Abstract
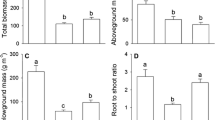
Phragmites australis (or common reed), one of the most widespread invasive species in wetlands of North America, has a high nitrogen (N) demand compared to native species. However, it is unclear how P. australis is able to meet this N demand, especially in systems with low soil N and limited N inputs. We evaluated whether deep rooting could be a mechanism by which P. australis accesses otherwise unused N pools, allowing it to circumvent nutrient competition and acquire the “missing N” needed to meet its N demand. We examined above and belowground N pools, as well as depth profiles of belowground biomass (to 3 m), in native and P. australis-dominated plant communities in two brackish marshes of the Chesapeake Bay. To evaluate whether deep roots contribute to P. australis meeting its high N demand, we amended soil porewater with 15N at four soil depths (10, 20, 40, and 80 cm) and measured the rate of 15N assimilation into plant biomass. We found that P. australis had 6–8 × the aboveground standing stock N, 2–3 × the total belowground biomass, and roots up to 3 × deeper than native plant communities (some exceeding 3 m). Our 15N tracer study demonstrated that P. australis acquired N from all measured soil depths, whereas N uptake by native plants was minimal below 20 cm. Our results demonstrate that deep rooting is a mechanism by which P. australis can access previously buried N pools, helping to satisfy its high N demand and thus fuel its invasion. Moreover, these results fundamentally challenge our understanding of biogeochemical processes deep within the soil profile given the presence of active roots at depths where they were previously thought to be largely inert.





Similar content being viewed by others
Data Availability
Data from this study are available in the Smithsonian Institution figshare repository (https://smithsonian.figshare.com) under https://doi.org/10.25573/serc.22116053.
References
Armstrong, J., W. Armstrong, P.M. Beckett, J.E. Halder, S. Lythe, R. Holt, and A. Sinclair. 1996. Pathways of aeration and the mechanisms and beneficial effects of humidity- and Venturi-induced convections in Phragmites australis (Cav) Trin ex Steud. Aquatic Botany 54: 177–197.
Bedford, B.L., M.R. Walbridge, and A. Aldous. 1999. Patterns in nutrient availability and plant diversity of temperate North American wetlands. Ecology 80: 2151–2169.
Bernal, B., J.P. Megonigal, and T.J. Mozder. 2017. An invasive wetland grass primes deep soil carbon pools. Global Change Biology 23: 2104-2116. https://doi.org/10.1111/gcb.13539.
Bernal, B. S. Kim, and T.J. Mozdzer. 2023. Species specific priming alters deep soil organic matter dynamics. Science of the Total Environment 859: 159956. https://doi.org/10.1016/j.scitotenv.2022.159956.
Bertness, M.D., P.J. Ewanchuk, and B.R. Silliman. 2002. Anthropogenic modification of New England salt marsh landscapes. Proceedings of the National Academy of Sciences of the United States of America 99: 1395–1398.
Blum, L.K. 1993. Spartina alterniflora root dynamics in a Virginia Marsh. Marine Ecology-Progress Series 102.
Caplan, J.S., R.N. Hager, J.P. Megonigal, and T.J. Mozdzer. 2015. Global change accelerates carbon assimilation by a wetland ecosystem engineer. Environmental Research Letters 10: 115006.
Chambers, R.M., D.T. Osgood, D.J. Bart, and F. Montalto. 2003. Phragmites australis invasion and expansion in tidal wetlands: Interactions among salinity, sulfide, and hydrology. Estuaries 26: 398–406.
Chambers, R.M., L.A. Meyerson, and K. Saltonstall. 1999. Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Botany 64: 261–273.
Chambers, R.M., T.J. Mozdzer, and J.C. Ambrose. 1998. Effects of salinity and sulfide on the distribution of Phragmites australis and Spartina alterniflora in a tidal saltmarsh. Aquatic Botany 62: 161–169.
Cott, G.M., J.S. Caplan, and T.J. Mozdzer. 2018. Nitrogen uptake kinetics and saltmarsh plant responses to global change. Scientific Reports 8: 5393.
