles at the seasonal and habitat unit type level, we used the next highest habitat level (substrata or strata) or pooled seasonal data to estimate capacities. We pooled data from all geographic regions to increase sample sizes for the frequency statistics and assumed distributions would be similar among regions. We tested this assumption using a non-parametric test of equality (Kolmogorov–Smirnov) to evaluate differences among distributions of densities for coastal Oregon and Washington observations compared to the full dataset at the species and habitat strata levels.
Given that detailed channel mapping data were not available for all estuary systems for current conditions, potential or historical extent, or future conditions with SLR, we developed power regressions to estimate capacity as a function of vegetated wetland area. We developed regressions from the subset of estuary systems with detailed channel mapping data (Coos Bay, Necanicum River, Nehalem River, Nestucca Bay, Siletz River, and Tillamook Bay). Capacities for these systems were derived from the 25th and 75th percentiles of juvenile Chinook and coho salmon (subyearling and yearling combined) spring densities and usable habitat areas by channel and marsh type. The estimated capacities were then regressed with current total vegetated marsh area to predict capacities (adjusted for usable area) for systems that lacked detailed channel mapping data. This approach assumes that allometric relationships between vegetated marsh area and channel areas are retained under historical or potential SLR scenarios. Studies have found that allometric relationships can be affected by anthropogenic processes and that impacted marshes may have fewer channels and outlets, as well as less channel area relative to marsh area (Hood 2014, 2015). Therefore, power regressions based on current marsh allometry may underestimate historical channel areas or overestimate channel areas under SLR scenarios.
Results
Salmon Density
Data specific to Oregon and Washington represented only about 25% of the available data and filtering to just Washington and Oregon would have reduced sample sizes and therefore our ability to develop density ranges for habitat strata and substrata, seasons, species, and life stages. Differences between the distributions of densities in coastal Oregon and Washington observations compared to the full dataset at the species and strata levels were not significant (Kolmogorov–Smirnov test: p ≥ 0.12) for all comparisons, except for subyearling coho salmon in estuaries (Kolmogorov–Smirnov test: p < 0.001). Although estuarine coho salmon densities were significantly lower in coastal Oregon and Washington observations compared to the full dataset, frequency statistics for densities were summarized and derived from the full dataset, with geographic area pooled for density statistics.
Across habitat types, reported densities were higher in large river and floodplain habitats compared to estuarine habitats for both subyearling and yearling Chinook and coho salmon (Fig. 3). Seasonal densities of subyearling Chinook salmon were highest in the summer in large river and floodplain habitats and in the spring for estuarine habitats (Fig. 3). Seasonal densities of subyearling coho salmon were highest in the fall/winter through spring in large river and floodplain habitats and in the spring in estuarine habitats (Fig. 3). Yearling coho densities were highest in the summer in large river and floodplain habitats, whereas yearling Chinook densities were highest in the fall/winter in large river and floodplain habitats. Yearling coho and Chinook densities were both highest in the spring in estuarine habitats (Fig. 3).
Box and whisker plots of subyearling and yearling Chinook (left) and coho (right) salmon densities (fish/hectare) for large river, floodplain (LR&FP, top), and estuarine habitat types (bottom) by season from all non-zero measurements of fish density for all geographic regions combined. The whiskers show the range of values (min–max, excluding outliers), the outside of the boxes shows the first and third quartiles, and the dark bar shows the median. Note, x-axis scales are different for each pane
Juvenile Chinook and coho salmon densities also varied among habitat substrata (see S2. Detailed fish density summary statistics). Summaries of density frequencies for large river and floodplain habitats are reported here for context, but these data were not used for capacity estimates in this study. Among large river and floodplain habitats, subyearling Chinook salmon density ranges (25th–75th percentile) were higher in habitats associated with backwaters (1093 to 8819 fish/ha) and large wood jams (1129 to 20,575 fish/ha) compared to densities in mainstem (208 to 2734 fish/ha), braids and side channels (290 to 869 fish/ha), and ponds and wetlands (186 to 2813 fish/ha). Subyearling coho salmon density ranges were higher in habitats associated with braids and side channels (963 to 12,021 fish/ha) and large wood jams (1374 to 11,054 fish/ha) compared to mainstem (303 to 3000 fish/ha), backwater (1129 to 8085 fish/ha), and ponds and wetlands (1001 to 7700 fish/ha). Yearling density ranges were lower for Chinook salmon in large river and floodplain habitats (41 to 190 fish/ha) than yearling coho salmon (260 to 6818 fish/ha) with all habitat types pooled. Among large river and floodplain substrata, yearling Chinook salmon density ranges were higher in habitats associated with large wood jams (110 to 431 fish/ha) compared to other habitat substrata (38 to 175 fish/ha). For yearling coho salmon, density ranges were higher in braids and side channels (2608 to 13,832 fish/ha) compared to other habitat substrata (39 to 6059 fish/ha).
Within estuarine habitats, subyearling density ranges (25th–75th percentile) were higher in distributary channel habitats compared to tidal channel habitats for both Chinook and coho salmon (see S2. Detailed fish density summary statistics). Subyearling Chinook salmon densities ranged from 57 to 514 fish/ha in distributary channels compared to 16 to 293 fish/ha in tidal channel habitats, and subyearling coho densities were 25 to 419 fish/ha in distributaries compared to 3 to 264 fish/ha in tidal channels. When marsh type was considered, subyearling densities were higher in forested riverine tidal (FRT) marsh types associated with tidal channels compared to other channel and marsh type combinations for both species. Subyearling densities ranged from 57 to 2316 fish/ha and 1026 to 4945 fish/ha for Chinook and coho, respectively, in tidal channels associated with FRT marshes compared to ranges of 17 to 533 fish/ha and 2 to 646 fish/ha for Chinook salmon and coho salmon, respectively, in other channel and marsh type combinations. Sample sizes for yearling Chinook salmon (n = 29) and coho salmon (n = 15) density observations in estuarine habitat types were considerably smaller than subyearling sample sizes (n = 632 and 170, respectively), and this limited our ability to describe patterns of densities among estuarine, channel, and marsh type substrata. With channel type and marsh type pooled, yearling Chinook salmon density ranges (7 to 200 fish/ha) were higher than yearling coho salmon densities (2 to 22 fish/ha) in estuarine habitats.
