Abstract
The lower Rio Grande is a river-dominated estuary that serves as the border between Texas, USA, and Tamaulipas, Mexico. River estuaries encompass the section of the river influenced by tidal exchange with the Gulf of Mexico, but the connection with the Rio Grande is intermittent and can be temporarily open or closed. During the 4.8-year study period, the river mouth was closed 30% of the time, mostly during average or dry climatic conditions, with the temporary closing of the river mouth being linked to hydrology. When the Rio Grande estuary is closed, salinity is low (1.5 psu compared to 4.8 psu when open), nitrate plus nitrite are low (4.4 μM compared to 31.5 μM when open), and ammonium is high (9.6 μM compared to 4.3 μM when open), but chlorophyll is similar (20 μg/L compared to 21 μg/L when open). Benthic macrofaunal abundance and biomass are higher when the river mouth is closed: 16,700 individuals m−2 and 3.3 g m−2 compared to 8800 individuals m−2 and 2.4 g m−2 when the Rio Grande river mouth is open. Benthic macrofaunal community structure is divided into two groups: chironomid larvae and Oligochaeta dominated when the river mouth was closed, whereas polychaetes Mediomastus ambiseta and Streblospio benedicti dominated when the river mouth was open. The implications of these results for managing freshwater flows are that the open and closed conditions each have a characteristic benthic macrofaunal community that is strongly influenced by system hydrology.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
River-dominated estuaries are narrow and drain directly into oceans rather than into semi-enclosed bays. Historical studies have stressed the importance of freshwater inflow to estuarine systems and have demonstrated the role of inflow as a major factor driving estuary functioning and health (Chapman 1966; Kalke 1981). Inflows serve a variety of important functions in estuaries, including the creation and preservation of low-salinity nurseries, sediment and nutrient transport, allochthonous organic matter inputs, and movement and timing of critical estuarine species (Longley 1994). The nursery habitat function is facilitated by tidal exchange with the adjacent sea for species with estuarine-dependent life cycles. Studies have also highlighted the importance of river outflow to shelf dynamics (Garvine 1974), fisheries (Aleem 1972), pollution (Tuholske et al. 2021), and connectivity among coastal components (Justić et al. 2021).
Estuaries are geologically and hydrologically diverse (Elliott and McLusky 2002; Montagna et al. 2013). Low-flow estuaries often have a connection to the sea that is intermittent, i.e., temporarily open and closed estuaries (TOCE), because low-flow rates can cause the basin to be cut off from its connection to the sea (Allen 1983), and sediment may deposit at the mouth of the estuary due to longshore transport (Hinwood and McLean 2015). The ecological effects of intermittent opening and closing on estuarine organisms are unclear. For example, in Australia, benthic community structure is different in open and closed estuaries of Australia, but open and closed estuaries are also a result of different catchment sizes (Hastie and Smith 2006). Small watersheds lead to a smaller area for rain to flow downstream and lead to lower inflow and a higher likelihood that estuaries are closed (Mondon et al. 2003). So it is possible that catchment size is the primary driver of benthic community patterns (Hastie and Smith 2006). Also, in Australia there were no correlations with physical classifications or linkages at appropriate spatial and temporal scales (Dye 2006). There is a need for further examination of ecological effects in intermittent estuaries.
The goal of the current study is to determine the effects of intermittent opening and closing of the Rio Grande, Texas, USA, estuary on benthic communities. Benthic infauna (body length > 0.5 mm) are especially sensitive to changes in inflow and can be useful in determining its effects on estuarine systems over time (Montagna and Kalke 1992, 1995; Montagna et al. 2013; Montagna 2021). Benthos are excellent indicators of environmental effects of a variety of stressors because they are abundant, diverse, and sessile. Benthos abundance, biomass, and diversity were measured to assess change over time. The hypothesis is that benthic community structure is different when the estuary is open versus closed estuary as they relate to differences in freshwater inflow and connection to the sea. In addition, relevant water quality and sediment variables (i.e., salinity, temperature, dissolved oxygen, nutrients, chlorophyll, grain size, and sediment carbon and nitrogen content) were measured during each sampling period to assess inflow effects on the overlying water column and sediments, which make up benthic habitat.
Methods
Field and Laboratory Analyses
The Rio Grande was sampled quarterly between 25 October 2000 and 6 August 2005. Previous benthic studies (Montagna and Li 2010) demonstrated quarterly sampling to be effective in capturing temporal benthic dynamics, while economizing on temporal replication. Quarterly sampling occurred every January, April, July, and October. The timing of the sampling captures the major seasonal inflow events and temperature changes in Texas estuaries (Montagna et al. 2011).
