Abstract
Estuaries are ecologically and economically important ecosystems but are threatened by non-native invasive species, including many species of submersed aquatic vegetation (SAV). Herbicides are the primary tool used to control SAV, but most field evaluations of herbicides have been conducted in lentic systems. Therefore, managers working in estuaries must base their SAV control programs largely on findings from systems fundamentally different from their own. We conducted a study in the Sacramento-San Joaquin Delta to determine efficacy of the widely used herbicide fluridone in an estuarine ecosystem. The primary goal of SAV removal was restoration of open water habitat for endangered Hypomesus transpacificus (Delta Smelt). Over 18 months and multiple sets of multi-week fluridone applications, we monitored concentrations of fluridone and responses by SAV across pairs of treated and reference sites. Fluridone concentrations in the water were generally below the 2–5 parts per billion required for SAV control. Monitoring demonstrated that these low water concentrations were likely due to dissipation by tides, despite use of pelleted fluridone formulations marketed for flowing water environments. Fluridone did, however, accumulate in sediment at concentrations hundreds of times higher than those measured in the water. Nonetheless, we did not observe lasting reductions in SAV abundance or changes in SAV community composition. By demonstrating lack of efficacy of one of the few herbicides permitted for use in this estuary, this study highlights the need for development of SAV management tools tailored to the challenges of hydrologically complex environments like estuaries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, invasive submersed aquatic vegetation (SAV) threatens coastal, estuarine, and freshwater ecosystems and the services they provide (Williams and Grosholz 2008; Gallardo et al. 2016; Prior et al. 2018). Many widespread SAV species, such as Egeria densa (Brazilian Waterweed), act as ecosystem engineers by altering fundamental processes, such as sediment dynamics and nutrient cycling (Jones et al. 1994; Work et al. 2020), and these changes in ecosystem function can significantly alter species assemblages (Havel et al. 2015; Guy‐Haim et al. 2018). Controlling invasive SAV has become an important part of managing many of these ecosystems, but the availability of suitable tools depends on the characteristics of the ecosystem (Hussner et al. 2017).
Estuaries are uniquely challenging systems for controlling SAV. They are among the most invaded aquatic ecosystems in the world with diverse communities of SAV species (Cohen and Carlton 1998; Williams and Grosholz 2008). They are heavily used by an array of stakeholders, including drinking water districts, farmers, shipping companies, and fisherman (Pinto et al. 2013; Jetter and Nes 2018). In addition, estuaries provide vital habitat for a rich diversity of estuary specialized native species (Edgar et al. 2000; Lotze et al. 2006; Needles et al. 2015). Many SAV control methods may be incompatible with the needs of these stakeholders (Younes and Galal-Gorchev 2000; Chiconela et al. 2013) or with protective regulations for imperiled species (Madsen and Kyser 2020). Estuaries also have complex hydrodynamics, which further limit control options (Getsinger et al. 1996, 2008; Hussner et al. 2017). Many forms of physical SAV removal provide only short-term control because they remove shoots but not root crowns (Howard-Williams et al. 1996). In addition, disturbance from physical removal produces large amounts of plant fragments (Hussner et al. 2017), which can spread infestation to new areas via tidal flows. Benthic barriers are effective for small infestations but cannot be used over large areas or those with high flows (Madsen 2000; Hofstra and Clayton 2012; Hussner et al. 2017). There are few biological control agents for SAV species (Coetzee et al. 2011; Pratt et al. 2019). Given all these constraints, estuary managers are left with few SAV control tools that meet the demands of their systems.
Field studies have demonstrated that herbicides can control SAV in a variety of environments (Fox and Haller 1992; Fox et al. 1994; Parsons et al. 2007). However, effectiveness of herbicide treatments depends on whether target concentrations can be maintained in the water column surrounding the vegetation for adequate amounts of time (Getsinger and Netherland 1997). These effective concentrations and exposure times are more easily achieved in lakes and other lentic systems because rates of herbicide dissipation are lower relative to those in flowing water environments such as estuaries (Fox and Haller 1992; Getsinger and Netherland 1997; Patten 2003). Slow-release pelleted formulations have been developed for some herbicides to improve concentration and exposure times in flowing water (Netherland et al. 1998; Koschnick et al. 2003; Bultemeier and Haller 2015), but there have been few studies of their effectiveness in hydrologically complex estuarine environments (Fox and Haller 1992; Santos et al. 2009; Caudill et al. 2019).
One of the herbicides commonly available in pellet form is fluridone, 1-methyl-3-phenyl-5-[3-trifluromethyl)-phenyl]-4-(1H)-pyridinone. It is a systemic herbicide that inhibits an enzyme essential to the synthesis of carotenoids and thereby limits the ability of plants to perform photosynthesis (Arnold 1979; Sprecher et al. 1998; Puri et al. 2007). Due to this mode of action, target concentrations of fluridone must be maintained for multiple months to reduce SAV biomass (Netherland et al. 1993; Madsen et al. 2002; Parsons et al. 2009). Fluridone has been widely used around the world for several decades (Hussner et al. 2017) because, when applied at low concentrations (e.g., 2–5 parts per billion (ppb)), it can control target SAV species with low impacts on non-target species, including native plants, agricultural crops, aquatic invertebrates, and fishes (Kay et al. 1994; Paul et al. 1994; Madsen et al. 2002; Getsinger et al. 2008; Archambault et al. 2015). Despite this widespread adoption of fluridone, no studies, to our knowledge, have been conducted in estuaries that monitor the fate of fluridone applications to link concentrations in the environment to SAV management outcomes.
This study was implemented as part of an ecosystem management effort in the Sacramento - San Joaquin Delta (hereafter referred to as “Delta”) for recovery of a critically endangered fish species, Hypomesus transpacificus (Delta Smelt). The main objectives were to implement invasive SAV management actions to restore open water habitat in this estuarine environment for Delta Smelt and to simultaneously evaluate the effectiveness of pelleted formulations of fluridone for this purpose. During the past two decades, fluridone has been heavily used for SAV control in areas of the Delta with major boat traffic (e.g., marinas, boat ramps), largely because of its low toxicity to non-target species, but it has only recently been utilized for wildlife habitat restoration projects. We evaluated fluridone by comparing pairs of treated and reference field sites, matched based on a suite of characteristics (e.g., SAV species composition, hydrology). Specifically, we determined (1) whether target concentrations of fluridone (2–5 ppb) could be maintained within the water column of tidally influenced sites, (2) how much fluridone was present in the bed sediments of the sites during the study, and (3) if the fluridone applications reduced the abundance of SAV.
Methods
Study System
The Delta is a tidal freshwater system representing the inland region of the San Francisco Estuary (Fig. 1). The system is home to more than 250 special-status species with legal or regulatory protection, including the endangered fish H. transpacificus (DSC 2013). The Delta also has been invaded by many SAV species, including Egeria densa, Potamogeton crispus (curlyleaf pondweed), Myriophyllum spicatum (Eurasian watermilfoil), and Cabomba caroliniana (Carolina fanwort) (Santos et al. 2011; Ta et al. 2017). SAV now covers over 30% of the area of waterways in the Central and North Delta (Ta et al. 2017), degrading habitat for native species. This rapid spread of non-native SAV species has recently prompted interest in expanding use of fluridone from primarily areas of heavy boat traffic to those targeted for habitat restoration for endangered species, hence the impetus for this study.
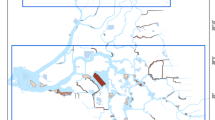
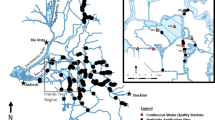
Map showing locations of the study sites and locations of sampling stations within sites. Autosampler stations were used at Little Hastings in October 2018 to determine the effect of tidal stage on water concentrations of fluridone. Fluridone stations were used to monitor water and sediment concentrations of fluridone throughout the study period. Vegetation stations were sampled for submersed aquatic vegetation biomass and species composition
The four sites selected for this study are in regions where H. transpacificus occurred historically (Sommer and Mejia 2013). Two sites were treated with fluridone and two were non-treated reference sites (Table 1). Prior to this study, none of these sites had ever been treated with fluridone as affirmed by records of the Division of Boating and Waterways (DBW), the only agency in the Delta permitted to apply this herbicide. All sites were tidally dynamic (tidal range: ~ 1 m), shallow (1–3 m deep), and contained SAV infestations dominated by E. densa. One pair of sites was in the North Delta (Treated: Little Hastings; Reference: French Island; Fig. 1). These sites are flooded islands surrounded by breached levees, and each is ~ 57 ha. The other pair was in the Central Delta (Treated: Decker Island; Reference: Fisherman’s Cut, Fig. 1). At the outset of the study in 2017, a reference site for the Central Delta could not be located, but in April 2018, Fisherman’s Cut was designated for this purpose. These two sites are small shallow shelves adjacent to channels (Decker Island: 11 ha, Fisherman’s Cut: 2 ha). The area targeted for SAV treatment at Decker Island was 7 ha. The other 4 ha of this site were occupied by Eichhornia crassipes (water hyacinth, floating aquatic vegetation), and Ludwigia spp. (water primrose, not identified to species, emergent aquatic vegetation). Restoration of this site for H. transpacificus included treatment of these two taxa with the herbicide 2,4-D after all fluridone treatments at this site were completed (5 applications; July 18–August 9, 2018). Pairing treatment and reference sites with similar water quality (Table 2), bathymetry, hydrology, size, and SAV composition minimized the influence of confounding effects from natural variation in SAV. Also, reference sites allowed for quantification of background levels of fluridone, potentially present due to tidal transport from other areas in the Delta.