Current, Historic, and Future Oregon Coast Estuarine Habitat Extent
Across the 16 estuaries we examined, current vegetated tidal marsh areas ranged from 11 to 903 ha, with most marsh area classified as EEM (average = 77%, SD = 16%) and an average of 13% ESS (SD = 12%) and 10% FRT (SD = 7%) (Fig. 4). Estimates of historical estuary extents ranged from 18 to 3493 ha among systems, with current extents representing 10–95% of historical extent (Fig. 4; see also S3. Estuarine habitat summaries). Current vegetated tidal wetland extents from PMEP’s West Coast USA Estuarine Biotic Habitat were strongly related to the current extent described by PMEP’s Indirect Assessment of West Coast USA Tidal Wetland Loss (linear regression: R2 = 0.95, slope = 0.92). For systems with SHSTMP mapped distributary and tidal channel features, channel edge lengths ranged from approximately 15 to 56 km for distributaries and 14 to 90 km for large tidal channels (Fig. 5). Small tidal channels were mapped as lines and their total length ranged approximately 5–105 km (Fig. 5). Distributary edge, large tidal channel edge, and small tidal channel length were predominately associated with EEM habitat (78–92% average), with an average of 4–7% of channel edge or length associated with ESS habitat. FRT habitat was associated with an average of 15% of distributary edge length, while only 4–7% of large tidal channel edge and small tidal channel length were associated with FRT habitat. Total large tidal channel edge and small tidal channel lengths were strongly correlated with total distributary edge length, with large tidal channel edge and small tidal channel lengths increasing with increasing distributary channel edge length (linear regression: R2 = 0.88 and 0.79, respectively). In addition, usable channel area was strongly related to vegetated marsh area (linear regression: R2 = 0.82, Fig. 6) and this relationship was used to predict usable channel area for systems without channel data.
Current and historic vegetated wetland area (vegetated wetland area), proportion of current vegetated marsh by marsh type (current marsh type; EEM, estuarine emergent marsh; ESS, estuarine scrub-shrub; and FRT, forested riverine tidal), and estimated capacities (estimated Chinook capacity and estimated Coho capacity) for juvenile Chinook and coho (subyearling and yearling combined) salmon based on spring (Apr–Jun) density ranges (left whisker, 25th percentile; closed diamond, median or 50th percentile; and right whisker, 75th percentile) for current and historic vegetated wetland extents. Estimates are based on regressions between capacity derived from systems with higher resolution channel area estimates and current vegetated marsh area
Current distributary and tidal channel edge lengths by channel type (current channel edge) and total estimated useable habitat area (usable habitat area) for systems with SHSTMP data, with estimated capacity (estimated capacity) for juvenile Chinook and coho (subyearling and yearling combined) salmon based on spring (Apr–Jun) density ranges (left whisker, 25th percentile; closed diamond, median or 50th percentile; and right whisker, 75th percentile) and estimated useable channel edge area by channel and marsh type
Changes in potential vegetated marsh area with SLR scenarios followed several patterns (Fig. 7): (1) increasing potential vegetated tidal wetland area with increasing SLR from 0.0 to 1.4 m (Chetco River, Elk River, Necanicum River, Rogue River, Salmon River, and Sixes River), (2) decreasing potential vegetated tidal wetland area with increasing SLR from 0.0 to 1.4 m (Coos Bay, Coquille River, Siuslaw River, Tillamook Bay, and Yaquina Bay), and (3) initial increases in potential vegetated tidal wetland area followed by a decline in potential area after 0.5–1.4 m SLR (Alsea Bay, Nehalem River, Nestucca Bay, Siletz Bay, and Umpqua River). Of the 16 systems considered, potential vegetated tidal wetland extent fell below current potential extent (0.0 m SLR) by the 1.4 m SLR scenario for 9 of the 16 systems (Alsea Bay, Coos Bay, Coquille River, Nehalem River, Nestucca Bay, Siuslaw River, Tillamook Bay, Umpqua River, and Yaquina Bay). Potential vegetated tidal wetland extent remained higher than the 0.0 m SLR scenario at the 1.4 m SLR scenario for all other systems. Estimated potential vegetated tidal wetland extent under the 0.0 ft SLR scenario from Brophy and Ewald (2017) was strongly correlated with estimated historical estuarine extent from PMEP’s Indirect Assessment of West Coast USA Tidal Wetland Loss data (linear regression: R2 = 0.99), with historical extent being about 16% higher than estimated current potential vegetated extent (linear regression: slope = 1.16). This regression was used to estimate historic extent for estuaries that were not included in PMEP’s Indirect Assessment of West Coast USA Tidal Wetland Loss data. Estuarine habitat summaries for tables of potential vegetated tidal wetland extent with each SLR scenario are available in S3.
Change in potential vegetated tidal wetland extent (areas that would be tidally influenced and within an elevation range that would support vegetation) with 0.0–1.42 m sea level rise (SLR) scenarios from Brophy and Ewald (2017) for coastal Oregon estuaries
Juvenile Salmon Capacity Estimates
Current juvenile (subyearling and yearling combined) capacities derived from usable habitat area for systems with detailed SHSTMP channel features (Coos Bay, Necanicum River, Nehalem River, Nestucca Bay, Siletz River, and Tillamook Bay) ranged from 1110 to 35,956 Chinook salmon and 1111 to 20,403 coho salmon using spring 25th and 75th percentile densities, respectively (Fig. 5). These capacity estimates were derived from detailed spatial data on channel and marsh type for systems with supporting data, and we found that the estimated capacities were strongly related to current vegetated marsh area (power regressions: R2 > 0.8, Fig. 8). The power regressions estimated current juvenile capacities ranging from 453 to 4291 (25th percentile) and 2902 to 33,817 (75th percentile) juvenile Chinook salmon and 271 to 2686 (25th percentile) and 2507 to 20,206 (75th percentile) juvenile coho salmon among coastal Oregon systems (Fig. 4).
Total juvenile capacity (subyearling and yearling combined) for Chinook (top) and coho (bottom) salmon from spring (Apr–Jun) density ranges at 25th, 50th, and 75th percentiles as a function of current vegetated marsh area among systems with SHSTMP mapped channel features (Coos Bay, Necanicum River, Nehalem River, Nestucca Bay, Siletz River, and Tillamook Bay). The capacities for these systems were derived from usable channel edge area and channel and marsh type. This relationship between capacities and total current vegetated marsh area for systems with SHSTMP mapped channel features (i.e., power regression equations) was used to estimate usable channel area adjusted capacities for estuaries that lacked SHSTMP mapped features and for historical and SLR scenario extent data that lacked channel area information
Capacities for historical vegetated tidal wetland extent were also estimated from the power regressions (Fig. 6) and ranged from 589 to 8555 (25th percentile) and 3869 to 71,844 (75th percentile) for Chinook salmon and 355 to 5428 (25th percentile) and 3201 to 38,337 (75th percentile) for coho salmon among coastal Oregon systems (Fig. 4). This translates to current capacities that are 28 to 97% and 33 to 98% of historic capacities for juvenile Chinook and coho salmon, respectively, based on the 75th percentile (Fig. 4).