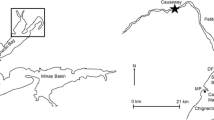
Three stations on the lower Rio Grande were chosen between the confluence with the Gulf of Mexico and Brownsville (Fig. 1). Stations A (25° 57.584ʹ N, 97° 13.662ʹ W), B (25° 57.796ʹ N, 97° 12.668ʹ W), and C (25° 57.720ʹ N, 97° 11.105ʹ W) were 12.6 km, 11.3 km, and 5.5 km from the Gulf of Mexico respectively. In April 2002, it was discovered that station C was not on the main channel of the river, but in a secondary meander channel that was situated north of the main channel. A new station, D (25° 57.610ʹ N, 97° 11.089ʹ W), was established in the main channel, approximately 100 m from station C. Sampling at station D began in July 2002 and continued quarterly to July 2005 (Table S1). After being missed in July 2002, sampling resumed at station C in October 2002. One additional station, E (25° 57.953ʹ N, 97° 10.420ʹ W), located 1.8 km (1.1 mi) downstream of station D and 5.1 km (3.2 mi) from the mouth, was added in October 2002.
Environmental samples were collected instantaneous and synoptically with benthic samples during each sampling period and all data is available online (Montagna 2022). Salinity (psu), conductivity (mS cm−1), temperature (°C), pH, and dissolved oxygen concentration (mg L−1) were measured using multiprobe sondes and water quality meters. Measurements were made both at the surface (0.1 m deep) and the bottom (0.1 to 0.2 m above the sediment–water interface). A YSI 6920 multiprobe sonde was used with accuracy as follows: dissolved oxygen (DO) ± 0.2 mg L−1, temperature ± 0.15 °C, pH ± 0.2 units, depth ± 0.02 m, and salinity greater of ± 1% of reading or ± 0.1 psu. Salinity levels were automatically corrected to 25 °C.
Water samples were also collected at the surface (by hand) and at the bottom (using a horizontal mounted Van Dorn bottle). Water for chlorophyll analysis was filtered onto glass fiber filters in the field, placed on ice (< 4.0 °C), and kept in the dark. Nutrient samples were filtered in the field to remove biological activity (0.45-μm polycarbonate filters) and also placed in the dark on ice (< 4.0 °C). In the laboratory, chlorophyll-a was extracted overnight and read on a Turner Model 10-AU fluorometer using a non-acidification technique (USEPA 1997; Welschmeyer 1994). Nutrient analysis was conducted using a LaChat QC 8000 ion analyzer with computer-controlled sample selection and peak processing. Nutrients measured were as follows (concentration ranges; QuikChem method): nitrate + nitrate (0.03–5.0 μM; 31–107-04–1-A), silicate (0.03–5.0 μM; 31–114-27–1-B), ammonium (0.07–3.57 μM; 31–107-06–5-A), and phosphate (0.03–2.0 μM; 31–115-01–3-A).
Sediment grain size analysis was performed using standard geologic procedures (Folk 1964). A 20 cm3 sediment sample was mixed with 50 mL of hydrogen peroxide and 75 mL of deionized water to digest organic material in the sample. The sample was wet sieved through a 62-μm mesh stainless steel screen using a vacuum pump and a Millipore Hydrosol SST filter holder to separate rubble and sand from silt and clay. After drying, the rubble and sand were separated on a 125-μm screen. The silt and clay fractions were measured using pipette analysis. Percent contribution by weight was measured for four components: rubble (e.g., shell hash), sand, and mud (silt + clay).
The proportions of organic and inorganic carbon and nitrogen content in the sediment were measured, as were carbon and nitrogen isotopes δ13C and δ15N, using a Finnigan Delta Plus mass spectrometer linked to a CE instrument NC2500 elemental analyzer. The system uses Dumas-type combustion chemistry to convert nitrogen and carbon in solid samples to nitrogen and carbon dioxide gases. These gases are purified by chemical methods and separated by gas chromatography. The stable isotopic composition of the separated gases is determined by a mass spectrometer designed for use with the NC2500 elemental analyzer. Standard material of known isotopic composition was run every tenth sample to monitor the system and ensure the quality of the analyses.
Benthos were sampled with a 6.7-cm diameter core tube (35.26 cm2) held by divers or with a coring pole Three replicate cores were taken within a 2-m radius at each station. Cores were sectioned at depth intervals of 0–3 cm and 3–10 cm. Samples were preserved in the field with 5% buffered formalin. In the laboratory, samples were sieved on 0.5-mm mesh screens, sorted, identified to the lowest taxonomic level possible, and counted. Dry weight biomass was measured by drying for 24 h at 55 °C, and then weighing. The carbonate shells of mollusks were dissolved using 1 N HCl and rinsed with fresh water before drying. Abundance and biomass were extrapolated to the number of individuals (ind.) or biomass (g) per m2, but diversity metrics were not extrapolated and are reported per sample (1/35.26 cm−2).