Fluridone Applications
Fluridone was applied by DBW staff at the two treated sites using standardized methods employed throughout the Delta region for SAV control (described in brief below) and in accordance with all regulatory permits (DBW 2018). Treatment was organized into treatment series, each consisting of weekly applications during 11- to 16-week periods. During each year, treatment series were completed between March and November, the period in which the control program is legally permitted to apply fluridone (DBW 2018). Applications made earlier in the year would likely result in higher efficacy but would potentially overlap with the presence of protected fish species. Weekly treatments were conducted to maintain fluridone concentration at target levels for sustained periods. Three treatment series were completed at Little Hastings: summer 2017 (June 7–Sept. 19; 14 applications), spring 2018 (March 19–July 2; 15 applications), and fall 2018 (September 17–November 26; 11 applications) (Table 1, Fig. S1). Decker Island received two treatment series: summer 2017 (June 6–September 18; 15 applications) and spring 2018 (March 26–June 18; 11 applications) (Table 1, Fig. S1). There was no treatment at Decker Island in fall 2018 because herbicide application would have conflicted with nearby wetland construction activities.
Fluridone pellets designed for flowing water applications were used to provide more consistent concentrations over time than would be possible with liquid formulations (Netherland et al. 1998). During a treatment series, pellets were distributed throughout each site weekly using boat-mounted air blowers at a nominal rate that ranged 10–20 ppb (Fig. S1). These application rates were based on estimated water volume of a site (area × mean depth; Decker Island: 7.2 ha × 2.1 m; Little Hastings: 57.1 ha × 1.5 m). In general, the strategy was to use a relatively quick-releasing formulation (Sonar® Q) for a few weeks at the beginning of a treatment series. Sonar® Q was followed with SonarOne®, which has a longer release time than Sonar® Q. A treatment series ended with Sonar® PR, which has the slowest release time. Pellets of all three formulations were composed of clay embedded with 5% fluridone by weight (SePRO Corporation, Carmel, IN). Water concentrations of fluridone were monitored weekly or biweekly at all sites during active treatment, in part, to inform subsequent application rates. The goal was to maintain ambient concentrations of 2–5 ppb in the water column during a treatment series. These low target concentrations were required by regulatory permits to protect listed wildlife (e.g., endangered fishes) and nearby agricultural crops irrigated with Delta water. Previous work in lakes has demonstrated these low concentrations effectively reduce abundance of target SAV species while minimizing potential negative effects on non-target aquatic species (e.g., Madsen et al. 2002).
Across 18 months and 40 application events, a total of 11,401 kg of pellets, containing 570 kg of fluridone, was distributed at Little Hastings (10.0 kg fluridone per ha or 6.6 kg per ha-m or 656 ppb). Across 18 months and 26 application events, 1453 kg of pellets, containing 73 kg of fluridone, was distributed at Decker Island (10.0 kg fluridone per ha or 4.7 kg per ha-m or 468 ppb). Note, the total quantity applied at Decker Island was lower than that of Little Hastings because Decker Island is a significantly smaller site and received one fewer treatment series.
Fluridone Concentrations in the Water Column
Fluridone concentrations were measured immediately prior to the first applications in 2017 at Little Hastings, French Island, and Decker Island to quantify potential background levels in the environment. These sites were subsequently monitored during each treatment series over the next 18 months to determine whether concentrations were maintained within the target range of 2–5 ppb (Table 1). In summer 2017, sites were sampled weekly. In spring 2018, sampling frequency was reduced to every 2 weeks due to limited staff resources. Because Fisherman’s Cut was added to the study in April 2018, it was only sampled for fluridone water concentrations during the last two sampling dates of the spring 2018 treatment series. In fall 2018, French Island and Little Hastings were sampled weekly. Decker Island was not treated in fall 2018, so Decker Island and Fisherman’s Cut were not sampled during this period.
Within each study site, water samples were collected from three stations at a depth of 1 m. Two stations were located at water quality monitoring stations, one in a dense SAV bed and the other in an adjacent open water area, while the third station was located in an SAV bed distant from the other stations to better capture spatial variation in fluridone concentrations (Fig. 1). The 60-mL samples were stored in opaque polyethylene bottles. Within 24 h of collection, samples were delivered to a University of California-Davis (UC-Davis) laboratory for fluridone analysis. This laboratory used an enzyme-linked immunosorbent assay (ELISA, also called FasTEST) to analyze all samples until May 2018 (Pandey et al. 2019). From June 2018 onward, they used high-pressure liquid chromatographic (HPLC) (Wickham et al. 2019). ELISA and HPLC results compare well across a wide range of fluridone concentrations (Netherland et al. 2002).
Fluridone Concentrations in the Sediment
Monthly sediment samples were collected to capture changes in sediment fluridone concentrations during treatment and non-treatment periods and to characterize sediment composition. Bed sediment samples were collected from October 2017 to June 2018, such that the first samples were collected within 2 weeks of the last application of the 2017 treatments series (when 2017 sediment concentrations would likely be at a maximum), most of the spring 2018 treatment series, and the intervening months with no treatment (Table 1). Therefore, samples were collected on nine dates from all sites, except Fisherman’s Cut, which was only sampled in June 2018. Sediment samples were collected by boat using a ponar dredge (15 × 15 cm opening, Forestry Suppliers Inc., MS, USA). Vegetation, invertebrates, and debris were removed from samples. No fluridone pellets were observed in samples. Half of each ~ 2 kg sample was used to measure the fluridone concentration and half was used for sediment physio-chemical composition analysis. The ponar dredge was thoroughly rinsed between samples. Samples were transported to the laboratory in a cooler and stored in the dark at 4 °C until analyzed. All samples were analyzed within 1 week.
Sediment fluridone analysis was conducted by the same laboratory at UC-Davis that analyzed the water samples. This laboratory developed methods specifically for fluridone analysis of sediment (Wickham et al. 2019). Fluridone was extracted from wet sediment using the QuEChERS method (Quick, Easy, Cheap, Effective, Rugged, and Safe) (Wickham et al. 2019), and concentrations were determined using high-performance liquid chromatography coupled with an ultraviolet detector (HPLC–UV). Because these methods were still under development, sediment fluridone concentration was not analyzed during the first few months of the study. Another UC-Davis laboratory measured percent organic carbon using the loss-on-ignition method (Nelson and Sommers 1996). In brief, sediment was dried at 55 °C, ground to a fine powder, dried at 105 °C, and ignited in a muffle furnace for 2 h at 360 °C. Percent weight loss of samples was measured, and percent organic carbon was calculated based on the assumption that organic matter is 58% carbon. Additional sediment physio-chemical characteristics were analyzed by the Bryte Soils Laboratory of the California Department of Water Resources (CDWR) in West Sacramento, CA using standard American Society for Testing and Materials methods for soil texture classification (ASTM D2487 2006) and mechanical particle size analysis (ASTM D422–63 2007).
Relationship Between Water Column Fluridone Concentrations and Tidal Stage
Data were collected at Little Hastings to determine the impact of tides on fluridone concentrations. Fluridone is released over weeks from pellets distributed over the sediment surface, so fluridone concentrations in the water likely vary with the ratio between sediment surface area (a constant) and water volume (varies with tides) as well as water residence time. These data were collected during the sixth week of the fall 2018 treatment series (Table 1). ISCO 6700 Portable Samplers (autosamplers) were positioned in three locations to capture spatial variation in fluridone concentrations (Fig. 1). They were programmed to collect water every 2 h beginning October 22, 2018 at 14:00 PST and ending October 24, 2018 at 06:00 PST, which spanned three tidal cycles. Autosamplers rinsed Teflon-lined tubing three times prior to collecting each sample into a 350-mL ISCO glass bottle in the ice filled holding chamber. There were 63 field samples (3 stations × 21 samples) plus 4 quality control samples. Quality control samples consisted of one field blank to test for fluridone contamination of the equipment (purified water; randomly assigned to one autosampler) and three reference samples to test for potential fluridone degradation (purified water spiked with 0.1500 ppb fluridone; one per autosampler). Half the samples were retrieved the morning of October 23, and the remaining samples were retrieved the morning of October 24. Samples were immediately transported on ice to the US Geological Survey (USGS) Pesticides Fate Research Laboratory in Sacramento, CA for fluridone extraction by liquid chromatography tandem mass spectrometry. For details about the analysis methodology, see Orlando et al. (2020). The blank indicated no contamination (i.e., a non-detect; detection limit: 0.0037 ppb), and the reference samples showed no clear signs of degradation (0.158 ppb, 0.158 ppb, and 0.138 ppb). Tidal stage data were downloaded from the USGS website for the nearby monitoring station (USGS 11455315; 38°14′34.84′′, -121°41′03.43′′; 530 m from the study site).
SAV Biomass and Community Composition
Biomass and species composition of SAV was monitored at Little Hastings, French Island, and Decker Island during the 2 weeks prior to the first fluridone applications (May 25 to June 7, 2017) to determine baseline conditions of the sites. Then, sites were sampled every 2 months until December 2018, after the last fluridone applications were completed (10 sampling events total) (Table 1). Sampling at Fisherman’s Cut was initiated in April 2018 (5 sampling events).
A point intercept sampling method was used (Madsen and Wersal 2018). ArcGIS was used to randomly select a set of stations at each site for SAV sampling, specifying a minimum of 30 m between any two points (except Fisherman’s Cut because of its small size). During 2017, 40 stations per site were sampled. During 2018, sampling was reduced to 20 stations per site (a random subset of the original 40 stations), based on a power analysis of the 2017 biomass data (Fig. 1). A sample was generally collected within a ~ 20-m radius of its corresponding station. This approach prevented the removal of vegetation for one sample from affecting biomass of samples collected in subsequent months.