Similar to historical habitat extents, SLR scenario capacity estimates were based on the power regressions between usable channel area adjusted capacities and vegetated marsh area (Fig. 8). Capacity estimates for future vegetated tidal wetland extents with 0.0–1.4 m sea level rise (SLR) scenarios followed three patterns (Fig. 9): (1) increasing capacity with increasing SLR from 0.0 to 1.4 m (Chetco River, Elk River, Necanicum River, Rogue River, and Sixes River), (2) initial increases in capacity with SLR from 0.23 to 0.75 m SLR followed by decreasing capacity with increasing SLR (Alsea Bay, Nehalem River, Nestucca Bay, Salmon River, Siletz Bay, Tillamook Bay, and Umpqua River), and (3) decreasing capacity with increasing SLR from 0.0 to 1.4 m (Coos Bay, Siuslaw River, and Yaquina Bay). For systems with increasing capacity with increasing SLR, capacities increased approximately 2 to 5 times initial capacities (Fig. 9). For systems with initially increasing potential extent, initial increases in capacity were generally small (less than 1.15 times initial capacity) but then declined to about 0.85 times initial capacities by the 1.4 m SLR scenario. Among the systems with initial increasing capacity, relative capacities remained above initial conditions at 0.0 m SLR for only one system (Siletz Bay) by the 1.4 m SLR scenario (Fig. 9), while the Salmon River, Nestucca Bay, and Nehalem River end up at or near initial capacities by the 1.4 m SLR. For systems with decreasing capacity with increasing SLR, we see declining capacities of 0.99 to 0.75 times initial capacities with increasing SLR (Fig. 9).
Changes in relative capacity from 0.0 m SLR scenario to 4.7 m SLR scenario based on potential vegetated wetland extent (areas that would be tidally influenced and within an elevation range that would support vegetation). Relative capacities are shown based on 25th–75th percentile spring (Apr–Jun) densities (25 to 75% capacity range, relative to 50% at 0.0 m SLR) and 50th percentile spring (Apr–Jun) densities (50% capacity). Values of 1.0 (dashed line) indicate the same capacity as the 0.0 m SLR scenario, values below 1.0 indicate lower capacity, and values above 1.0 indicate increased capacity. Capacity estimates were derived from regressions between total vegetated marsh area and estimated capacity (adjusted by usable habitat area) from a subset of systems
Discussion
The compiled density data showed general patterns of decreasing densities from large river and floodplain habitats to estuarine habitats, with considerable variation in densities among substrata within these habitats. We found that historic and current juvenile salmon capacity of Oregon estuaries varies and was directly related to tidal marsh area. Likewise, the effects of SLR on estuarine habitat and juvenile capacities vary among estuaries and follow the pattern of changes in vegetated tidal wetland habitat extent. Below, we discuss our results in the context of our research questions including how salmon densities vary among habitat types, comparisons of current and historical capacity estimates for coastal Oregon systems, and implications for restoration planning. We then use a case study to demonstrate how the habitat expansion approach can be used as a tool to support recovery planning and restoration evaluation in coastal Oregon estuaries where significant losses of estuarine habitat have occurred (Brophy et al. 2019), as well as other systems. Lastly, we discuss limitations, data gaps, and next steps for developing and refining the tools and data developed in this study to support restoration planning for recovery of coastal Oregon salmon populations.
How Does Juvenile Salmon Density Differ Among Various Habitat Types?
Juvenile Chinook and coho densities generally decreased from large river and floodplain habitats to estuary habitats, with considerable variation in densities among substrata within these habitats and seasons (Figs. 3 and S2. Detailed fish density summary statistics). Higher densities in large river and floodplain habitats compared to estuarine habitats were consistent with decreasing densities with dispersion and mortality as juveniles rear and migrate downstream from their natal habitats to estuaries (Healey 1982; Beamer et al. 2005; Bottom et al. 2005). Differences in densities among substrata were consistent with fish preferences and differences in habitat types needed by juvenile salmon at different life stages (e.g., Healey 1982; Beamer et al. 2005; Bottom et al. 2005) and with the influence of landscape connectivity on fish density patterns in estuaries (e.g., Beamer et al. 2005; Greene et al. 2021). For example, subyearling Chinook and coho salmon densities were higher in distributary channels compared to tidal channels, when considered without marsh type. Distributaries are more connected to natal population sources and can have higher densities compared to adjacent tidal channel habitats, while marsh type can significantly influence fish use of tidal channel habitats (Greene et al. 2021). However, when we considered marsh type, the highest densities were in tidal channels associated with forested riverine tidal wetlands (see S5 in supplemental materials). The density ranges for subyearling Chinook salmon in forested riverine tidal wetlands greatly exceeded distributary densities, but usable channel areas for distributaries were greater than usable channel areas associated with tidal channels for all estuaries with channel data (Fig. 5). Therefore, the relative composition of channel and marsh types can significantly influence capacity estimates.
Such variations in densities among habitat types are a key premise of the habitat expansion approach, which seeks to estimate potential benefits from restoration, or capacities under a variety of habitat scenarios (Roni et al. 2010, 2018; Beechie et al. 2015; Ehinger et al. 2015; Bond et al. 2019; Baker et al. 2020; Pess and Jordan 2019; See et al. 2021). These observed variations among substrata also provide support for considering finer scale habitat information, when possible, but we demonstrate application of the habitat expansion approach where two resolutions of spatial data were available (e.g., with detailed data on estuarine channel features or with only vegetated tidal marsh data).
What Is the Current Juvenile Chinook and Coho Salmon Capacity of Coastal Oregon Estuaries and How Does This Compare to Historic Capacity?
The historic and current juvenile salmon capacity of Oregon estuaries varied by estuary and was directly related to tidal marsh area. Current estimated capacities are 28 to 97% and 33 to 98% of historic capacities for juvenile Chinook and coho salmon, respectively, based on the 75th percentile. This is likely a result of a decline in available vegetated tidal marsh area compared to historic extents. Based on recent mapping of coastal Oregon’s estuaries (Brophy et al. 2019; PMEP 2019), current vegetated tidal marsh areas range from 11 to 903 ha (Fig. 4). These current extents represent approximately 10–95% of historical extent. The current extent of coastal Oregon’s smaller estuaries represents a greater proportion of the historic extent (e.g., Salmon River, Rogue River, and Alsea Bay) compared to larger systems, where the current extent represents a smaller percent of the historical extent (e.g., Coos Bay and Coquille River).