Hydrology
The approach used to assess temporal trends is to compare periods when the river mouth is open or closed, and to determine climate influences during wet or dry months. Wet and dry month thresholds were determined using freshwater inflow data from a hydrological station ~ 70 km upstream from the benthic sampling stations, near Brownsville, Texas, which is managed by the International Boundary and Water Commission (Station 08–4750.00; www.ibwc.gov/wad/DDQBROWN.htm). Daily flow was smoothed by averaging the 30 days prior to and including each daily flow value. This 30-day criterion was used to account for the lag in benthic response after a freshwater event (Montagna and Kalke 1992). To classify wet and dry periods, the 30-day daily flow means were calculated using the 20-year period from 1985 to 2005. Macrofauna sample dates were deemed to be in “dry” weather conditions if the date sampled was in the lower 25% of 30-day daily flow mean, and in “wet” weather conditions if the date sampled was in the higher 75% of 30-day daily flow mean.
Statistical Analyses
A one-way block analysis of variance (ANOVA) was run on replicates to test for differences in benthic response among stations and dates. Sampling dates are the main effect because the main goal is to test for differences over time. Stations are blocks because they were incomplete and are mainly a form of replication for date effects. No interaction exists because this is an incomplete block design. Linear contrasts were used to test for differences between open and closed periods, and wet and dry periods. Statistical analyses were performed using SAS software (SAS 2017). For benthic analyses, the sections were summed to a depth of 10 cm, and abundance and biomass were log-transformed prior to analysis. Species diversity was not transformed.
Multivariate analyses were used to analyze species distributions and how environmental variables affect distributions. The water column structure and sediment structure were each analyzed using principal component analysis (PCA). PCA reduces multiple environmental variables into component scores, which describe the variance in the data set to discover the underlying structure in a data set. The first two principal components were used. Spearman rank correlations between principal component scores were calculated to examine the relationship between sediment and water column data. All variables were averaged by date-station and standardized to a normal distribution with a mean of 0 and variance of 1 prior to analysis so that the relative scale of each variable did not affect the analysis.
Macrofaunal community structure was analyzed with non-metric multi-dimensional scaling (nMDS) analysis using a Bray–Curtis similarity matrix among stations or station-date combinations. The resulting nMDS plot represents the macrofaunal community relationship among stations spatially so that the distances among stations are directly related to the similarities in macrofaunal species compositions among those same stations (Clarke et al. 2014). Relationships within each nMDS were highlighted with a cluster analysis using the group average method, based on Bray–Curtis similarity matrices. Cluster analysis was displayed as similarity contours on the nMDS plots and in dendrograms, both using percentage similarity among factors. Significant differences between each cluster were tested with the SIMPROF permutation procedure using a significance level of 0.05. Data were square root transformed prior to analysis using Primer software (Clarke and Gorley 2015).
Results
During the first week of February 2001, a sand bar formed and closed the mouth of the Rio Grande, stopping exchange with the Gulf of Mexico. The mouth was artificially opened with a backhoe on 18 July 2001 by the International Boundary and Water Commission (US State Department); however, it closed again in November 2001. The mouth of the Rio Grande was manually opened again on 9 October 2002 at Boca Chica Beach but closed on 15 October 2002. On 2 November 2002, a large rainstorm occurred near the river mouth, east of Brownsville, Texas, which caused enough flow pressure to breach the berm, restoring exchange between the river and the sea. The Rio Grande mouth has remained open from that date to the end of the study period (Randy Blankenship, personal communication, 20 May 2003). The mouth was open when the Rio Grande was sampled in late November 2002. Based on available reports, the river mouth was not blocked during the sampling period (October 2002 to July 2003), in fact heavy rain occurred in October to November 2002 that delayed sampling of stations C and E for a month (Fig. 2A). Salinity change over time is a function of both Gulf of Mexico exchange and river flow. River flow was low from the beginning of the sampling period in 2000 until late September 2003 (Fig. 2B). A series of large flood events occurred from September to November 2003, April to July 2004, September to October 2004, and July 2005.
Overall average salinities among all stations and periods (both open and closed) in the Rio Grande were low (4.0 ± 3.9 (mean ± SD); Table 1), and mean temperatures were high (25.2 ± 4.1 °C). Depths were shallow (0.3 ± 0.3 m). Ammonium levels were lower (5.6 ± 12.4 μM) than nitrite + nitrate (25.8 ± 25.7 μM). The nitrogen to phosphorous ratio (N:P) was 5.3 because phosphate was only 5.9 μM. The average chlorophyll-a concentration was high at 21.0 ± 15.2 μg/L.
The PCA for water quality variables found 48% of the variance is explained by two new variables, PC1 and PC2 (Fig. 3). The first and second principal components (PC1 and PC2) explained 30% and 18% of the variation within the data set. Low temperature and high dissolved oxygen, chlorophyll, and pH were inversely related along PC1, which explains seasonal differences of winter and summer. The PC loading vectors for low-salinity and high dissolved inorganic nitrogen and silicate lined up with PC2, explaining freshwater input, which dilutes salinity while delivering nutrients.