Sample collection was completed using the methods of Johnson and Newman (2011). Briefly, the steps were as follows: (1) lower a long-handled, double-headed thatch rake vertically to the bottom (rake head width of 35 cm with fourteen 5.5-cm-long metal teeth on either side), (2) turn the rake three times on its vertical axis while in contact with the bottom, (3) pull the rake straight up to the surface while continuing to rotate it. In high density SAV areas, the maximum capacity of the rake was sometimes exceeded, and therefore biomass may have been underestimated in some instances. Percent of total sample volume that each SAV species comprised was recorded to the nearest 10%. Samples were transported in garbage bags at ambient temperatures during the 1-h drive to the laboratory where they were stored for up to a week in a refrigerator prior to processing.
Samples were processed to estimate biomass following the methods described in Bickel and Perrett (2015). In brief, samples were rinsed to remove sediment and macroinvertebrates. Once water ran clear through the sample, it was placed into an 18.9-L salad spinner and rotated 20 times (1 revolution per second) to remove excess water. Wet biomass was measured with a bench scale (Ohaus ES BV Bench Series, accuracy of 1.0 g). This method produces values comparable in consistency to those obtained from dry biomass (Bickel and Perrett 2015). To estimate species-specific biomass, total wet biomass of a sample was multiplied by the proportion of volume of each species in the sample.
Changes in SAV Distribution
Hyperspectral airborne imagery was collected over the full Delta, in part, to determine whether fluridone applications reduced the site-wide coverage of SAV at the two treated sites in this study compared to the reference sites. In the Delta, SAV coverage generally reaches a maximum in the fall season, so imagery was first collected during October 8–9, 2016 (8 months before this study). During this survey, Airborne Visible/Infrared Imaging Spectometer - Next Generation sensor (430 bands: 350–2500 nm, bandwidth: 5–7 nm) was used by the National Aeronautics and Space Administration Jet Propulsion Laboratory (Pasadena, CA, USA) to collect imagery at a 2.5 m × 2.5 m ground resolution. The sites were imaged again during October 6–9, 2018, which was 4 months after treatment at Decker Island ended and in the middle of the final treatment series at Little Hastings (Table 1). During this survey, the HyMap sensor (126 bands: 450–2500 nm, bandwidth: 10–15 nm) was used by the HyVista Corporation (Sydney, Australia) to collect imagery at a 1.7 m × 1.7 m resolution. A diverse suite of inputs was derived from these images to capture reflectance properties across different regions of the electromagnetic spectrum, which track biophysiological characteristics useful for distinguishing types of plants. These intermediate inputs were generated using IDL scripts (IDL 8.01, ITT Visual Information Solutions) in ENVI (ENVI 4.8, ITT Visual Information Solutions).
Concurrent with image collection in both years, species composition data were collected at points across the Delta region (2016: 637 points, 2018: 950 points). The dominant type of vegetation was recorded at each location (3 × 3 m minimum). Yearly field data were divided into training and validation subsets for image classification and independent validation of class maps. Training and validation polygons were overlaid on the raster images with generated inputs, and corresponding pixels within the raster images were extracted using the R statistical computing language (R Core Team 2019; version 4.0.2) and packages “sp” (version 1.4.5), “rgdal” (version 0.5.5), and “rgeos” (version 1.5.23).
Training data were fed into a Random Forests classifier (packages “raster”: version 3.4.5 and “randomforest”: version 4.6.14). The best fit class type (e.g., SAV, open water) for each pixel was chosen based on consistency across tree predictions. Accuracy of the final maps was assessed using confusion matrices and Kappa coefficients (Congalton 1996; Rosenfield and Fitzpatrick-Lins 1986). The area of SAV was calculated per year per site as the number of pixels classified as SAV multiplied by the area of a single pixel. For additional details about the imagery analysis, see Khanna et al. (2018).
Statistical Analysis
All statistical analyses were conducted using the R statistical computing language (R Core Team 2019). All tests were evaluated using α = 0.05. Most responses were analyzed with linear mixed models (LMM) using the package “nlme” (Pinheiro et al. 2019). For analyses with fluridone concentrations as responses, non-detects were included as zeros. P-values for LMMs were derived using likelihood ratio tests based on maximum likelihood estimation (Zuur et al. 2013). When pairwise tests were conducted between study sites and/or sampling dates, p-values were adjusted using the Benjamini–Hochberg false discovery rate controlling method to account for multiple comparisons (Benjamini and Hochberg 1995).
Fluridone Concentrations in the Water Column
An LMM was conducted with fluridone concentrations as the response and site as the predictor. Sites included in the analysis were Little Hastings, French Island, and Decker Island (insufficient data for Fisherman’s Cut). For this and other analyses, study site rather than treatment assignment (treated vs reference site) was used as the main predictor because there were only two replicates per treatment assignment. Analysis included data from the Summer 2017 and Spring 2018 treatment periods because these were the periods when both Little Hastings and Decker Island were treated (no treatment of Decker Island in Fall 2018). The LMM included sampling station as a random effect to account for autocorrelation among samples due to repeated measurements (Zuur et al. 2017).
Fluridone Concentrations in the Sediment
An LMM was conducted with sediment concentrations as the response and three predictors: site, treatment (i.e., binary predictor: treatment period vs. non-treatment period), and percent organic carbon of the sediment. Both percent organic carbon and percent clay (particles < 0.005 mm) were considered as candidate predictors to represent sediment characteristics because fluridone bonds strongly to both. Due to significant collinearity, only percent organic carbon was included in the model. Sampling station was included as a random effect. As with water column concentrations, there was not sufficient data to include Fisherman’s Cut. Two outliers for sediment concentrations were excluded (both > 500 ppb higher than all other values; Decker Island: n = 1, Little Hastings: n = 1).
Relationship Between Water Column Fluridone Concentrations and Tidal Stage
An LMM was conducted with concentrations of fluridone in the water column as the response and tidal stage as the predictor. Station was included as a random effect.
SAV Biomass and Community Composition
Comparisons were made among three dates within each site to determine if biomass or composition changed over time. Comparisons were also made between the two sites within each regional pair (North Delta, Central Delta) on each date to determine if sites became less similar over time due to differences in treatment assignment (treated vs reference). The three dates were June 2017 (pre-treatment), June 2018 (during active treatment and 1 year after study initiation), and December 2018 (1 week after all fluridone applications were completed). For all SAV analyses, the 2017 data were randomly subsetted to 20 samples per site and date to assure sample sizes were comparable to those in the 2018 data. To determine if biomass changed in response to treatment, t-tests were conducted between relevant pairs of sites and dates. To determine if species composition changed in response to treatment, comparisons between sites and dates were made with pairwise Permutational Multivariate Analysis of Variance (PERMANOVA, McArdle and Anderson 2001) based on the “adonis2” function of the “vegan” package (Oksanen et al. 2019). Bray–Curtis dissimilarity indices based on square root transformed estimated wet biomass data were generated, and for each comparison, 999 independent permutations were run. The assumption of homogeneity of group dispersions was tested and confirmed for all comparisons using the “betadisper” function in the “vegan” package.
Results
Fluridone Concentrations in the Water Column
Fluridone was detected in 84% of the 259 samples collected during the active treatment periods (detection limit = 0.02 ppb). Detections were frequent across all four sites, including both treated sites (Little Hastings: 87% of 96 samples, Decker Island: 95% of 65 samples) and non-treated reference sites (French Island: 74% of 96 samples, Fisherman’s Cut: 88% of 8 samples). In addition, fluridone was detected in the two samples collected at each site immediately prior to the first applications in 2017 (concentrations in ppb: Little Hastings = 0.03, 0.05; French Island = 0.05, 0.07; Decker Island = 0.10, 1.12; Fisherman’s Cut = NA). Fluridone detection at reference sites and in pre-treatment samples suggests the presence of background levels of fluridone from other sources, which have been documented in studies of contaminant loads in this estuary (Orlando et al. 2020).
Site was a significant predictor of fluridone concentrations (site: χ2 = 7.20, p = 0.03). Pairwise comparisons indicated that all three sites included in the analysis differed significantly from one another (all adjusted p < 0.01; Fig. 2). Mean concentration at Little Hastings was 0.90 ppb ± 1.15 ppb SD, which was 1.5 × and 4.9 × higher than that of Decker Island and French Island, respectively. The limited data for Fisherman’s Cut also indicated low concentrations compared to the two treated sites (Fig. 2).
Concentrations of fluridone in the sediment and water column by study site. Each point represents the mean of three samples. The target concentration range is shown in both plots as a gray band bordered by dashed lines (note difference in y-axis range between plots). Sediment samples were collected monthly for nine consecutive months. Water samples were collected every 1 to 2 weeks during periods of active treatment. There are few data points for Fisherman’s Cut because this reference site was added to the study near the end of treatment for Decker Island. DI, Decker Island, FC, Fisherman’s Cut, FI, French Island, LH, Little Hastings
Though concentrations at treated sites were higher than those of reference sites, treated site concentrations were still generally low relative to the target range of 2–5 ppb (Fig. 2). Specifically, the minimum target concentration of 2 ppb was exceeded in only 12% of samples collected at treated sites (Little Hastings: 14%, Decker Island: 9%), and there were no instances in which all three samples collected from a study site on a given date were simultaneously above this minimum concentration (see electronic supplementary material Figs. S2–S4 for time series of water concentrations by site).