Given that fixed scalars were used to expand habitat areas by juvenile salmon density ranges, patterns of estimated capacity among systems track with differences in current and historic habitat extent (Fig. 4). Although significant variations in density ranges occurred within habitat strata and substrata, capacity estimates would benefit from detailed classification of habitat types. Our results also provide support for estimating capacity based on higher order aggregations of habitat areas. For example, we estimated capacity for a subset of systems using detailed channel mapping data and estimated usable habitat areas by marsh and channel type. This assumed that fish typically prefer slow-water edge habitats and habitats with optimal velocities, depth, and cover (Nickelson 2012; Beechie et al. 2017; Lowery et al. 2017). However, we found that capacities derived from usable habitat area by channel and marsh type were strongly correlated with vegetated marsh areas (R2 > 0.8, Fig. 8). This pattern is useful for comparisons among systems and for estimating benefits from restoration of tidal wetland extent, given that detailed information on channel areas is not needed to estimate capacities. We took advantage of this relationship in our analysis to estimate current and historic capacities for systems without detailed channel mapping data (Fig. 4).
How Will Sea Level Rise Affect Estuarine Habitat Extent and Capacity Estimates?
Our results indicate the effects of sea level rise on habitat extent and juvenile Chinook and coho capacity might vary among estuaries of coastal Oregon. We found that capacity, based on future vegetated tidal wetland extent with 0.0–1.4 m sea level rise scenarios (2070), tracked with predicted changes in potential vegetated tidal marsh extent, and demonstrated three patterns—increasing, decreasing, or initial increases followed by a decrease (Fig. 7, Fig. 9). In systems where an increase in capacity was predicted with increasing SLR (Chetco River, Elk River, Necanicum River, Rogue River, Salmon River, and Sixes River), lower gradient valley slopes might allow increasing inundation of potentially tidally influenced habitat within an elevation range that would support vegetation. The new inundation regime could outpace losses at the delta front due to increased inundation and result in a net increase in potential vegetated marsh area (Brophy and Ewald 2017). These systems could be considered more resilient to potential SLR, although existing flood control and development infrastructure would need to be considered given that the SLR scenarios only model potential extent and do not account for levees, dikes, or other artificial features that may constrain tidal connectivity (Brophy and Ewald 2017). In other systems, vegetated tidal wetland extent and estimated potential capacity decrease with increasing SLR (Coos Bay, Siuslaw River, and Yaquina Bay). These systems are more confined and therefore more sensitive to changes in water elevation and inundation regime (Brophy and Ewald 2017). However, some systems (Alsea Bay, Nehalem River, Nestucca Bay, Salmon River, Siletz Bay, Tillamook Bay, and Umpqua River) exhibit an initial increase followed by a decrease in vegetated tidal wetland extent as SLR increases (Brophy and Ewald 2017).
What Are the Implications for Restoration Planning?
Current and historic estuarine extent and habitat types are an important consideration in restoration planning for coastal Oregon salmon populations. Coastal Oregon’s estuaries vary in both their current extent and their potential or historical extent due to differences in land use histories and their geomorphic setting, and these differences should be considered when developing recovery plans and restoration strategies. As current vegetated tidal marsh extents approach historical extents, we assume less potential for restoration of lateral connectivity (e.g., dike breaching and setbacks designed to increase extent of tidally influenced habitat) to increase capacity. In these places, restoration could focus on improving habitat quality (e.g., increased channel area or vegetated marsh area in subsided marshes) as opposed to increasing the extent of tidally flooded habitat. Where greater differences exist between current and potential extent, restoration of lateral connectivity to increase tidal wetland habitat extent can increase habitat capacity relative to current capacity. However, the actual area should also be considered given that a fixed restoration project area would translate to different proportional increases in potential tidal wetland area, and therefore capacity, while the absolute increases would be the same. For example, restoration of tidal flooding to 100 ha in the Nestucca Bay would nearly double the 135 ha of current extent tidal wetland habitat, and this would increase the proportional extent from 20 to 35% of historical extent, or a 15% increase. In contrast, restoration of tidal flooding to 100 ha in a larger system like Tillamook Bay would only increase the proportional area of current tidal wetland habitat by approximately 5% (29 to 34%). Therefore, restoration strategies and recovery targets should consider the population recovery goals (e.g., increase in capacity needed to meet recovery targets) along with the current and potential area of tidal wetland habitat.
Our approach assumes a linear response between habitat area and capacity and overlooks synergistic effects of restoration projects (Nickelson 2012). For example, the location of restoration sites in an estuarine system can impact tidal and riverine forcing as well as the distribution and extent of mixohaline habitats (Hall et al. 2018b), and restoration of multiple sites may have benefits that exceed the sum of the capacities that would be derived from each project area alone (Chamberlin et al. 2021; Diefenderfer et al. 2021). Improving connectivity between patches of rearing habitat in a highly degraded estuary by strategically locating restoration sites may also have cumulative effects on survival and capacity (Diefenderfer et al. 2021). Restoration of tidal wetland may also improve habitat quality indirectly through improvements in ecosystem processes and services that benefit salmon, even if the habitats are not directly accessible for rearing (Diefenderfer et al. 2021). Furthermore, densities in estuarine habitats are a function of landscape connectivity, with densities generally decreasing as the complexity of pathways or bifurcation order from the source increases (Beamer et al. 2005). Our estimates of capacity do not account for landscape connectivity, but we hypothesize that aggregating observed densities provides a proxy for habitat capacity. Future studies could refine estimates of capacity by developing connectivity metrics to support evaluation of restoration strategies and priorities (e.g., Beamer et al. 2005; Chamberlin et al. 2021).
Future estuarine extent and habitat types are also an important consideration in restoration planning for coastal Oregon salmon populations. Variations in underlying geomorphology as well as levee, dike, and other flood control and development infrastructure may constrain the landward migration of tidal marshes as SLR increases the inundation frequency and duration of lower elevation marshes (Brophy et al. 2019). Therefore, understanding resiliency to climate change and SLR can provide important context for restoration and recovery planning. Our estimates of rearing capacity track predicted changes in vegetated tidal marsh habitat extent under SLR scenarios, given that capacities were a function of predicted vegetated wetland extent. Therefore, these patterns can be used in combination with the amount of current habitat relative to historic habitat to determine the degree to which SLR may impact potential benefits from restoration. Given uncertainties in predicted SLR and climate change in general, we focused on relative changes and the direction of change rather than the magnitude of change in rearing capacity. In this way, the results can be used to identify systems that are more or less sensitive to potential climate change. For example, estimated current vegetated tidal wetland extents for the Coquille River estuary were near the average of all 16 systems, but historical extent was the highest among all systems considered. Therefore, the Coquille River represents a system with an average rearing capacity but has the highest potential rearing capacity among the systems considered. However, the Coquille River estuary was also among the most sensitive to SLR, with a 1.4 m SLR scenario reducing potential vegetated tidal wetland area by approximately 51%. Therefore, the Coquille River estuary represents the greatest opportunity for increased capacity while also being among the most vulnerable to SLR.