The opening and closing of the river mouth did not appear to affect water quality because the samples collected when the mouth was open or closed ranged across the entire freshwater inflow axis (i.e., PC2; Fig. 4A). Samples collected in winter and spring often had positive PC1 values and samples collected in summer and fall mostly had negative PC1 values (Fig. 4B). Again, seasonal samples ranged across the entire PC2 axis indicating there were no seasonal effects on the freshwater inflow response. Samples collected during wet periods aligned with the freshwater inflow axis because most wet period samples had positive PC2 values and average and dry periods had negative PC2 values (Fig. 4C). Thus, inflow is related to PC2 (i.e., the salinity/nutrient axis), there is some link between season and PC1 (i.e., the DO/temperature axis), and opened/closed does not appear to be correlated with either PCA axis.
Classification of samples based on principal component (PC) scores based on water quality loadings (Fig. 3). A Symbols are when river mouth is open (O) or closed (C). B Season where winter = 1, spring = 2, summer = 3, and fall = 4. C Climatic period where average = A, dry = D, and W = wet
The Rio Grande sediments are sandy (52%) with low porewater content (36%) and low total organic carbon (0.6%); nitrogen content was nearly unmeasurable (0.06%) (Table 1). The PCA for Rio Grande sediments explained 85% of the variance in the dataset (Fig. 5A). Mud versus sand content drove the response, explaining 62% of the variance. Percent nitrogen, porewater percent content, and total organic carbon content correlated strongly with mud content. The carbon isotope variable was the only one loading on PC2, which explained 23% of the variance. Sediment samples were collected in fall only. There were no differences among wet periods and dry periods. There was some evidence that river mouth opening influenced sediment characteristics because 6 of 7 samples when the mouth was closed were negative for PC1, meaning they were sandier (Fig. 5B).
Benthic macrofaunal abundance, biomass, and diversity varied over time (Fig. 6). All benthic metrics were similar among all stations (Table 2). Abundance averaged about 10,600 ind./m2 and biomass averaged 2.58 g/m2 overall (Table 1). Analyses focused on change over time and there were differences in abundance and biomass with opening and closing of the river mouth and with wet periods as compared to average and dry periods (Table 2A). There were higher benthic macrofaunal abundance and biomass when the river mouth was closed (16,678 ind./m2 and 3.27 g/m2 compared to 8762 ind./m2 and 2.36 g/m2 respectively), but species richness was similar during closed and open periods (4.2 and 3.9 species/sample respectively) (Table 2B). Abundance, biomass, and richness decreased from dry to average to wet climatic periods (Table 2C).
Non-metric multidimensional scaling (nMDS) analysis indicates there is complex seriation over time related to the opening and closing of the river mouth and climatic periods (Fig. 7). This difference is illustrated by large shifts from July 2003 (03–07) to November 2003 (03–11), which was a transition from an open-average period to an open-wet period; and from April 2002 (02–04) to July 2002 (02–07), which is a transition from a closed-average period to a closed-dry period. The two most different samples (i.e., the furthest left versus the furthest right) were July 2002 during a closed-dry period and November 2003 during an open-wet period.
Benthic community structure change by non-metric multidimensional scaling over time. Benthic macrofaunal species abundances are averaged for each sampling date. Symbols are for when the river mouth is open (open) or closed (filled) and for dry (triangle), average (square), and wet (circle) periods averaged where flow is averaged over 30 days prior to sampling. Data labels are the year-month when sampling occurred
Benthic macrofaunal diversity in the Rio Grande was low with only a total of 43 species found in all samples, but four of the species were insect larvae or nymphs (Table S1). Of the 43, 11 were dominant under various conditions (Tables 3 and S2). Chironomidae larvae were dominant when the river was closed and conditions were dry. The dominant polychaetes during dry conditions were Mediomastus ambiseta and Streblospio benedicti. S. benedicti was more abundant when the river mouth was open, while Oligochaeta were more abundant when the river mouth was closed.
Biotic responses were further linked to abiotic drivers using correlation analysis among the PC scores for samples and the benthic responses at the time (Table 4). Abundance (r = −0.35, p ≤ 0.0010), biomass (r = −0.45, p < 0.0001), richness (r = −0.45, p < 0.0001), N1 diversity (r = −0.31, p ≤ 0.0038), and Hʹ diversity (r = −0.33, p ≤ 0.0025) were inversely correlated with water column PC2, meaning that freshwater inflow was related to decreases in benthic metrics. There were no correlations between water column PC1 and any benthic metric, meaning that season did not drive benthic responses. There were no relationships between the benthic metrices and sediment PC scores; therefore, neither sediment type nor biogeochemical variables drive benthic response in the Rio Grande.
Discussion
The intermittent nature of the Rio Grande is caused by reduced inflow to the system. During average and dry climatic periods, a sand bar can form at the mouth of the river and block exchange with the Gulf of Mexico. This leads to a transformation of the estuary into a lake. This lake-like effect was evidenced by the decreasing salinities over the course of the study period when the climate was wetter after fall 2003. Following drought, the Rio Grande closed in 2001 and was re-opened in 2003 and 2004 and consequently, salinities returned to estuary conditions (Fig. 2).