Fluridone Concentrations in the Sediment
Fluridone was detected in 63% of the 78 samples collected during the nine months of monthly monitoring (detection limit = 81 ppb). Detections occurred at the two treated sites (Little Hastings: 72% of 25 samples, Decker Island: 76% of 25 samples) and one of the reference sites (French Island: 48% of 25 samples, Fisherman’s Cut: 0% of 3 samples). Timing of sampling during a treatment vs. non-treatment period was not a significant predictor of sediment concentrations (timing: χ2 = 10.47, p = 0.23). Sediment concentrations remained relatively high throughout the non-treatment period between the summer 2017 and spring 2018 treatment series (see electronic supplementary material Fig. S5 for time series of sediment concentrations by site). However, site was a significant predictor (site: χ2 = 5.79, p = 0.05, Fig. 2). Pairwise comparisons indicated the site effect was driven by the difference between Decker Island and French Island (adjusted p = 0.008). Mean concentration at Decker Island was 437 ppb ± 537 ppb SD by mass, which was 3.1 × higher than that of French Island. Little Hastings and French Island did not differ significantly in concentrations despite differences in treatment assignment. This finding suggests the transport of fluridone to French Island from outside this reference site. There was insufficient data for Fisherman’s Cut. Mean sediment concentrations were several orders of magnitude higher than mean water concentrations (treated sites: Decker Island = 744 × , Little Hastings = 327 × ; reference site: French Island = 762 ×).
Though sediment constituents that bind with fluridone were abundant at the sites, there was no significant relationship between sediment characteristics and sediment concentrations of fluridone (percent organic carbon: χ2 = 0.37, p = 0.54). Sediment at the two North Delta sites was relatively high in clay (particle size < 0.005 mm; Little Hastings: 47.4% ± 6.7% SD; French Island: 52.6% ± 7.9% SD) and organic carbon (Little Hastings: 5.2% ± 1.5% SD; French Island: 5.8% ± 1.9% SD). The treated site in the Central Delta, Decker Island, contained sandier sediment with less clay (28.1% ± 10.3% SD) and organic carbon (4.1% ± 1.5% SD). Sediment composition data were not collected for Fisherman’s Cut.
Relationship Between Water Column Fluridone Concentrations and Tidal Stage
Fluridone was detected in all 63 samples (detection limit: 0.0037 ppb), but concentrations were low (0.53 ppb ± 0.27 ppb SD) with none falling within the target range of 2–5 ppb. Although concentrations were low, they were significantly negatively correlated with tidal stage (Fig. 3; mean fluridone concentration = −0.39 * tidal stage + 3.15, R2 = 0.63; tidal stage: χ2 = 18.01, p < 0.0001) (see electronic supplementary material Fig. S6 for paired time series of fluridone concentrations and tidal stage).
SAV Biomass
In total, 886 samples containing a total wet biomass of 1156 kg were collected. Little Hastings and French Island did not differ from one another in biomass in June 2017 (pre-treatment) or in December 2018 (post-treatment) (both p > 0.1). However, biomass at Little Hastings was lower than that of French Island in June 2018 (adjusted p < 0.0001; Fig. 4), which was 1 year after initiation of treatment and during a treatment period. Decker Island and Fisherman’s Cut did not differ from one another for either date compared (June 2018, December 2018; both p > 0.1). In addition, there were no significant changes in biomass within any of the four study sites among any of the three focal dates (all p > 0.1).
Time series of mean submersed aquatic vegetation (SAV) biomass sampled with a long-handled rake at the four study sites. In the North Delta, treated and reference sites were Little Hastings and French Island respectively. In the Central Delta, treated and references sites were Decker Island and Fisherman’s Cut, respectively. Each point represents the mean of ~ 20 samples. Error bars are standard errors. Shaded background regions indicate periods of weekly fluridone application. The series for Fisherman’s Cut is truncated because sampling at this site was not initiated until 2018
SAV Community Composition
Nine species were detected across the four sites. Egeria densa dominated the communities, particularly at the Central Delta sites, Decker Island and Fisherman’s Cut (Fig. 5). At the North Delta sites, Little Hastings and French Island, Ceratophyllum demersum and Elodea canadensis (common waterweed) were also frequently highly abundant. Myriophyllum spicatum and Potamogeton crispus, when present, were typically at relatively low abundances. Najas guadalupensis (Southern Naiad), Potamogeton nodosus (American pondweed), Stuckenia pectinata (sago pondweed), and Cabomba caroliniana were all rare, composing an estimated combined 0.5% of the total biomass collected during the study (grouped into the “Other” category in Fig. 5). Four of these species are not native to the Delta: E. densa, M. spicatum and P. crispus, and C. caroliniana.
Time series of submersed aquatic vegetation (SAV) community composition sampled with a long-handled rake at the four study sites. Top two panels show the North Delta sites: LH, Little Hastings; FI, French Island. Bottom two panels show the Central Delta sites: DI, Decker Island; FC, Fisherman’s Cut. Each bar represents data from ~ 20 samples. The series for Fisherman’s Cut is truncated because sampling at this site was not initiated until 2018
The community composition at Little Hastings differed between June 2017 (pre-treatment) and June 2018 (1 year after initiation of treatment), based on pairwise PERMANOVA (adjusted p = 0.016; Fig. 5). This difference was driven by an increase in E. densa and decrease in M. spicatum, P. crispus, and E. canadensis (Fig. 5). However, the composition at Little Hastings did not differ between June 2017 (pre-treatment) and December 2018 (post-treatment) (adjusted p = 0.8). Little Hastings and non-treated French Island did not differ from each other in composition in June 2017 (adjusted p = 0.2) but did differ in June 2018 (adjusted p = 0.0005) and December 2018 (adjusted p = 0.009). French Island did not exhibit within-site differences among the three focal dates (all adjusted p > 0.08). For Decker Island and Fisherman’s Cut, there were no differences in composition within sites between dates (all adjusted p > 0.09) or between sites within dates (all adjusted p > 0.1).
Changes in SAV Distribution
Total surface area of SAV increased at most of the sites between October 2016 (pre-treatment) and October 2018 (near end of study), based on hyperspectral imagery. The largest percentage increases occurred at the two treated sites, with SAV area increasing by 34.2% (38.6 to 51.8 ha) and 59.8% (3.7 to 5.9 ha) at Little Hastings and Decker Island, respectively (Fig. S7). French Island exhibited an increase of 25.5% (31.5 to 39.6 ha), and Fisherman’s Cut exhibited no detectable change (1.1 ha at both times periods; imagery for reference sites not shown).
Discussion
We evaluated efficacy of fluridone for control of SAV in the freshwater tidal Sacramento-San Joaquin Delta. Over 18 months, pelleted fluridone treatments were applied to dense SAV stands in our study sites using standard operational procedures. We monitored fluridone concentrations in the environment and responses by SAV communities. We found that concentrations of fluridone in the sediment were high, but concentrations in the water column were generally below the target range, likely because of dissipation of fluridone by tides. We did not observe lasting reductions in SAV abundance or large changes in SAV community composition. Consequently, this project did not achieve the management goal of restoring open water habitat for endangered Hypomesus transpacificus. However, this study highlights the importance of using a science-based approach for evaluating tools in the field at an operational scale and underscores the ongoing challenges of managing SAV in tidal systems. Fluridone has been one of the primary tools for SAV control in the Delta since 1999, but no previous study in this tidal system has paired intensive monitoring of the fate of fluridone and responses by the SAV community to evaluate efficacy and investigate the mechanisms underlying it.
Environmental Concentrations of Fluridone
Fluridone concentrations in the sediment were frequently high. Monthly mean values were typically well over 100 ppb, even in months between treatment series. High concentrations could have resulted from interactions between pelleted fluridone and major constituents of the sediment. Sediment at our sites contained significant amounts of clay and organic carbon. A laboratory experiment using a quantity of fluridone pellets that would theoretically produce a 10-part-per-million (ppm) aqueous solution demonstrated that < 1% of fluridone binds to sediment composed of sand, while > 90% of fluridone binds to sediment composed of clay or organic peat (Mossler et al. 1993). In a laboratory experiment related to our study, a 10-ppm aqueous solution of fluridone had an estimated half-life of 48 days in French Island sediment but a half-life of only 38 days in a mixture of 25% French Island sediment and 75% pure sand (maintained at 20 °C with no light) (Wickham et al. 2020). If fluridone released from pelleted formulations becomes bound to bed sediments, it could remain there for long periods because it would have low exposure to ultraviolet photolysis and likely would be less bioavailable for microbial metabolism and plant absorption (Bailey and White 1964; Muir and Grift 1982; Wickham et al. 2020). These high sediment concentrations of fluridone could negatively affect benthic organisms such as mollusks, zooplankton, and macroinvertebrates (Crosby and Tucker 1966; Yi et al. 2011; Archambault et al. 2015).
Sediment concentrations were sometimes highly variable. On some dates, concentrations varied among the stations within a site by more than 500 ppb (e.g., Decker Island in November 2017). Pelleted formulations likely distribute fluridone in the sediment in a more spatially heterogeneous way than do liquid formulations, which may explain this high variation in measurements in some cases. West et al (1983) generally observed higher and more variable sediment concentrations in ponds treated with pelleted fluridone than with liquid fluridone. They posited that some of this variation was likely due to the presence of fluridone pellets in some of their sediment samples. Samples we collected in April to June 2018 could have contained pellets because these were months of active treatment, and a mostly disintegrated clay pellet would be indistinguishable from the surrounding clay dominated sediment. However, pellets would not have been present during the November 2017 to March 2018 sediment sampling (5 of 9 sampling events) because there was no treatment during this period (2017 treatments ended September 19), and pellets decompose within a few weeks of application (Mossler et al. 1993). Therefore, presence of fluridone pellets in samples cannot fully explain the high concentrations we sometimes observed.