Furthermore, the possible landward migration of vegetated tidal wetland extent should also be considered in restoration. However, Brophy and Ewald (2017) noted that connectivity was not considered in the delineation of potential vegetated tidal wetland habitat. Infrastructure (e.g., dikes and developed areas) may impact both restoration potential and vulnerability to SLR, which were not considered in our analysis or the potential extent data. Where systems are vulnerable to SLR due to infrastructure but sufficient opportunity for restoration exists, a balanced restoration strategy that includes restoring tidal connectivity to the higher elevation margins or landward extent of the estuary could increase SLR resiliency. Sea level rise represents just one aspect of climate change that will influence fish habitat. Other factors and processes linked to climate change and SLR, including salt intrusion and tidal forcing, storm surge, precipitation regimes and timing, and temperature, can influence the structure and extent of estuarine habitats as well as the timing and duration of juvenile salmon migration and rearing patterns (Mantua et al. 2010; Chamberlin et al. 2021). In addition, deep surface processes (e.g., subsidence and hydrostatic rebound) as well as shallow surface processes (e.g., compaction, sedimentation, and erosion) can impact elevations and potential vegetated tidal wetland extents over time (Montillet et al. 2018; Brophy and Ewald 2017; Oregon Coastal Management Program (OCMP) 2017). We only considered the impacts of SLR on potential vegetated tidal wetland, yet consideration of other factors would improve evaluations of potential gains from restoration and resilience to climate change. Furthermore, although we compiled density data for large river and floodplain habitats, regional-scale spatial data describing historic or future conditions with climate change were not readily available. While some data are available on larger river habitat features for coastal Oregon systems (e.g., Beechie et al. 2018), development of such data would allow application of our approach to large river and floodplain habitats for coastal Oregon populations.
Restoration Evaluation Case Study
The fish density data and habitat expansion approach used in this study can be used as a tool to support recovery planning and restoration evaluation in Oregon and other systems. To demonstrate an application of the habitat expansion approach, we compared our 25th and 75th percentile densities to subyearling Chinook salmon densities measured in several tidal marshes in the Salmon River estuary. Gray (2005) reported subyearling Chinook salmon densities for several restoration sites where dikes or tide gates were breached to restore tidal connectivity to marsh habitat and one reference site where tidal connectivity had not been impacted. Densities reported by Gray (2005) at the restored sites averaged 51 fish/ha compared to 177 fish/ha at the reference site based on monitoring in estuarine emergent marsh tidal channels in the spring (Apr–Jun). Comparing these densities to our frequency statistics, we found that the restoration sites fell within the 25–50th percentile (30–103 fish/ha), while observed densities at the reference marsh fell within the 50–75th percentile (103–295 fish/ha) for estuarine emergent marsh tidal channels in the spring. This supports the concept that restored sites may have a lower rearing capacity in the early stages of a restoration trajectory, and that ranges of densities can be used to estimate potential responses to restoration along a recovery trajectory or with different functional scenarios (e.g., recently restored compared to a desired reference condition). This is useful from a recovery planning and evaluation perspective given that restoration or recovery targets can be quantified in terms of a range of potential responses as well as potential response time lags to a desired future condition.
Considerations and Future Research Needs
Our approach has assumptions, limitations, and data gaps that are worth noting to inform application and interpretation of our results. First, due to population declines, observations of juvenile fish densities used in the habitat expansion may be biased low (Bond et al. 2019). Therefore, while we assume the 75th percentile represents a conservative proxy for desired future conditions or fully seeded habitats with respect to juvenile habitat capacity, this density may not represent the historic capacity. Other studies have used a 90th percentile for Chinook salmon parr in freshwater (Bond et al. 2019; See et al. 2021), but little justification is provided, and additional research is needed to determine what percentile of distribution best represents capacity. Furthermore, our ranges were derived from all non-zero measurements of juvenile salmon densities and therefore our results are an expected range of juvenile fish densities where and when fish occur rather than instantaneous densities. For restoration planning purposes such as identification, prioritization, or comparisons of relative benefits of restoration strategies, we posit that the relative capacities derived from the habitat expansion approach are more important than the percentile used to estimate capacities.
We also compiled reported densities from a range of sampling methods (e.g., beach seines, fyke trapping, lampara nets) and assumed that densities could be aggregated if fish per unit area could be calculated. We acknowledge that capture efficiencies can vary greatly among sampling methods, which may introduce some error in the frequency statistics and habitat expansions. However, capture efficiencies were rarely reported, and filtering data to a subset of methods or stratifying density statistics by sampling method would significantly impact sample sizes. Consistent assessment and reporting of capture efficiency in future monitoring studies would support further refinement of habitat expansion methods to estimate capacities.
Likewise, our analysis did not account for residency time and therefore did not produce an estimate of total potential fish production. Juvenile salmon residence time varies by species and habitat type, and from freshwater to marine environments (Healey 1982, 1991; Beamer et al. 2005; Chamberlin et al. 2022). For example, subyearling Chinook and coho salmon may spend considerable time rearing in estuarine habitats, presumably from weeks to months, while yearlings may spend little time rearing in estuarine or nearshore habitats (Simenstad et al. 1982; Beamer et al. 2005; Koski 2009). If the average residence time and duration of rearing are known, the density statistics and habitat area estimates developed in this study could be expanded to estimate total rearing capacity in terms of fish produced or supported in a year. For instance, Beamer et al. (2005) reported subyearling Chinook salmon delta rearing fry residence times as an average of 34.2 days, with a duration of 90 days in the Skagit River estuary. Our analysis indicated that the 75th percentile of subyearling Chinook salmon densities were 418/ha when all estuarine habitat types were combined, which would equate to a total annual rearing capacity of 1100 fish/ha, or (418 fish/ha ∙ 90 days)/34.5 days when accounting for rearing duration and residence time (Chamberlin et al. 2022). This annual productive capacity could then be multiplied by usable estuarine habitat area to estimate total fish produced from a system or project, and marine survival estimates could even be applied to adjust these capacities and estimate total adult production per unit estuary area. However, residence times vary among species, life histories, and populations as do marine survival rates; therefore, additional research is needed to develop appropriate scalars for capacity and adult production for Oregon’s coastal estuaries (e.g., Nickelson 2012; Jorgenson et al. 2021).