Eller, F., H. Skalova, J.S. Caplan, G.P. Bhattarai, M.K. Burger, J.T. Cronin, W.Y. Guo, E.L. Hazelton, K.M. Kettenring, C. Lambertini, M.K. McCormick, L.A. Meyerson, T. J. Mozdzer, P. Pysek, B.K. Sorrell, D.F. Whigham, and H. Brix. 2017. Cosmopolitan species as ecophysiological models for responses to global change: the common reed Phragmites australis. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2017.01833.
Faillace, C.A., J.S. Caplan, J.C. Grabosky, and P.J. Morin. 2018. Beneath it all: Size, not origin, predicts belowground competitive ability in exotic and native shrubs. Journal of the Torrey Botanical Society 145: 30–40.
Findlay, S.G., S. Dye, and K. Kuehn. 2002. Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22: 616–625.
Gale, M.R., and D.F. Grigal. 1987. Vertical root distributions of northern tree species in relation to successional status. Canadian Journal of Forest Research 17: 829–834.
Jackson, R.B., J. Canadell, J.R. Ehleringer, H.A. Mooney, O.E. Sala, and E.D. Schulze. 1996. A global analysis of root distributions for terrestrial biomes. Oecologia 108: 389–411.
Keller, J.K. A.A. Wolf, P.B. Weisenhorn, B.G. Drake, and J.P. Megonigal. 2009. Elevated CO2 affects porewater chemistry in a brackish marsh. Biogeochemistry 96: 101–117. https://doi.org/10.1007/s10533-009-9347-3.
Kettenring, K.M., S. de Blois, and D.P. Hauber. 2012. Moving from a regional to a continental perspective of Phragmites australis invasion in North America. AoB Plants 2012: pls040.
Kettenring, K.M., M.K. McCormick, H.M. Baron, and D.F. Whigham. 2010. Phragmites australis (common reed) invasion in the Rhode River subestuary of the Chesapeake Bay: Disentangling the effects of foliar nutrients, genetic diversity, patch size, and seed viability. Estuaries and Coasts 33: 118–126.
Kettenring, K.M., M.K. McCormick, H.M. Baron, and D.F. Whigham. 2011. Mechanisms of Phragmites australis invasion: Feedbacks among genetic diversity, nutrients, and sexual reproduction. Journal of Applied Ecology 48: 1305–1313.
King, R.S., W.V. Deluca, D.F. Whigham, and P.P. Marra. 2007. Threshold effects of coastal urbanization on Phragmites australis (common reed) abundance and foliar nitrogen in Chesapeake Bay. Estuaries and Coasts 30: 469–481.
Langley, J.A., and J.P. Megonigal. 2010. Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature 466: 96–99.
Langley, J.A., K.L. McKee, D.R. Cahoon, J.A. Cherry, J.P. Megonigal, and C.B. Field. 2009. Elevated CO2 stimulates marsh elevation gain, counterbalancing sea-level rise. Proceedings of the National Academy of Sciences of the United States of America 106: 6182–6186.
Lissner, J., and H.-H. Schierup. 1997. Effects of salinity on the growth of Phragmites australis. Aquatic Botany 55: 247–260.
McCormick, M., K. Kettenring, H. Baron, and D. Whigham. 2010. Extent and reproductive mechanisms of Phragmites australis spread in brackish wetlands in Chesapeake Bay, Maryland (USA). Wetlands 30: 67–74.
McKinley, D.C., J.C. Romero, B.A. Hungate, B.G. Drake, and J.P. Megonigal. 2009. Does deep soil N availability sustain long-term ecosystem responses to elevated CO2? Global Change Biology 15: 2035–2048.
Meyerson, L., and J. Cronin. 2013. Evidence for multiple introductions of Phragmites australis to North America: Detection of a new non-native haplotype. Biological Invasions 15: 2605–2608.
Meyerson, L.A., K. Saltonstall, L. Windham, E. Kiviat, and S. Findlay. 2000a. A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecology and Management 8: 89–103.
Meyerson, L.A., K.A. Vogt, and R.M. Chambers. 2000b. Linking the success of Phragmites to the alteration of ecosystem nutrient cycles. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 827–844. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Mozdzer, T.J., and J.C. Zieman. 2010. Ecophysiological differences between genetic lineages facilitate the invasion of non-native Phragmites australis in North American Atlantic coast wetlands. Journal of Ecology 98: 451–458.
Mozdzer, T.J., and J.P. Megonigal. 2012. Jack-and-Master trait responses to elevated CO2 and N: a comparison of native and introduced Phragmites australis. Plos One 7: e42794.