We did not observe a simple relationship between the amount of fluridone applied and the concentrations measured in sediment. Sediment concentrations did not increase monotonically with cumulative amounts of fluridone applied, and sediment concentrations did not significantly differ between treatment and non-treatment periods. Mean concentrations generally remained high throughout the 6 months between the summer 2017 and spring 2018 treatment series (mid-September to mid-March). In addition, sediment concentrations were relatively low during the final sediment sampling period in June 2018, even though this was during a period of treatment. Environmental factors known to affect the half-life of fluridone may help to explain these patterns. Persistence of fluridone bound to sediment during the non-treatment period may have been due, in part, to the lower water temperatures (mean: 13.0 °C; SD: 3.2 °C) and shorter days (range: 9 h 28 min to 11 h 48 min) characteristic of this time of year, which would reduce degradation rates by bacterial metabolism and ultraviolet photolysis. Conversely, during the month of June 2018, water temperatures were higher (mean: 21.5 °C, SD: 1.2 °C) and day length was at an annual maximum (up to 14 h 51 min). In a laboratory experiment, Wickham et al. (2020) found that decreasing water temperature from 20 to 4 °C increased the estimated half-life of a 10-ppm aqueous solution of fluridone in French Island sediment from 1.8 to 5.1 months (maintained in dark conditions). In a similar laboratory experiment, Wickham et al. (2020) observed a significant reduction in the half-life of fluridone in water with increasing ultraviolet light intensity (e.g., 27.3 d with 1000 µW/cm2; 48.1 d with 25 µW/cm2) but no effect of ultraviolet light intensity (up to 1000 µW/cm2) on the degradation rate of fluridone in French Island sediment (12 h light: 12 h dark, 20 °C). West et al. (1983) applied fluridone pellets at 1.12 kg ha−1 in six ponds spanning a range of locations across North America and found sediment concentrations up to 0.18 kg ha−1 (roughly 360–900 ppb) in samples collected 6 months after the treatment. In another field study, two ponds were treated with 100 ppb of aqueous fluridone and the half-life of fluridone in the sediment was about 17 weeks (Muir and Grift 1982). Taken together, these findings suggest that dynamics of sediment concentrations of fluridone were complex and likely due to variation in multiple factors, including those that we measured (e.g., sediment composition, temperature) and others that we did not (e.g., water residence time, uptake rates by SAV, and spatial heterogeneity in fluridone pellet distribution).
Despite high sediment concentrations, concentrations in the water column were generally below the target range of 2–5 ppb. The autosampler data from Little Hastings showed a decrease in fluridone concentrations in the water with increasing tidal stage, suggesting that incoming tides diluted fluridone and outgoing tides likely transported it away from the sites. A previous study conducted in the Delta on dissipation rates of Rhodamine WT dye indicated that high tidal flow rates may represent a significant barrier to achieving required herbicide concentration and exposure times for effective SAV control in many areas of the Delta (Anderson 1999). Slow-release pelleted formulations are designed to help overcome the obstacles associated with water exchange (Netherland et al. 1998). However, we found that target concentrations were difficult to maintain in the water column of these tidally dynamic sites, despite weekly treatments with application rates up to 20 ppb of these pelleted formulations.
Fluridone concentrations were generally higher at treated sites compared to reference sites. However, we detected fluridone at times (i.e., pre-treatment period) and in locations (i.e., reference sites) we did not necessarily expect it. None of our four sites had been previously treated with fluridone, and aside from perhaps Fisherman’s Cut, there would have been no reason to treat them. DBW primarily treats areas of importance to boaters (e.g., marinas, boat ramps), not sites like these targeted for wildlife habitat restoration. It is likely that fluridone was transported via tides to these sites from other treated areas of the Delta. During the 2017–2018 study period, DBW applied 184,353 kg of pellets containing 9218 kg of fluridone at sites across the Delta, including our two treated sites (DBW 2017, 2018). According to the manufacturer, fluridone can travel up to 1.6 km from where it is applied. Based on DBW records, there is only one site within a 1.6-km radius of our sites that has been treated. A private boat ramp located 1.2 km from the reference site, French Island, was treated during the 2 years of this study (but not prior), so this is the most likely external source of fluridone for this site. Fluridone could also have entered French Island from its downstream companion treated site, Little Hastings, but hydrodynamic modeling conducted prior to the study suggested a low probability of this. For the other two study sites, specific external sources remain unclear. The watershed for the Delta consists of a complex and intensively used patchwork of public and private property, which makes external fluridone sources difficult to trace. The USGS laboratory that analyzed some of our water samples for fluridone (see Methods section) also analyzed water samples at up to five sites across the North Delta during the summer and fall months of 2016–2018 and found that, among the 53 pesticides detected, fluridone was one of the dominant contaminants (Orlando et al. 2020). For example, at a sampling site 30 km upstream from Little Hastings Tract, fluridone concentrations up to 0.184 ppb were measured, which is similar to some of our pre-treatment concentrations. The possibility of background levels of fluridone is one of the reasons we included sampling at reference sites and sampling prior to the first round of treatments.
SAV Responses
We did not observe lasting reductions in SAV abundance in this estuarine environment in response to fluridone applications. Studies in lakes indicate fluridone is effective at controlling many of the non-native invasive SAV species present in the Delta, including Egeria densa (Parsons et al. 2009), Potamogeton crispus (Johnson et al. 2012), and Myriophyllum spicatum (Madsen et al. 2002; Wagner et al. 2007; Parsons et al. 2009). In these lake studies, fluridone concentrations in the water were maintained within a range of 2–12 ppb for periods generally lasting at least 60 days. It was possible to achieve effective concentration and exposure times in the lakes with just one to three applications and using a liquid formulation of fluridone, presumably because of the high water residence times characteristic of these systems. Compared to these previous studies, we used slower releasing formulations (pellets), higher application rates (up to 20 ppb per application to achieve a 2–5 ppb in water concentration), more frequent applications (weekly), and longer application periods (up to 16 weeks per treatment series). Nonetheless, we were unsuccessful in sustaining concentrations within our target range in the water column. There were no cases in which all three stations within a site were above the 2-ppb threshold on the same day, and it was rare for measurements from a given station to exceed 2 ppb for even two consecutive weeks. In another field study in the Delta, fluridone pellets were applied at four sites weekly at rates of 5–20 ppb over 12–16 weeks in an effort to achieve water concentrations of 2–5 ppb (Madsen et al. 2021). At one site, fluridone did not reduce biomass of E. densa, the target species. At the other three sites, a significant reduction in biomass was achieved 2 months after treatment, compared to non-treated reference sites, but this effect disappeared by 4 months post treatment. Their findings that fluridone had either a transient effect or no effect on SAV abundance are similar to ours. The challenges encountered in the Delta with maintaining target fluridone concentrations and exposure times have been documented in other systems with complex flows. In a 2-year study conducted in a chain of lakes in a river, liquid fluridone was applied at a nominal rate of 10–12 ppb over a 13-week period each year to control hydrilla (Fox et al. 1994). This nominal rate was not successfully maintained throughout the target region in either year of the study due to difficulties in predicting effects of flows, though the below target concentrations did still reduce presence of hydrilla in both years. Also, field studies of fluridone pellets in other systems have documented inconsistent efficacy even when target concentrations are achieved (Getsinger and Netherland 1997).
Based on hyperspectral imagery, there was generally a net increase in area occupied by SAV at our sites during the 18-month study period. This was a counterintuitive result for the two treated sites. In the North Delta, both treated and reference sites showed an increase in SAV area, so it is likely this increase was due to natural variation in SAV abundance in the region, regardless of fluridone applications. In the Central Delta, the treated site showed a large percent increase in surface area occupied by SAV, while the reference site did not. The increase at this treated site may be partly explained by the 2,4-D applications made to patches of Eichhornia crassipes and Ludwigia spp. at this site during 2018. Areas classified as these taxa in fall 2016 became classified as SAV in fall 2018, indicating an expansion of SAV area or at least a rise to dominance of SAV in those areas. This increase in SAV in response to control of other types of aquatic vegetation highlights one of the many challenges of managing SAV and has been documented in previous studies (Santos et al. 2009; Khanna et al. 2012).
We did not observe lasting changes in the SAV species composition in response to the fluridone applications, which we also attribute to low fluridone concentrations in the water. Previous work suggests that, in some cases, sustained low concentrations of fluridone can shift the SAV community from one dominated by non-native species to one dominated by native species. For example, 2 months of maintaining fluridone concentrations of 2–5 ppb in the water with a liquid formulation effectively controlled M. spicatum in Michigan lakes for 2 years after treatment without reducing native SAV species diversity or cover (Madsen et al. 2002). During a 12-year study in the Delta, fluridone pellets were applied in a large tidal water body to achieve nominal concentrations of 2.5–3.5 ppb during 8–16 weeks per year during most years (Caudill et al. 2019). During that time, the SAV community shifted from one dominated by E. densa to one composed of a diverse mix of species, including seven native species. It is difficult to know how much the fluridone applications contributed to this community shift because there were no non-treated reference sites available for comparison, and measurements of fluridone water concentrations were not included in the manuscript. However, it is possible that this previous Delta study documented a change in SAV community because of the significantly greater number of years of treatment compared to our study.