Few studies we reviewed distinguished between hatchery and naturally produced Chinook salmon, so it is likely that our database includes a mix of natural and hatchery origin fish (e.g., Jones et al. 2018). The presence and magnitude of hatchery releases in a system can influence capacities; therefore, natural origin densities may be biased low in systems with larger hatchery influence. Residence time and abundance of hatchery fish may also influence the life history patterns of natural origin fish and therefore seasonal and habitat densities (e.g., Chamberlin et al. 2021). Similarly, our approach did not consider potential competitive interactions between juvenile Chinook and coho salmon whereby capacities could be inversely related (e.g., as the abundance of one species approaches capacity, the habitat capacity for the other species decreases). We assumed that by using observed densities from a broad geographic range where the species (natural and hatchery) co-occur, the potential bias due to competitive interactions is minimized. However, additional data on hatchery fish densities as well as overlaps in species occurrences is needed to address these questions directly.
Detailed channel mapping for coastal Oregon’s estuaries is critical to the refinement of capacity estimates and potential restoration benefits or habitat scenarios over time. The approaches used in Beechie et al. (2018) have been fully implemented in the Puget Sound, but this scale of mapping has not been completed for the Oregon Coast. Although previous studies have found that channel areas are strongly related to marsh area, considerable geographic variation can occur and habitats in an impaired or newly restored state may have significantly different channel areas than reference conditions or desired future conditions (Hood 2014, 2015). Channel areas can also vary among marsh types and estimates of usable channel area could be refined or scaled based on channel size criteria (e.g., Beamer et al. 2005). Therefore, we recommend continued development of detailed channel mapping for coastal Oregon’s estuaries, as developed by Beechie et al. (2018), as well as possible reconstruction or estimation of historical channel areas (potentially integrating relationships from Hood 2014 and Hood 2015) to support refinements of capacity estimates. In addition, our approach does not account for variations in depth, which can influence habitat capacity and fish use (e.g., Beamer et al. 2005). This would require integration of elevation data (e.g., LiDAR) to refine estimates of usable habitat area or to adjust densities or capacities based on depth.
Fish sampling in additional habitat types will also help refine capacity estimates. Much of the data that is available on fish use of estuarine habitats comes from sampling of distributary channels or tidal channels and little from sampling of flooded marsh or unvegetated tidal flats. We generally assume that fish using the flooded marsh or unvegetated tidal flats are concentrated in distributary and tidal channels that remain wetted during low tides, although density estimates could be biased depending on tidal height at the time of sampling. Few studies have sampled salt marsh at high tide because of the difficulty in sampling these habitats consistently (Hood et al. 2018), and for similar reasons, limited data on fish use of unvegetated tidal flats were available. The question of how to enumerate fish produced in salt marsh and tidal flat habitats to quantify potential benefits of restoring those habitats remains an ongoing research question. This could be addressed with a focused sampling effort of both salt marsh and tidal flats as well as distributaries and tidal channels at different tide heights and cycles.
Summary and Conclusions
We synthesized existing data on juvenile Chinook and coho salmon in estuarine and other habitats to assist with restoration and recovery planning in Oregon and elsewhere. The habitat expansion approach and habitat-specific juvenile density ranges developed in this study provide a simple and robust tool to support estimation of potential benefits from restoration or habitat scenarios. This is an important contribution to salmon recovery and restoration efforts, which currently do not have strong biological metrics to evaluate alternative restoration designs or strategies. As a result, economic or physical metrics (i.e., hydrology, scour, velocity, or habitat area) drive evaluations and design choices, and these do not necessarily directly link to habitat capacity. The methods we developed in this study will provide a better understanding of the link between habitat improvement and salmon recovery and can be further refined when paired with connectivity models and improved mapping of current, restored, and future habitat extent and quality as well as by collecting additional data on juvenile salmon use, density, and residence time in various estuarine and floodplain habitats.
Data Availability
All spatial data used to estimate habitat area extents were derived from data developed by others (Brophy and Ewald 2017; Beechie et al. 2018; PMEP 2019, Brophy et al. 2019), and all juvenile density statistics were derived from other sources (see S1. Reference list for fish density). No data were collected or mapped as part of this project.
References
Apgar, T.M., J.E. Merz, B.T. Martin, and E.P. Palkovacs. 2020. Alternative migratory strategies are widespread in subyearling Chinook salmon. Ecology of Freshwater Fish 30 (1): 125–139.
Baker, M., A. Domanski, T. Hollweg, J. Murray, D. Lane, K. Skrabis, R. Taylor, T. Moore, and L. DiPinto. 2020. Restoration scaling approaches to addressing ecological injury: The habitat-based resource equivalency method. Environmental Management 65: 161–177.
Beamer, E., A. McBride, C. Greene, R. Henderson, G. Hood, K. Wolf, K. Larsen, C. Rice, and K. Fresh. 2005. Delta and nearshore restoration for the recovery of wild Skagit River Chinook salmon: linking estuary restoration to wild Chinook salmon populations. Skagit Chinook Recovery Plan, Skagit River System Cooperative Research Program, LaConner, Washington.
Beechie, T.J., C. Fogel, C. Nicol, and G.R. Pess. 2018. Monitoring large river, floodplain, and delta habitat status and trends in the Oregon coast coho evolutionarily significant Unit: FY 2018 progress report. NOAA Fisheries, Seattle, Washington.
Beechie, T., H. Imaki, J. Greene, A. Wade, H. Wu, G. Pess, P. Roni, J. Kimball, J. Stanford, P. Kiffney, and N. Mantua. 2013. Restoring salmon habitat for a changing climate. River Research and Applications 29: 1535–1467.
Beechie, T.J., G. Pess, H. Imaki, A. Martin, J. Alvarez, and D. Goodman. 2015. Comparison of potential increases in juvenile salmonid rearing habitat capacity among alternative restoration scenarios, Trinity River, California. Restoration Ecology 23: 75–84.
Beechie, T.J., O. Stefankiv, B. Timpane-Padgham, J.E. Hall, G.R. Pess, M. Rowse, M. Liermann, K. Fresh, and M.J. Ford. 2017. Monitoring salmon habitat status and trends in Puget Sound: development of sample designs, monitoring metrics, and sampling protocols for large river, floodplain, delta, and nearshore environments. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-NWFSC-137.
Bond, M.H., T.G. Nodine, T.J. Beechie, and R.W. Zabel. 2019. Estimating the benefits of widespread floodplain reconnection for Columbia River Chinook salmon. Canadian Journal of Fisheries and Aquatic Sciences 76 (7): 1212–1226.