Mozdzer, T. J., and J.S. Caplan. 2018. Complementary responses of morphology and physiology enhance stand scale production under elevated CO2 and nitrogen. Functional Ecology 32(7): 1784–1796.
Mozdzer, T.J., J. Brisson, and E.L.G. Hazelton. 2013. Physiological ecology and functional traits of North American native and Eurasian introduced Phragmites australis lineages. AoB Plants 5: plt048.
Mozdzer, T., J. Zieman, and K. McGlathery. 2010. Nitrogen uptake by native and invasive temperate coastal macrophytes: Importance of dissolved organic nitrogen. Estuaries and Coasts 33: 784–797.
Mozdzer, T.J., J.A. Langley, P. Mueller, and J.P. Megonigal. 2016. Deep rooting and global change facilitate spread of invasive grass. Biological Invasions. https://doi.org/10.1007/s10530-016-1156-8.
Neubauer, S.C., I.C. Anderson, and B.B. Neikirk. 2005. Nitrogen cycling and ecosystem exchanges in a Virginia tidal freshwater marsh. Estuaries 28: 909–922.
Pimentel, D., R. Zuniga, and D. Morrison. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52: 273–288.
Raich, J.W., and K.J. Nadelhoffer. 1989. Belowground carbon allocation in forest ecosystems: Global trends. Ecology 70: 1346–1354.
Rickey, M.A., and R.C. Anderson. 2004. Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinata. Journal of Applied Ecology 41: 888–896.
Rundel, P.W., I.A. Dickie, and D.M. Richardson. 2014. Tree invasions into treeless areas: Mechanisms and ecosystem processes. Biological Invasions. https://doi.org/10.1007/s10530-013-0614-9.
Saltonstall, K. 2002. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences of the United States of America 99: 2445–2449.
Saunders, C., J.P. Megonigal, and J. Reynolds. 2006. Comparison of belowground biomass in C3- and C4-dominated mixed communities in a Chesapeake Bay brackish marsh. Plant and Soil 280: 305–322.
Solorzano, L. (1969). Determination of ammonia in natural waters by the phenol hypochlorite method. Limnology and Oceanography 14(5): 799–801.
Templer, P., S. Findlay, and C. Wigand. 1998. Sediment chemistry associated with native and non-native emergent macrophytes of a Hudson River marsh ecosystem. Wetlands 18: 70–78.
Valiela, I., J.M. Teal, and W. Sass. 1973. Nutrient retention in salt marsh plots experimentally fertilized with sewage sludge. Estuarine and Coastal Marine Science 1: 261–269.
Windham, L. 2001. Comparison of biomass production and decomposition between Phragmites australis (common reed) and Spartina patens (salt hay grass) in brackish tidal marshes of New Jersey, USA. Wetlands 21: 179–188.
Windham, L., and L.A. Meyerson. 2003. Effects of common reed (Phragmites australis) expansions on nitrogen dynamics of tidal marshes of the northeastern US. Estuaries 26: 452–464.
Windham, L., and J.G. Ehrenfeld. 2003. Net impact of a plant invasion on nitrogen-cycling processes within a brackish tidal marsh. Ecological Applications 13: 883–896.
Windham, L., and R.G. Lathrop. 1999. Effects of Phragmites australis (common reed) invasion on aboveground biomass and soil properties in brackish tidal marsh of the Mullica River, New Jersey. Estuaries 22: 927–935.
Acknowledgements
The authors would like to thank A. Peresta, G. Peresta, and R.N. Hager for field and laboratory assistance. We also thank the editor and two anonymous reviewers for constructive feedback on the manuscript.
Funding
This work was supported with funding from the National Science Foundation Division of Environmental Biology (DEB-2051602, DEB-2051598, DEB-2051343, DEB-1457100, DEB-0950080, DEB-1557009), Maryland Sea Grant (SA7528082, SA7528114-WW), the University of Maryland, the Smithsonian Institution, and Bryn Mawr College.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kenneth Dunton
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mozdzer, T.J., Meschter, J., Baldwin, A.H. et al. Mining of Deep Nitrogen Facilitates Phragmites australis Invasion in Coastal Saltmarshes. Estuaries and Coasts 46, 998–1008 (2023). https://doi.org/10.1007/s12237-022-01146-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01146-x