Differences in water concentrations of fluridone between our study and most other field studies likely explain the lack of SAV control efficacy in our study. There is a strong linear relationship between concentrations of fluridone in the water and concentrations absorbed by SAV shoot tissues (Vassios et al. 2017). Maintaining effective fluridone concentrations in the water intermittently can significantly reduce SAV chlorophyll levels provided that effective concentrations are sustained for at least 3 days between periods up to 12 days of reduced concentrations (Netherland 2015). However, it is unlikely that minimum target concentrations were frequently maintained for multi-day periods in this tidally dynamic system. In the absence of sufficiently sustained effective concentrations in the water, this slow acting systemic herbicide is unlikely to damage photosynthetic tissues enough to reduce SAV biomass. It is less clear why high sediment concentrations of fluridone did not affect SAV biomass or composition because most SAV species are rooted in the sediment (Ceratophyllum demersum is an exception). There is little research on root absorption rates of fluridone from sediment by different SAV species, likely because most studies use liquid formulations that are primarily absorbed by shoots (Vassios et al. 2017). Perhaps fluridone absorbed by SAV roots is released into the water column from shoots before it causes tissue damage if water concentrations are low. Perhaps fluridone is not absorbed well by roots of the SAV species present at these sites. It is also possible that fluridone bound to sediment is significantly less bioavailable for absorption by plants compared to fluridone in water (Bailey and White 1964; Mossler et al. 1993).
Conclusions
By integrating a diversity of techniques and tools, this study provides a unique evaluation of the efficacy and environmental fate of fluridone in an estuarine ecosystem. Our findings suggest that maintaining target concentration and exposure times may not be possible in environments with strong tidal influence, even with repeated applications of pelleted formulations marketed for flowing water use. The results of this detailed fluridone efficacy study conducted at a small number of sites are similar to the results of a Delta-wide efficacy analysis based on remote sensing data, which showed that the impacts of fluridone on SAV at a variety of sites are limited and transient (Khanna et al., in review). To identify SAV sites in estuarine systems where pelleted fluridone will be effective, targeted research is necessary to determine thresholds for water residence time (Fox and Haller 1992; Getsinger et al. 1996) and perhaps also appropriate sediment types (Mossler et al. 1993). This study also highlights the importance of monitoring fluridone in sediment because it can accumulate at high concentrations, persist for months, and likely move with tides to non-treated areas. Lack of fluridone efficacy at these sites means there are limited remaining tools permitted for large scale SAV control in the Delta, complicating efforts to protect and restore habitat for endangered H. transpacificus. The small remaining population of this fish species may be restricted to areas where SAV is excluded by turbidity, salinity, and water depth (Sommer and Mejia 2013; Durand et al. 2016).
It is critical to develop a toolbox for SAV control in tidal systems that is not primarily reliant on a single herbicide. Estuaries comprise a complex mix of habitats, and each habitat type requires a combination of SAV control tools tailored to its unique characteristics and challenges. Development of additional tools is also important because long-term reliance on fluridone can result in fluridone resistance in SAV species (Netherland and Jones 2015). A rotation of suitable herbicides reduces the risk of herbicide resistance. Importantly, new herbicides should undergo sufficient pilot-scale field testing in tidal systems to assure system appropriateness (Getsinger and Netherland 1997). The call for SAV control tools for hydrodynamically complex systems that also minimize impacts on non-target protected species is not new. Getsinger et al. (2008) identified this need, suggesting development of herbicides to supplement fluridone and integration of chemical and non-chemical approaches. In the Delta, DBW recently received approval from permitting agencies to conduct small scale field evaluations (up to 8 ha) of a suite of tools not previously used in this ecosystem. This includes physical control tools (benthic barriers, diver assisted suction removal), herbicides (endothall, carfentrazone-ethyl, flumioxazin, and florpyrauxifen-benzyl), and tools for evaluating and increasing herbicide concentrations and exposure times in this tidal environment (dyes, temporary vertical barriers). It will take years to move from this initial evaluation phase to a broader application phase with these tools, and it is possible that some will be deemed unsuitable for widespread use due to cost, ineffectiveness, or toxicity concerns. The need for developing, implementing, and integrating tools will only increase through time because invasive SAV continues to expand in estuaries (Gillard et al. 2017; Ta et al. 2017; Nunes et al. 2020), threatening native ecosystems and local economies (Herbold et al. 2014; Havel et al. 2015; Jetter and Nes 2018).
Data Availability
All of the data associated with this study have been published on the Environmental Data Initiative (EDI) repository. https://portal.edirepository.org/nis/mapbrowse?packageid=edi.1079.1.
References
Anderson, Lars W.J. 1999. Egeria invades the Sacramento-San Joaquin Delta. Aquatic Nuisance Species Digest 3: 37–40.
Archambault, J.M., C.M. Bergeron, W.G. Cope, R.J. Richardson, M.A. Heilman, J.E. Corey III, M.D. Netherland, and R.J. Heise. 2015. Sensitivity of freshwater molluscs to hydrilla-targeting herbicides: providing context for invasive aquatic weed control in diverse ecosystems. Journal of Freshwater Ecology 30. Taylor & Francis: 335–348. https://doi.org/10.1080/02705060.2014.945104.
Arnold, W. 1979. Fluridone - a new aquatic herbicide. Journal of Aquatic Plant Management 17: 30–33.
ASTM International. 2006. Standard Practice for Classification of Soils for Engineering Purposes. D 2487-06. West Conshohocken, PA: ASTM International.
ASTM International. 2007. Standard Test Method for Particle-Size Analysis of Soils. ASTM D422-63. West Conshohocken, PA: ASTM International.
Bailey, G.W., and J.L. White. 1964. Soil-pesticide relationships, adsorption and desorption of organic pesticides by soil colloids, with implications concerning pesticide bioactivity. Journal of Agricultural and Food Chemistry 12. American Chemical Society: 324–332. https://doi.org/10.1021/jf60134a007.
Benjamini, Yoav, and Yosef Hochberg. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological 57: 289–300.
Bickel, Tobias Oliver, and Christine Perrett. 2015. Precise determination of aquatic plant wet mass using a salad spinner. Canadian Journal of Fisheries and Aquatic Sciences 73: 1–4.
Bultemeier, B.W., and W.T. Haller. 2015. Endothall, triclopyr and fluridone granular release profiles under static and aerated water conditions. Journal of Aquatic Plant Management 53: 197–201.
Caudill, Jeffrey, Ajay R. Jones, Lars Anderson, John D. Madsen, Patricia Gilbert, Scott Shuler, and Mark A. Heilman. 2019. Aquatic plant community restoration following the long-term management of invasive Egeria densa with fluridone treatments. Management of Biological Invasions 10: 473–485. https://doi.org/10.3391/mbi.2019.10.3.05.
Chiconela, T.F., T.J. Koschnick, and W.T. Haller. 2013. Effect of diquat in irrigation water on germination and vegetative growth of rice. Journal of Agricultural and Biological Science 8: 8.
Coetzee, J.A., A. Bownes, and G.D. Martin. 2011. Prospects for the biological control of submerged macrophytes in South Africa. African Entomology 19. BioOne: 469–487.
Cohen, Andrew N., and James T. Carlton. 1998. Accelerating invasion rate in a highly invaded estuary. Science 279: 555–558. https://doi.org/10.1126/science.279.5350.555.
Congalton, R.G. 1996. Accuracy assessment: a critical component of land cover. In Gap analysis: a landscape approach to biodiversity planning, 119–131. Bethesda, Maryland: American Society for Photogrammetry and Remote Sensing.
Crosby, D.G., and R.K. Tucker. 1966. Toxicity of aquatic herbicides to Daphnia magna. Science 154. American Association for the Advancement of Science: 289–291. https://doi.org/10.1126/science.154.3746.289.
DBW. 2017. Submersed Aquatic Vegetation Control Program Annual Monitoring Report. Sacramento, CA: California Department of Parks and Recreation Division of Boating and Waterways.
DBW. 2018. Submersed Aquatic Vegetation Control Program Annual Monitoring Report. Sacramento, CA: California Department of Parks and Recreation Division of Boating and Waterways.
DSC. 2013. The Delta Plan: Ensuring a Reliable Water Supply for California, a Healthy Delta Ecosystem, and a Place of Enduring Value. Sacramento, CA: Delta Stewardship Council.
Durand, John, William Fleenor, Richard McElreath, Maria J. Santos, and Peter Moyle. 2016. Physical controls on the distribution of the submersed aquatic weed Egeria densa in the Sacramento–San Joaquin Delta and implications for habitat restoration. San Francisco Estuary and Watershed Science 14.
Edgar, Graham J, Neville S. Barrett, David J. Graddon, and Peter R. Last. 2000. The conservation significance of estuaries: a classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study. Biological Conservation 92. Elsevier: 383–397.
Fox, A., and W. Haller. 1992. Improving herbicide efficacy in spring-fed tidal canals by timing and application methods. Journal of Aquatic Plant Management 30: 58–62.
Fox, Alison M., W.T. Haller, and D.G. Shilling. 1994. Use of fluridone for hydrilla management in the Withlacoochee River, Florida. Journal of Aquatic Plant Management 32: 47–55.
Gallardo, Belinda, Miguel Clavero, Marta I. Sánchez, and Montserrat Vilà. 2016. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology 22: 151–163. https://doi.org/10.1111/gcb.13004.