Bottom, D.L., C.A. Simenstad, J. Burke, A.M. Baptista, D.A. Jay, K.K. Jones, E. Casillas, and M.H. Schiewe. 2005. Salmon at river’s end: the role of the estuary in the decline and recovery of Columbia River salmon. NOAA Technical Memorandum NMFS-NWFSC-68, Northwest Fisheries Science Center, Seattle, Washington.
Brophy, L.S., and M.J. Ewald. 2017. Modeling sea level rise impacts to Oregon’s tidal wetlands: maps and prioritization tools to help plan for habitat conservation into the future. Report prepared for MidCoast Watersheds Council, Newport, Oregon.
Brophy, L.S., C.M. Greene, V.C. Hare, B. Holycross, A. Lanier, W.N. Heady, K. O’Connor, H. Imaki, T. Haddad, and R. Dana. 2019. Insights into estuary habitat loss in the western United States using a new method for mapping maximum extent of tidal wetlands. PLoS ONE 14 (8): e0218558.
Burgess, S., K. Ross, C. Clark, M. Krall, D. Arterburn, and J. Hall. 2019. Lower mainstem Nooksack River salmon habitat assessment. Report prepared for Whatcom County Flood Control Zone District, Bellingham, Washington.
Chamberlin, J.W., W.T. Zackey, and O. Stefankiv. 2022. Estimating rearing capacity for juvenile Chinook salmon in tidal deltas: implications for recovery planning in the Snohomish delta. Report for the Estuary and Salmon restoration Program (ESRP), Olympia, Washington, Project #14–2303.
Chamberlin, J., J.E. Hall, T. Zackey, F. Leonetti, M. Rustay, and C.A. Rice. 2021. Broad scale spatial and temporal variability in Chinook salmon abundance reflects important life history distinctions relevant to restoration in an estuarine landscape. Estuaries and Coasts. https://doi.org/10.1007/s12237-021-00994-3.
Davis, M.J., I. Woo, C.S. Ellings, S. Hodgson, D.A. Beauchamp, G. Nakai, and S.E.W. De La Cruz. 2022. A climate-mediated shift in the estuarine habitat mosaic limits prey availability and reduces nursery quality for juvenile salmon. Estuaries and Coasts 45: 1445–1464.
Diefenderfer, H.L., G.D. Steyer, M.C. Harwell, A.J. LoSchiavo, H.A. Neckles, D.M. Burdick, G.E. Johnson, K.E. Buenau, E. Trujillo, and J.C. Callaway. 2021. Applying cumulative effects to strategically advance large-scale ecosystem restoration. Frontiers in Ecology and the Environment 19 (2): 108–117.
Ehinger, S.I., J.P. Fisher, R. McIntosh, D. Molenaar, and J. Walters. 2015. Working draft, April 2015: use of the Puget Sound nearshore habitat values model with habitat equivalency analysis for characterizing impacts and avoidance measures for projects that adversely affect critical habitat of ESA-listed Chinook and chum salmon. National Marine Fisheries Commission, Oregon-Washington Coastal Office, Lacey, Washington.
Finn, R.J., L. Chalifour, S.E. Gergel, S.G. Hinch, D.C. Scott, and T.G. Martin. 2021. Quantifying lost and inaccessible habitat for Pacific salmon in Canada’s Lower Fraser River. Ecosphere 12 (7): e03646.
Gray, A. 2005. The Salmon River estuary: restoring tidal inundation and tracking ecosystem response. Doctoral dissertation. University of Washington, Seattle.
Greene, C.M., E. Beamer, J. Chamberlin, G. Hood, M. Davis, K. Larsen, J. Anderson, R. Henderson, J. Hall, M. Pouley, T. Zackey, S. Hodgson, C. Ellings, and I. Woo. 2021. Landscape, density-dependent, and bioenergetic influences upon Chinook salmon in tidal delta habitats: comparison of four Puget Sound estuaries. Final Report prepared for Estuary Salmon and Restoration Program, ESRP Report 13–1508.
Greene, C.M., K. Blackhart, J. Nohner, A. Candelmo, and D.M. Nelson. 2015. A national assessment of stressors to estuarine fish habitats in the contiguous USA. Estuaries and Coasts 38 (3): 782–799.
Hall, J.E., T.P. Khangaonkar, C.A. Rice, J. Chamberlin, T. Zackey, F. Leonetti, M. Rustay, K. Fresh, A. Kagley, and M. Rowse. 2018a. Characterization of salinity and temperature patterns in a large river delta to support tidal wetland habitat restoration. Northwest Science 92 (1): 36–52.
Hall, J.E., C.M. Greene, O. Stefankiv, J.H. Anderson, B. Timpane-Padgham, T.J. Beechie, and G.R. Pess. 2018b. Large river habitat complexity and productivity of Puget Sound Chinook salmon. PLoS ONE 13 (11): e0205127.
Hall, J., and P. Roni. 2018. Literature review summary results to support SBI (Salmon Benefit Index) development. Memorandum prepared for the Puget Sound Partnership, Tacoma, Washington.
Healey, M.C. 1982. Juvenile Pacific salmon in estuaries: The life support system. In Estuarine comparisons, ed. V.S. Kennedy, 315–341. New York: Academic Press.
Healey, M.C. 1991. Life history of Chinook salmon. In Pacific salmon life histories, ed. C. Groot and L. Margolis, 311–393. Vancouver, British Columbia: University of British Columbia Press.
Hillman, T., P. Roni, and J. O’Neal. 2016. Effectiveness of tributary habitat enhancement projects. Report prepared for the Bonneville Power Administration, Portland, Oregon.
Hood, W.G. 2014. Differences in tidal channel network geometry between reference marshes and marshes restored by historical dike breaching. Ecological Engineering 71: 563–573.
Hood, W.G. 2015. Geographic variation in Puget Sound tidal channel planform geometry. Geomorphology 230: 98–108.
Hood, W.G., E.M. Beamer, and R. Henderson. 2018. Juvenile salmon density on marsh surfaces versus within tidal channels. Salish Sea Ecosystem Conference, Seattle, WA. April 6, 2018.
Jones, K.K., T.J. Cornwell, D.L. Bottom, S. Stein, and K.J. Anlauf-Dunn. 2018. Population viability improves following termination of Coho salmon hatchery releases. North American Journal of Fisheries Management 38 (1): 39–55.
Jorgensen, J.C., C. Nicol, C. Fogel, and T.J. Beechie. 2021. Identifying the potential of anadromous salmonid habitat restoration with life cycle models. PLoS ONE 16 (9): e0256792.