The Texas coast lies in the northwestern Gulf of Mexico. There is a climatic gradient with decreasing rainfall from the northeast to the southwest that results in decreasing freshwater inflow to the Texas coast (Montagna et al. 2013). Even though the Rio Grande is the southwestern-most estuary along the gradient, it does have lower salinity ranges and higher nutrient and chlorophyll concentrations than the lagoons and bays to the north (Palmer et al. 2011). For example, during the same 5-year period, Rio Grande averages were as follows: salinity 4 psu, ammonium 5.6 µm, nitrite + nitrate 26.0 µm, phosphate 5.9 µm, and chlorophyll 21.0 µg/L; in contrast, Lavaca Bay (an open lagoon-like bay connected to the Lavaca River) averages were as follows: salinity 16 psu, ammonium 1.4 µm, nitrite + nitrate 3.6 µm, phosphate 1.3 µm, and chlorophyll 8.8 µg/L.
During sampling, it was noted that the Rio Grande has a large amount of cyanobacteria and filamentous green algae, which likely adds to the productivity and deposition of detritus to the system. This is supported by the isotope values in sediments. The isotope values of sediments of δ15N (7 ppt) and δ13C (-20 ppt) indicate the organic matter in sediments is likely derived mainly from deposited algae based on comparative information found in Fry (2006).
The higher chlorophyll and nutrients in the Rio Grande are correlated to higher average benthic macrofaunal abundance and diversity compared to permanently open estuaries in Texas such as Lavaca Bay. For example, during the same 5-year period, Rio Grande macrofauna averages were biomass 2.6 g/m2 and abundance 10,600 ind./m2. In contrast, Lavaca Bay macrofauna averages were biomass 0.7 g/m2 and abundance 3800 ind./m2 (Palmer et al. 2011). In contrast, macrofauna diversity was identical with both Hʹ of 0.81. This is consistent with the finding that there are differences in macrofauna between open and closed estuaries in New South Wales, Australia (Hastie and Smith 2006).
Benthic macrofaunal biomass and diversity in the river-dominated Rio Grande estuary were lower than in other freshwater habitats along the Texas coast. For example, in the Nueces Delta marsh (connected to the Nueces River) biomass averaged 1.7 g/m2 and Hʹ averaged 0.69 over 1 year between October 1998 and October 1999; however, abundance in the Nueces marsh was higher, 13,100 ind./m2 (Palmer et al. 2002). Rincon Bayou is the main stem of the Nueces Delta marsh and a hydrological restoration project was constructed to enhance the connection with the Nueces River to lower salinity (Ward et al. 2002; Montagna et al. 2009). Rincon Bayou data (Montagna and Nelson 2009) were downloaded for the same time as the current study (October 2001–August 2005), and the averages were salinity 9.7, biomass 0.5 g/m2, abundance 4800 ind./m2, and diversity 0.5 Hʹ. Thus, long-term average salinity in Rincon Bayou was a little more than twice as high as the Rio Grande, but mean macrofauna abundance, biomass, and diversity were much (4.7 times, 2.3 times, and 1.7 times respectively) lower in than Rincon Bayou. The difference is due to a lack of connection to the Gulf of Mexico in Rincon Bayou, and more episodic floods where the large swings in diversity can act as a disturbance (VanDiggelen and Montagna 2016, Montagna et al. 2018).
The macrobenthic community in the Rio Grande was dominated by Chironomidae larvae and Oligochaetes when the river mouth was closed and the polychaetes Mediomastus ambiseta and Streblospio benedicti when the mouth was open (Table 3). Thus, community structure changed over time in a serial fashion (Fig. 7). Wet periods were dominated by Mediomastus ambiseta and Chironomidae larvae. The lack of dominance by mollusks contradicts previous studies, which found freshwater inflow events lead to dominance by suspension feeding bivalve species (Montagna and Kalke 1995; Montagna et al. 2002). Overall, the Rio Grande appears to be more influenced by freshwater inflow than the connection with the Gulf of Mexico. The lack of strong exchange with the Gulf of Mexico in 2001 caused the Rio Grande River to change from an estuarine ecosystem to a freshwater ecosystem, but from late 2002 through 2004 the system returned to brackish conditions. Species diversity increased with increasing salinity. Diversity in river-dominated estuaries was lower than in lagoonal estuaries in Texas (Palmer et al. 2011). These results are consistent with those of previous studies in that species diversity increases from nearly freshwater to seawater conditions (Montagna and Kalke 1992; Mannino and Montagna 1997; Palmer et al. 2002; Ysebaert et al. 2003). One possible mechanism explaining low diversity in small estuaries is due to fluctuating water levels during drought or flood (Adams et al. 1992; Montagna et al. 2018). Another explanation for the low diversity of intermittent estuaries is that food chains are short with high rates of connectivity and high rates of cannibalism (Mendonça, and Vinagre 2018).