Getsinger, K.D., A.M. Fox, and W.T. Haller. 1996. Herbicide application technique development for flowing water: summary of research accomplishments. Miscellaneous Paper A-96–3. Vicksburg, MS: U.S. Army Engineer Waterways Experiment Station.
Getsinger, K.D., and M.D. Netherland. 1997. Herbicide concentration/exposure time requirements for controlling submersed aquatic plants: summary of research accomplishments. Miscellaneous Report A-97–2. Vicksburg, MS: Army Engineer Waterways Experiment Station.
Getsinger, Kurt D., M.D. Netherland, C.E. Grue, and T.J. Koschnick. 2008. Improvements in the use of aquatic herbicides and establishment of future research directions. Journal of Aquatic Plant Management 46: 32–41.
Gillard, Morgane, Gabrielle Thiébaut, Carole Deleu, and Boris Leroy. 2017. Present and future distribution of three aquatic plants taxa across the world: decrease in native and increase in invasive ranges. Biological Invasions 19. Springer: 2159–2170.
Guy‐Haim, Tamar, Devin A. Lyons, Jonne Kotta, Henn Ojaveer, Ana M. Queirós, Eva Chatzinikolaou, Christos Arvanitidis, Serena Como, Paolo Magni, and Andrew J. Blight. 2018. Diverse effects of invasive ecosystem engineers on marine biodiversity and ecosystem functions: a global review and meta‐analysis. Global Change Biology 24. Wiley Online Library: 906–924.
Havel, John E., Katya E. Kovalenko, Sidinei Magela Thomaz, Stefano Amalfitano, and Lee B. Kats. 2015. Aquatic invasive species: Challenges for the future. Hydrobiologia 750: 147–170. https://doi.org/10.1007/s10750-014-2166-0.
Herbold, Bruce, Donald M. Baltz, Larry Brown, Robin Grossinger, Wim Kimmerer, Peggy Lehman, Charles (Si) Simenstad, Carl Wilcox, and Matthew Nobriga. 2014. The role of tidal marsh restoration in fish management in the San Francisco Estuary. San Francisco Estuary and Watershed Science 12. https://doi.org/10.15447/sfews.2014v12iss1art1.
Hofstra, Deborah E., and John S. Clayton. 2012. Assessment of benthic barrier products for submerged aquatic weed control. Journal of Aquatic Plant Management 101–105.
Howard-Williams, Clive, Anne-Maree Schwarz, and Virginia Reid. 1996. Patterns of aquatic weed regrowth following mechanical harvesting in New Zealand hydro-lakes. Hydrobiologia 340: 229–234. https://doi.org/10.1007/BF00012760.
Hussner, A., I. Stiers, M.J.J.M. Verhofstad, E.S. Bakker, B.M.C. Grutters, J. Haury, J.L.C.H. van Valkenburg, et al. 2017. Management and control methods of invasive alien freshwater aquatic plants: A review. Aquatic Botany 136: 112–137. https://doi.org/10.1016/j.aquabot.2016.08.002.
Jetter, Karen M., and Kjersti Nes. 2018. The cost to manage invasive aquatic weeds in the California Bay-Delta. Agricultural and Resource Economics Update 21: 9–11.
Johnson, James A., Ajay R. Jones, and Raymond M. Newman. 2012. Evaluation of lakewide, early season herbicide treatments for controlling invasive curlyleaf pondweed (Potamogeton crispus) in Minnesota lakes. Lake and Reservoir Management 28. Taylor & Francis: 346–363. https://doi.org/10.1080/07438141.2012.744782.
Johnson, James A., and R.M. Newman. 2011. A comparison of two methods for sampling biomass of aquatic plants. Journal of Aquatic Plant Management 49: 1–8.
Jones, Clive G., John H. Lawton, and Moshe Shachak. 1994. Organisms as ecosystem engineers. In Ecosystem Management, 130–147. Springer.
Kay, S.H., David W. Monks, Steve T. Hoyle, and Darren K. Robinson. 1994. Sensitivity of tobacco (Nicotiana tabacum) and vegetable crop seedlings to fluridone in irrigation water. Weed Technology 8. [Cambridge University Press, Weed Science Society of America]: 250–257.
Khanna, Shruti, Maria J. Santos, Jennifer D. Boyer, Kristen D. Shapiro, Joaquim Bellvert, and Susan L. Ustin. 2018. Water primrose invasion changes successional pathways in an estuarine ecosystem. Ecosphere 9: e02418. https://doi.org/10.1002/ecs2.2418.
Khanna, Shruti, Maria J. Santos, Erin L. Hestir, and Susan L. Ustin. 2012. Plant community dynamics relative to the changing distribution of a highly invasive species, Eichhornia crassipes: a remote sensing perspective. Biological Invasions 14. Springer: 717–733.
Koschnick, T. J., W. T. Haller, V. V. Vandiver, and U. Santra. 2003. Efficacy and residue comparisons between two slow-release formulations of fluridone. Journal of Aquatic Plant Management 41. Lehigh Acres: Aquatic Plant Management Soc, Inc: 25–27.
Lotze, Heike K., Hunter S. Lenihan, Bruce J. Bourque, Roger H. Bradbury, Richard G. Cooke, Matthew C. Kay, Susan M. Kidwell, Michael X. Kirby, Charles H. Peterson, and Jeremy BC. Jackson. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312: 1806–1809.
Madsen, John D. 2000. Advantages and Disadvantages of Aquatic Plant Management Techniques. LakeLine 20.
Madsen, John D., Kurt D. Getsinger, R. Michael Stewart, and Chetta S. Owens. 2002. Whole lake fluridone treatments for selective control of Eurasian watermilfoil: II. Impacts on submersed plant communities. Lake and Reservoir Management 18: 191–200.
Madsen, John D., and Guy B. Kyser. 2020. Herbicides for management of water hyacinth in the Sacramento-San Joaquin River Delta California. Journal of Aquatic Plant Management 58: 98–104.
Madsen, J. D., C. Morgan, J. Miskella, G. Kyser, P. Gilbert, J. O’Brien, and K. D. Getsinger. 2021. Brazilian egeria herbicide mesocosm and field trials for managing the Sacramento–San Joaquin River Delta. Journal of Aquatic Plant Management 59s: 90–97.
Madsen, J. D., and R. M. Wersal. 2018. Proper survey methods for research of aquatic plant ecology and management. Journal of Aquatic Plant Management 90–96.
McArdle, B.H., and M.J. Anderson. 2001. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 82: 290–297.
Mossler, M.A., D.G. Shilling, K.E. Milgram, and W.T. Haller. 1993. Interaction of formulation and soil components on the aqueous concentration of fluridone. Journal of Aquatic Plant Management 31: 257–260.
Muir, Derek C.G., and Norbert P. Grift. 1982. Fate of fluridone in sediment and water in laboratory and field experiments. Journal of Agricultural and Food Chemistry 30. American Chemical Society: 238–244. https://doi.org/10.1021/jf00110a006.
Needles, Lisa A, Sarah E. Lester, Richard Ambrose, Anders Andren, Marc Beyeler, Michael S. Connor, James E. Eckman, Barry A. Costa-Pierce, Steven D. Gaines, and Kevin D. Lafferty. 2015. Managing bay and estuarine ecosystems for multiple services. Estuaries and Coasts 38. Springer: 35–48.
Nelson, D.W., and L.E. Sommers. 1996. Chapter 34: total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 3, 1001–1006. Chemical Methods-SSSA Book Series No. 5. Madison, WI: Soil Science Society of America and American Society of Agronomy.
Netherland, M.D., D. Sisneros, A.M. Fox, and W.T. Haller. 1998. Field Evaluation of Low-dose Metering and Polymer Endothall Applications and Comparison of Fluridone Degradation from Liquid and Slow-release Pellet Applications. Technical Report A-98–2. U.S. Army Engineer Waterways Experiment Station.
Netherland, M.D. 2015. Laboratory and greenhouse response of monoecious hydrilla to fluridone. Journal of Aquatic Plant Management 53: 178–184.
Netherland, Michael D., K.D. Getsinger, and E.G. Turner. 1993. Fluridone concentration and exposure time requirements for control of Eurasian watermilfoil and hydrilla. Journal of Aquatic Plant Management 31: 189–189.
Netherland, Michael D., David R. Honnell, Alicia G. Staddon, and Kurt D. Getsinger. 2002. Comparison of immunoassay and HPLC for analyzing fluridone concentrations: new applications for immunoassay techniques. Lake and Reservoir Management 18. Taylor & Francis: 75–80. https://doi.org/10.1080/07438140209353931.
Netherland, Michael D., and Dean Jones. 2015. Fluridone-resistant hydrilla (Hydrilla verticillata) is still dominant in the Kissimmee Chain of Lakes, FL. Invasive Plant Science and Management 8: 212–218. https://doi.org/10.1614/IPSM-D-14-00071.1.
Nunes, Monique, Janine B. Adams, and Lara van Niekerk. 2020. Changes in invasive alien aquatic plants in a small closed estuary. South African Journal of Botany 135: 317–329. https://doi.org/10.1016/j.sajb.2020.09.016.
Oksanen, Jari F., Guillaume Blanchet, Roeland Kindt, Pierre Legendre, Peter R. Minchin, R.B. O’Hara, Gavin L. Simpson, et al. 2019. vegan: Community Ecology Package (version 2.5–6). Comprehensive R Archive Network.
Orlando, J.L., M. De Parsia, C. Sanders, M. Hladik, and J. Frantzich. 2020. Pesticide concentrations associated with augmented flow pulses in the Yolo Bypass and Cache Slough Complex, California. USGS Numbered Series 2020–1076. Reston, VA: U.S. Geological Survey. https://doi.org/10.3133/ofr20201076.