Koski, K.V. 2009. The fate of coho salmon nomads: The story of an estuarine rearing strategy promoting resilience. Ecology and Society 14 (1): 4.
Lotze, H.K., H.S. Lenihan, B.J. Bourque, R.H. Bradbury, R.G. Cooke, M.C. Kay, S.M. Kidwell, M.X. Kirby, C.H. Peterson, and J.B.C. Jackson. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312 (5781): 1806–1809.
Lowery, E.D., J.N. Thompson, E. Connor, D. Pflug, B. Donahue, and J.P. Shannahan. 2017. Seasonal distribution and habitat associations of salmonids with extended juvenile freshwater rearing in different precipitation zones of the Skagit River, WA. Seattle City Light, Washington.
Magnusson, A., and R. Hilborn. 2003. Estuarine influence on survival rates of coho (Oncorhynchus kisutch) and Chinook salmon (Oncorhynchus tshawytscha) released from hatcheries on the US Pacific Coast. Estuaries 26 (4): 1094–1103.
Mantua, N., I. Tohver, and A. Hamlet. 2010. Climate change impacts on streamflow extremes and summertime stream temperature and their possible consequences for freshwater salmon habitat in Washington State. Climatic Change 102 (1–2): 187–223.
Montillet, J.P., T.I. Melbourne, and W.M. Szeliga. 2018. GPS vertical land motion corrections to sea-level rise estimates in the Pacific Northwest. Journal of Geophysical Research: Oceans 123 (2): 1196–1212.
Morley, S.A., J.D. Toft, and K.M. Hanson. 2012. Ecological effects of shoreline armoring on intertidal habitats of a Puget Sound urban estuary. Estuaries and Coasts 35 (3): 774–784.
National Marine Fisheries Service (NMFS). 2016. Endangered Species Act Recovery Plan for Oregon Coast coho salmon.
National Marine Fisheries Service (NMFS). 2021. 12-Month Findings on petitions to list spring-run Oregon Coast Chinook salmon and spring-run southern Oregon and northern California coastal Chinook salmon as threatened or endangered under the endangered species act. Federal Register 86:156 (17 August 2021) 45970–45974.
Nickelson, T. 2012. Futures analysis for wetlands restoration in the Coquille River basin: how many adult coho salmon might we expect to be produced? Report prepared for The Nature Conservancy, Seattle, Washington.
Nickelson, T.E., and P.W. Lawson. 1998. Population viability of coho salmon, Oncorhynchus kisutch, in Oregon coastal basins: application of a habitat-based life cycle model. Canadian Journal of Fisheries and Aquatic Sciences 55 (11): 2383–2392.
Oregon Coastal Management Program (OCMP). 2017. Sea level rise exposure inventory for Oregon’s estuaries.
Pacific Marine and Estuarine Fish Habitat Partnership (PMEP). 2019. West Coast USA estuarine biotic habitat. https://www.pacificfishhabitat.org/data/estuarine-biotic-habitat.
Pess, G., and C.E. Jordan, editors. 2019. Characterizing watershed-scale effects of habitat restoration actions to inform life cycle models: case studies using data-rich vs. data-poor approaches. NOAA Technical Memorandum NMFS-NWFSC, Seattle, Washington.
Pess, G., S. Morley, J.L. Hall, and R.K. Timm. 2005. Monitoring floodplain restoration. In Monitoring stream and watershed restoration, ed. P. Roni, 127–165. Bethesda, Maryland: American Fisheries Society.
QGIS Development Team. 2009. QGIS Geographic Information System. Open Source Geospatial Foundation. http://qgis.org.
Reeves, G. H., F. H. Everest, and T. E. Nickelson. 1989. Identification of physical habitats limiting the production of coho salmon in western Oregon and Washington. U.S. Forest Service PNW-GTR 245. Portland, Oregon.
Roni, P., P.J. Anders, T.J. Beechie, and D.J. Kaplowe. 2018. Review of tools for identifying, planning, and implementing habitat restoration for Pacific salmon and steelhead. North American Journal of Fisheries Management 38 (2): 355–376.
Roni, P., T.J. Beechie, and R. E., Bilby, F. E. Leonetti, M. M. Pollock, and G. P. Pess. 2002. A review of stream restoration techniques and a hierarchical strategy for prioritizing restoration in Pacific Northwest watersheds. North American Journal of Fisheries Management 22: 1–20.
Roni, P., T. Beechie, G. Pess and K. Hanson. 2014. Fish-habitat relationships and effectiveness of habitat restoration. NOAA Technical Memorandum NMFS-NWFSC-127, Seattle, Washington.
Roni, P., J.E. Hall, S.M. Drenner, and D. Arterburn. 2019. Monitoring the effectiveness of floodplain habitat restoration: A review of methods and recommendations for future monitoring. Wiley Interdisciplinary Reviews: Water 6 (4): e1355.
Roni, P., K. Hanson, and T. Beechie. 2008. Global review of physical and biological effectiveness of stream rehabilitation. North American Journal of Fisheries Management 28: 856–890.
Roni, P., G. Pess, S.T. Beechie, and S. Morley. 2010. Estimating changes in coho salmon and steelhead abundance from watershed restoration: How much restoration is needed to measurably increase smolt production? North American Journal of Fisheries Management 30: 1469–1484.
See, K.E., M.W. Ackerman, R.A. Carmichael, S.L. Hoffmann, and C. Beasley. 2021. Estimating carrying capacity for juvenile salmon using quantile random forest models. Ecosphere 12 (3): e03404.
Simenstad, C.A., and J.R. Cordell. 2000. Ecological assessment criteria for restoring anadromous salmonid habitat in Pacific Northwest estuaries. Ecological Engineering 15 (3–4): 283–302.
Simenstad, C.A., K.L. Fresh, and E.O. Salo. 1982. The role of Puget Sound and Washington coastal estuaries in the life history of Pacific salmon: An unappreciated function. In Estuarine comparisons, ed. V.S. Kennedy, 343–364. New York: Academic Publishers.
Acknowledgements
We would like to thank Derek Arterburn and Michelle Krall with development of the fish density database and spatial data analysis used in this study.
Funding
The funding for this project was provided by The Nature Conservancy. Development of the database was funded primarily by The Nature Conservancy, with some earlier literature reviews funded by the Puget Sound Partnership.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mark S. Peterson.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hall, J., Roni, P., Ross, K. et al. Estimating Juvenile Salmon Estuarine Carrying Capacities to Support Restoration Planning and Evaluation. Estuaries and Coasts 46, 1046–1066 (2023). https://doi.org/10.1007/s12237-023-01185-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01185-y