In the Rio Grande, hydrology had more influence in shaping benthic community dynamics than the intermittent opening and closing of the inlet with the Gulf of Mexico. This is consistent with the studies on benthos from Australia (Hastie and Smith 2006). Not surprisingly, water column dynamics also appeared to be more influenced by river flow and hydrology than the intermittent nature of the estuary. Both the abiotic and biotic responses were driven by hydrological changes over time. The benthic community in the Rio Grande was relatively homogenous with distance from the sea (Table S1). In contrast, studies on intermittent estuaries in New South Wales, Australia, found the heterogeneity with distance from the sea (Dye 2006).
Management Implications
The Rio Grande is a river with historical significance as well as being the 2000-km-river border between two North American countries. The International Amistad Reservoir separates the river into upper and lower portions that are hydrologically distinct (RGBBEST 2012). The flows from Fort Quitman, Texas (~ 100 km downstream from El Paso, Texas) to the Gulf of Mexico are divided between the USA and Mexico by a 1944 treaty. Flows in the lower Rio Grande have been reduced by reservoirs, and irrigation. The lower 80 km of the Rio Grande is tidally influenced.
In 2007, the Texas Legislature passed Senate Bill 3, which required environmental flow standards be developed for major river basins and estuarine systems. A group of scientists named the Lower Rio Grande Basin and Bay Expert Science Team (RGBBEST) was tasked with an environmental flow analysis to recommend an environmental flow regime adequate to support a “sound ecological environment” for the Lower Rio Grande. The RGBBEST (2012) defined a sound ecological environment as one that (1) maintains native species, (2) is sustainable, and (3) is a current condition. Based on a combination of historical conditions, altered hydrology, and temporarily open and closed estuary conditions, the RGBBEST (2012) determined that the Lower Rio Grande was an unsound environment.
The current study provides information pertinent to the management of environmental flows to the coast and to the question of reopening tidal connections. Hydrology affects hydrography, meaning the volume of fresh water flowing into an estuary is related to declines in salinity and increased concentrations of nitrate, nitrite, and phosphate, and estuaries are sinks for these nutrients. The implication is that these ecosystems have a characteristic community that is strongly influenced by hydrology of the systems (Lill et al. 2013). Intermittent estuaries will require different approaches for setting environmental flow standards because of the alternating influences of watershed forcing and connections to the sea (Stein et al. 2021). Decisions to reopen a closed river mouth are often based on benefits to improve fisheries, or reduce flooding, nutrients, or algal blooms (Conde et al. 2015). The higher diversity and marine-estuarine community structure indicates it is desirable to maintain the Rio Grande as an open estuary, and this may help restore the historical conditions of the habitat.
Data Availability
All data is available on line at https://doi.org/10.7266/xzyn67pf.
References
Adams, J.B., W.T. Knoop, and G.C. Bate. 1992. The distribution of estuarine macrophytes in relation to freshwater. Botanica Marina 35: 215–226.
Aleem, A.A. 1972. Effect of river outflow management on marine life. Marine Biology 15: 200–2008. https://link.springer.com/content/pdf/10.1007/BF00383550.pdf.
Allen, K.R. 1983. Introduction to the Port Hacking Estuary project. In Synthesis and modelling of intermittent estuaries, ed. W.R. Cuff and M. Tomczak Jr., 1–6. Berlin: Springer. https://books.google.com/books?hl=en&lr=&id=10juCAAAQBAJ&oi=fnd&pg=PA1&dq=intermittent+estuaries&ots=1dceQgU27M&sig=LrqLBG2YvfK7sNPzn6FNbbaHFBg.
Chapman, E.R. 1966. The Texas basins project. In A symposium on estuarine fisheries, vol. 95, ed. R.F. Smith, A.H. Swartz, and W.H. Massmann, 83–92. American Fisheries Society Special Publ. No. 3., 154 pp.
Clarke, K.R., R.N. Gorley, P.J. Somerfield, and R.M. Warwick. 2014. Change in marine communities: An approach to statistical analysis and interpretation, 3rd ed. Plymouth: Primer-E.
Clarke, K.R., and R.N. Gorley. 2015. Primer v7: User Manual/Tutorial. Plymouth, United Kingdom: Primer-E.
Conde, D., J. Vitancurt, L. Rodríguez-Gallego, D. de Álava, N. Verrastro, C. Chreties, S. Solari, L. Teixeira, X. Lagos, G. Piñeiro, L. Seijo, H. Caymaris, and D. Panario. 2015. Solutions for sustainable coastal lagoon management: from conflict to the implementation of a consensual decision tree for artificial opening. In Coastal zones, ed. J. Baztan, O. Chouinard, B. Jorgensen, P. Tett, J.-P. Vanderlinden, and L. Vasseur, 217–250. Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-12-802748-6.00013-9.
Dye, A.H. 2006. Is geomorphic zonation a useful predictor of patterns of benthic infauna in intermittent estuaries in New South Wales, Australia? Estuaries and Coasts 29: 455–464. https://doi.org/10.1007/BF02784993.