Pandey, Pramod, Jeffrey Caudill, Sarah Lesmeister, Yawen Zheng, Yi Wang, Marie Stillway, Krista Hoffmann, et al. 2019. Assessing glyphosate and fluridone concentrations in water column and sediment leachate. Frontiers in Environmental Science 7. https://doi.org/10.3389/fenvs.2019.00022
Parsons, Jenifer K., A. Couto, K.S. Hamel, and G.E. Marx. 2009. Effect of fluridone on macrophytes and fish in a coastal Washington lake. J Aquat Plant Manage 47: 31–40.
Parsons, Jenifer K., K.S. Hamel, and R. Wierenga. 2007. The impact of diquat on macrophytes and water quality in Battle Ground Lake, Washington. Journal of Aquatic Plant Management 45: 35–39.
Patten, Kim. 2003. Persistence and non-target impact of imazapyr associated with smooth cordgrass control in an estuary. Journal of Aquatic Plant Management 41: 1–6.
Paul, E.A., H.A. Simonin, J. Symula, and R.W. Bauer. 1994. The toxicity of diquat, endothall, and fluridone to the early life stages of fish. Journal of Freshwater Ecology 9. Taylor & Francis: 229–239. https://doi.org/10.1080/02705060.1994.9664890.
Pinheiro, Jose, Douglas Bates, Saikat DebRoy, and Deepayan Sarkar. 2019. nlme: Linear and Nonlinear Mixed Effects Models (version 3.1–140). Comprehensive R archive network.
Pinto, R., V.N. de Jonge, J.M. Neto, T. Domingos, J.C. Marques, and J. Patrício. 2013. Towards a DPSIR driven integration of ecological value, water uses and ecosystem services for estuarine systems. Ocean & Coastal Management 72: 64–79. https://doi.org/10.1016/j.ocecoaman.2011.06.016.
Pratt, Paul D., John C. Herr, Raymond I. Carruthers, and Guillermo Cabrera Walsh. 2019. Complete development on Elodea canadensis (Hydrocharitaceae) eliminates Hydrellia egeriae (Diptera, Ephydridae) as a candidate biological control agent of Egeria densa (Hydrocharitaceae) in the USA. Biocontrol Science and Technology 29. Taylor & Francis: 405–409.
Prior, Kirsten M., Damian C. Adams, Kier D. Klepzig, and Jiri Hulcr. 2018. When does invasive species removal lead to ecological recovery? Implications for management success. Biological Invasions 20: 267–283. https://doi.org/10.1007/s10530-017-1542-x.
Puri, Atul, Gregory E. MacDonald, William T. Haller, and Megh Singh. 2007. Growth and reproductive physiology of fluridone-susceptible and-resistant hydrilla (Hydrilla verticillata) biotypes. Weed Science 55: 441–445.
R Core Team. 2019. R: A language and environment for statistical computing (version 3.6.1). Vienna, Austria: R Foundation for Statistical Computing.
Rosenfield, G.H., and K. Fitzpatrick-Lins. 1986. A coefficient of agreement as a measure of thematic classification accuracy. Photogrammetric Engineering and Remote Sensing 52: 223–227.
Santos, Maria J., Lars W. Anderson, and Susan L. Ustin. 2011. Effects of invasive species on plant communities: An example using submersed aquatic plants at the regional scale. Biological Invasions 13: 443–457. https://doi.org/10.1007/s10530-010-9840-6.
Santos, Maria J., Shruti Khanna, Erin L. Hestir, Margaret E. Andrew, Sepalika S. Rajapakse, Jonathan A. Greenberg, Lars W. J. Anderson, and Susan L. Ustin. 2009. Use of hyperspectral remote sensing to evaluate efficacy of aquatic plant management. Invasive Plant Science and Management 2: 216–229. https://doi.org/10.1614/IPSM-08-115.1.
Sommer, Ted, and Francine Mejia. 2013. A place to call home: a synthesis of Delta Smelt habitat in the upper San Francisco Estuary. San Francisco Estuary and Watershed Science 11.
Sprecher, Susan L., Michael D. Netherland, and A.B. Stewart. 1998. Phytoene and carotene response of aquatic plants to fluridone under laboratory conditions. Journal of Aquatic Plant Management 36: 111–120.
Ta, Jenny, Lars W.J. Anderson, Mairgareth A. Christman, Shruti Khanna, David Kratville, John D. Madsen, Patrick J. Moran, and Joshua H. Viers. 2017. Invasive aquatic vegetation management in the Sacramento–San Joaquin River Delta: status and recommendations. San Francisco Estuary and Watershed Science 15.
Vassios, Joseph D., Scott J. Nissen, Tyler J. Koschnick, and Mark A. Hielman. 2017. Fluridone, penoxsulam, and triclopyr absorption and translocation by Eurasian watermilfoil (Myriophyllum spicatum) and Hydrilla (Hydrillla verticillata). Journal of Aquatic Plant Management 55: 58–64.
Wagner, Kelly I., J. Hauxwell, P.W. Rasmussen, F. Koshere, P. Toshner, K. Aron, D.R. Helsel, S. Toshner, S. Provost, and M. Gansberg. 2007. Whole-lake herbicide treatments for Eurasian watermilfoil in four Wisconsin lakes: Effects on vegetation and water clarity. Lake and Reservoir Management 23: 83–94.
West, Sheldon D., Robert O. Burger, George M. Poole, and Daniel H. Mowrey. 1983. Bioconcentration and field dissipation of the aquatic herbicide fluridone and its degradation products in aquatic environments. Journal of Agricultural and Food Chemistry 31: 579–585. https://doi.org/10.1021/jf00117a028.
Wickham, Patrick, Pramod Pandey, Thomas Harter, and Samuel Sandovol-Solis. 2020. UV light and temperature induced fluridone degradation in water and sediment and potential transport into aquifer. Environmental Pollution 265: 114750. https://doi.org/10.1016/j.envpol.2020.114750.
Wickham, Patrick, Latika Singh, Pramod Pandey, Sarah Lesmeister, Patricia Gilbert, Michael Kwong, Jeffrey Caudill, Jon O’Brien, Sagor Biswas, and Swee Teh. 2019. Development of extraction and detection method for fluridone in water and sediment by HPLC-UV. AMB Express 9. https://doi.org/10.1186/s13568-019-0807-4
Williams, Susan L., and Edwin D. Grosholz. 2008. The invasive species challenge in estuarine and coastal environments: Marrying management and science. Estuaries and Coasts 31: 3–20. https://doi.org/10.1007/s12237-007-9031-6.
Work, Paul A., Maureen Downing-Kunz, and Judith Z. Drexler. 2020. Trapping of suspended sediment by submerged aquatic vegetation in a tidal freshwater region: Field observations and long-term trends. Estuaries and Coasts. https://doi.org/10.1007/s12237-020-00799-w.
Yi, S.A., B.M. Francis, W.M. Jarrell, and D.J. Soucek. 2011. Toxicological effects of the aquatic herbicide, fluridone, on male water mites (Hydrachnidiae: Arrenurus: Megaluracarus). Ecotoxicology 20: 81–87. https://doi.org/10.1007/s10646-010-0558-0.
Younes, M., and H. Galal-Gorchev. 2000. Pesticides in drinking water—a case study. Food and Chemical Toxicology 38: S87–S90. https://doi.org/10.1016/S0278-6915(99)00132-5.
Zuur, Alain F., Joseph M. Hilbe, and Elena N. Ieno. 2013. Beginner’s guide to GLM and GLMM with R: a frequentist and Bayesian perspective for ecologists. Highland Statistics Ltd.
Zuur, Alain F., Elena N. Ieno, and Anatoly A. Saveliev. 2017. Beginner’s Guide to Spatial, Temporal and Spatial-Temporal Ecological Data Analysis with R-INLA. Vol. Volume I: Using GLM and GLMM. Newburgh, UK: Highland Statistics Ltd.
Acknowledgements
We are grateful to Karen Gehrts, Edward Hard, and Ted Sommer for the help in securing funding and for providing staff resources for this project. We thank the many staff of the California Department of Water Resources (CDWR), California Department of Fish and Wildlife (CDFW), and DBW who assisted with collecting and processing samples. We thank the UC-Davis Center for Spatial Technologies and Remote Sensing for help generating hyperspectral vegetation classification maps. We thank the USGS Water Science Center Pesticide Fate Research Group for analyzing fluridone concentration in water samples. We thank the CDWR Bryte Soils Laboratory for analyzing sediment characteristics. This project was supported by funds from CDFW, CDWR, and DBW. DBW executed the herbicide applications in this study under the National Pollutant Discharge Elimination System Statewide General Permit (CAG990005) issued by the State Water Resources Control Board, US Fish and Wildlife Service Biological Opinion (08FBDT00-2018-F-0029-1), National Oceanic and Atmospheric Administration National Marine Fisheries Service Biological Opinion (WCR-2017-8268), and California Department of Fish and Wildlife Streambed Alteration Agreement (1600-2015-0132-R3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kenneth Dunton
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rasmussen, N., Conrad, J.L., Green, H. et al. Efficacy and Fate of Fluridone Applications for Control of Invasive Submersed Aquatic Vegetation in the Estuarine Environment of the Sacramento-San Joaquin Delta. Estuaries and Coasts 45, 1842–1860 (2022). https://doi.org/10.1007/s12237-022-01079-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01079-5