Elliott, M., and D.S. McLusky. 2002. The need for definitions in understanding estuaries. Estuarine, Coastal and Shelf Science 55: 815–827. https://doi.org/10.1006/ecss.2002.1031.
Folk, R.L. 1964. Petrology of sedimentary rocks, 155. Austin: Hemphill’s Press.
Fry, B. 2006. Stable isotope ecology. New York: Springer.
Garvine, R.W. 1974. Physical features of the Connecticut River outflow during high discharge. Journal of Geophysical Research 79: 831–846. https://agupubs.onlinelibrary.wiley.com/doi/pdfdirect/10.1029/JC079i006p00831.
Hastie, B.F., and S.D.A. Smith. 2006. Benthic macrofaunal communities in intermittent estuaries during a drought: Comparisons with permanently open estuaries. Journal of Experimental Marine Biology and Ecology 330: 356–367. https://doi.org/10.1016/j.jembe.2005.12.039.
Hinwood, J.B., and E.J. McLean. 2015. Predicting the dynamics of intermittently closed/open estuaries using attractors. Coastal Engineering 99: 64–72. https://doi.org/10.1016/j.coastaleng.2015.02.008.
Justić, D., V. Kourafalou, G. Mariotti, S. He, R. Weisberg, 17 others. 2021. Transport processes in the Gulf of Mexico along the river-estuary-shelf-ocean continuum: A review of research from the Gulf of Mexico Research Initiative. Estuaries and Coasts 45: 621–657. https://doi.org/10.1007/s12237-021-01005-1.
Kalke, R.D. 1981. The effects of freshwater inflow on salinity and zooplankton populations at four stations in the Nueces-Corpus Christi and Copano-Aransas Bay systems, TX from October 1977-May 1975. In Proceeding of the International Symposium on Freshwater Inflow to Estuaries, ed. R.D. Cross and D.L. Williams, 454–471. Washington: U.S. Dept. Int. Fish & Wildlife.
Lill, A.W.T., M. Schallenberg, A. Lal, C. Savage, and G.P. Closs. 2013. Isolation and connectivity: Relationships between periodic connection to the ocean and environmental variables in intermittently closed estuaries. Estuarine, Coastal and Shelf Science 128: 76–83. https://doi.org/10.1016/j.ecss.2013.05.011.
Longley, W.L., ed. 1994. Freshwater inflows to Texas bays and estuaries: Ecological relationships and methods for determination of needs, 386. Austin: Texas Water Development Board and Texas Parks and Wildlife Department.
Mannino, A., and P.A. Montagna. 1997. Small-scale spatial variation of macrobenthic community structure. Estuaries 20: 159–173.
Mendonça, V., and C. Vinagre. 2018. Short food chains, high connectance and a high rate of cannibalism in food web networks of small intermittent estuaries. Marine Ecology Progress Series 587: 17–30. https://doi.org/10.3354/meps12401.
Mondon, J., J. Sherwood, and F. Chandler. 2003. Western Victorian Estuaries Classification Project. Deakin University, Western Coastal Board, Parks Victoria, Corangamite and Glenelg Hopkins Catchment Management Authorities.
Montagna, P. 2022. Rio Grande water and sediment quality 2000-2005. Gulf of Mexico Research Initiative Information and Data Cooperative (GRIIDC), Harte Research Institute, Texas A&M University–Corpus Christi. https://doi.org/10.7266/xzyn67pf.
Montagna, P.A. 2021. How a simple question about freshwater inflow to estuaries shaped a career. Gulf and Caribbean Research 32: ii–xiv. https://doi.org/10.18785/gcr.3201.04.
Montagna, P.A., and R.D. Kalke. 1992. The effect of freshwater inflow on meiofaunal and macrofaunal populations in the Guadalupe and Nueces Estuaries, Texas. Estuaries 15: 307–326.
Montagna, P.A., and R.D. Kalke. 1995. Ecology of infauna Mollusca in south Texas estuaries. American Malacological Bulletin. 11: 163–175.
Montagna, P.A., R.D. Kalke, and C. Ritter. 2002. Effect of restored freshwater inflow on macrofauna and meiofauna in upper Rincon Bayou, Texas, USA. Estuaries 25: 1436–1447.
Montagna, P., G. Ward, and B. Vaughan. 2011. The importance and problem of freshwater inflows to Texas estuaries. In Water Policy in Texas: Responding to the Rise of Scarcity, ed. R.C. Griffin, 107–127. Washington: The RFF Press.
Montagna, P.A., C. Chaloupka, E.A. DelRosario, A.M. Gordon, R.D. Kalke, T.A. Palmer, and E.L. Turner. 2018. Managing environmental flows and water resources. WIT Transactions on Ecology and the Environment 215: 177–188. https://doi.org/10.2495/EID180161.
Montagna, P.A., and J. Li. 2010. Effect of freshwater inflow on nutrient loading and macrobenthos secondary production in Texas lagoons. In Coastal lagoons: critical habitats of environmental change, ed. M.J. Kennish and H.W. Paerl, 513–539. Boca Raton: CRC Press, Taylor & Francis Group.
Montagna, P.A., and K. Nelson. 2009. Observation data model (ODM) for Rincon Bayou, Nueces Delta. Final report to the Coastal Bend Bays & Estuaries Program, Project # 2008–18, 9. Corpus Christi, Texas, USA: Harte Research Institute, Texas A&M University-Corpus Christi. Accessed 17 December 2022 from https://tamucc-ir.tdl.org/handle/1969.6/90073.
Montagna, P.A., E.M. Hill, and B. Moulton. 2009. Role of science-based and adaptive management in allocating environmental flows to the Nueces Estuary, Texas, USA. In Ecosystems and Sustainable Development VII, ed. C.A. Brebbia and E. Tiezzi, 559–570. Southampton: WIT Press. https://doi.org/10.2495/ECO090511.
Montagna, P.A., T.A. Palmer, and J. Beseres Pollack. 2013. Hydrological changes and estuarine dynamics. In SpringerBriefs in Environmental Sciences, 94. New York: Springer New York. https://doi.org/10.1007/978-1-4614-5833-3.
Palmer, T.A., P.A. Montagna, and R.D. Kalke. 2002. Downstream effects of restored freshwater inflow to Rincon Bayou, Nueces Delta, Texas, USA. Estuaries 25: 1448–1456.
Palmer, T.A., P.A. Montagna, J.B. Pollack, R.D. Kalke, and H.R. DeYoe. 2011. The role of freshwater inflow in lagoons, rivers, and bays. Hydrobiologia 667: 49–67. https://doi.org/10.1007/s10750-011-0637-0.
Rio Grande, Rio Grande Estuary, and Lower Laguna Madre Basin and Bay Expert Science Team (RGBBEST). 2012. Environmental Flows Recommendations Report. Final Submission to the Environmental Flows Advisory Group, Rio Grande, Rio Grande Estuary, and Lower Laguna Madre Basin and Bay Stakeholders Committee, and Texas Commission on Environmental Quality. Accessed 17 December 2022 from https://tamucc-ir.tdl.org/handle/1969.6/90073.
SAS Institute, Incorporated. 2017. SAS/STAT® 14.3 User’s Guide, 10801. Cary, NC: SAS Institute Inc.
Stein, E.D., E.M. Gee, J.B. Adams, K. Irving, and L. Van Niekerk. 2021. Advancing the science of environmental flow management for protection of temporarily closed estuaries and coastal lagoons. Water 13: 595. https://doi.org/10.3390/w13050595.
Tuholske, C., B.S. Halpern, G. Blasco, J.C. Villasenor, M. Frazier, and K. Caylor. 2021. Mapping global inputs and impacts from of human sewage in coastal ecosystems. PLoS ONE 16 (11): e0258898. https://doi.org/10.1371/journalpone.0258898.
USEPA. 1997. Method 445.0, In vitro determination of chlorophyll a and pheophytin a in marine and freshwater algae by fluorescence. Cincinnati, OH: USEPA, National Exposure Research Laboratory.
VanDiggelen, A.D., and P.A. Montagna. 2016. Is salinity variability a benthic disturbance in estuaries? Estuaries and Coasts 39: 967–980. https://doi.org/10.1007/s12237-015-0058-9.
Ward, G.H., M.J. Irlbeck, and P.A. Montagna. 2002. Experimental river diversion for marsh enhancement. Estuaries 25: 1416–1425. https://doi.org/10.1007/BF02692235.
Welschmeyer, N.A. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnology and Oceanography. 39: 1985–1992.
Ysebaert, T., P.M.J. Herman, P. Meire, J. Craeymeersch, H. Verbeek, and C.H.R. Heip. 2003. Large-scale spatial patterns in estuaries: Estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuarine Coastal and Shelf Science. 57: 335–355.
Acknowledgements
The authors acknowledge significant contributions by Rick Kalke and Larry Hyde for taxonomic work and field collections. Hudson DeYoe, University of Texas-Rio Grande Valley, performed sampling in the Rio Grande. The work for this report was supported by the Texas Water Development Board, Research and Planning Fund, Research Grant, authorized under the Texas Water Code, Chapter 15, and as provided in §16.058 and §11.1491. This support was administered by the Board under interagency cooperative contract number 2006-483-026. This study benefitted by partial support from the National Oceanic and Atmospheric Administration, Office of Education Educational Partnership Program award (NA16SEC4810009). Its contents are solely the responsibility of the award recipient and do not necessarily represent the official views of the US Department of Commerce, National Oceanic and Atmospheric Administration.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Patricia Ramey
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montagna, P.A., Palmer, T.A. & Pollack, J.B. Effect of Temporarily Opening and Closing the Marine Connection of a River Estuary. Estuaries and Coasts 46, 2208–2219 (2023). https://doi.org/10.1007/s12237-022-01159-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01159-6