Abstract
The ratio of stable carbon and nitrogen isotopes in the suspended particulate matter has been widely used to study processes occurring in the marine ecosystem. At the same time, the signals provided by isotope ratios in coastal ecosystems can be difficult to interpret, due to several, often contradictory processes taking place simultaneously. In this study, we hypothesized that the carbon and nitrogen isotopic variation is predominantly affected by seasonally occurring phytoplankton species succession in the Gulf of Riga, Baltic Sea. Cyclical seasonal patterns were observed for carbon and nitrogen isotopic compositions of both SPM and phytoplankton data. Enrichment of heavy isotopes in the Gulf of Riga took place during spring phytoplankton bloom (from on average between + 7.1 and + 8.8 ‰, and between − 23.7 and − 21.9 ‰ for δ15N and δ13C, respectively) and pooled at significantly lower values (from + 3.1 to + 5.1 ‰ and from − 28.7 to − 25.1 ‰ for δ15N and δ13C, respectively) for the rest of the year. At the same time, the spatial gradient of isotope ratios was sporadic and inconclusive. The results showed that terrestrial and anthropogenic input to particulate matter is negligible from spring to autumn. Multivariate analysis revealed that the observed seasonal variability was indeed driven by variation in phytoplankton species composition. The diatoms, dinoflagellates, and the ciliate Mesodinium rubrum facilitated enrichment of 15N and 13C in spring. In contrast, atmospheric nitrogen fixation by cyanobacteria and the assimilation of their released nutrients by other organisms resulted in lower δ15N values during summer. This variability requires careful considerations for conducting food web studies in temperate coastal and estuarine environments during high phytoplankton biomass periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Suspended particulate matter (SPM) is a dynamic pool of both living and non-living particles that can have a role in the functioning of food webs, nutrient and contaminant cycling, and system productivity, particularly in coastal and estuarine environments (Cresson et al. 2012; Golubkov et al. 2017; Jędruch et al. 2017; Xu et al. 2019). The amount and composition of SPM in estuarine and coastal systems are affected by various external sources like riverine inflows, coastal erosion, and atmospheric deposition, as well as internal processes like primary production and organic matter mineralization.
The opportunities presented by variable isotopic composition in different sources, like δ13C ratio of + 10‰ in dissolved inorganic carbonates (Wimmer et al. 2013) versus δ13C ratio of − 28 to − 27‰ in terrestrial SPM material (Marcelina et al. 2018; Winogradow et al. 2019), has been successfully explored to characterize extent of terrestrial influence in estuarine and continental shelf waters (McKinney et al. 2010; van de Merwe et al. 2016; Jędruch et al. 2017). Similarly, temporal 15N depletion of SPM due to fixation of atmospheric nitrogen by diazotrophs or seasonally observable enriched SPM δ values has been used to characterize impact of internal processes (Rolff 2000; Montoya et al. 2002; Landrum et al. 2011; Marcelina et al. 2018; Winogradow et al. 2019).
At the same time, the limited number of seasonal studies of carbon and nitrogen isotope variation presents somewhat controversial evidence demonstrating from none or very limited seasonal change of δ13C and δ15N (Harmelin-Vivien et al. 2008; van de Merwe et al. 2016) to substantial seasonal fluctuations (Remeikaite-Nikiene et al. 2017; Marcelina et al. 2018). Therefore, it can be assumed that in some areas, the change in isotope ratio, caused by external sources, is offset by changes created by seasonally occurring internal processes or vice versa. However, there are only a few seasonal studies, like Remeikaite-Nikiene et al. (2017), that attempts to link seasonal changes of external sources, e.g., river runoff, with internal processes, e.g., algae blooms, to substantiate such an assumption. Therefore, the present study aims to evaluate factors and processes that control spatial and temporal changes of stable isotope ratios in the SPM pool in an estuarine like gulf, i.e., the Gulf of Riga, Baltic Sea.
In the Baltic Sea surface, SPM isotopic content is largely controlled by the presence or absence of phytoplankton that incorporates dissolved nutrients into SPM (Winogradow et al. 2019). As dissolved nutrient concentrations in the water column decrease, phytoplankton exhibits less discrimination to absorbing isotopically enriched and energetically more consuming dissolved carbon species, e.g., bicarbonate or atmospheric CO2 (Golubkov et al. 2017).
In the case of nitrogen, the dissolved nitrogen in spring is rapidly incorporated into particulate phase by phytoplankton growth. This reveals how isotope kinetics influence the bulk particulate δ15N value—as the dissolved nitrogen pool is depleted, fractionation of isotopes decreases as well and the particulate δ15N values increase over spring bloom (Savoye et al. 2003). When nitrogen limiting conditions occur in the summer, diazotrophic cyanobacteria (most commonly Aphanizomenon sp., Nodularia spumigena, and Dolichospermum sp.) become an important dissolved nitrogen source in the surface layers of the Baltic Proper (Karlson et al. 2015). These photosynthetic bacteria can cause a significant drop in δ15N value by fixating isotopically depleted atmospheric nitrogen (δ15N = 0‰). Furthermore, other organisms can incorporate the dissolved ammonia and organic nitrogen freshly generated by cyanobacteria into SPM (Ploug et al. 2010).
This let us hypothesize that the seasonal variation of SPM carbon and nitrogen isotope ratios in the study area is predominantly affected by the seasonal succession and the biomass of phytoplankton species. We also hypothesized that the effect of freshwater runoff on isotopic composition of SPM is greatest in the area directly receiving it with fading signal strength further away in transitional waters. To test these hypotheses, we conducted a temporary and spatially resolved study of carbon and nitrogen stable isotope composition of SPM together with phytoplankton species composition, and concentrations of chlorophyll a.
Materials and Methods
Study Area
The Gulf of Riga is a relatively shallow, semi-enclosed sub-basin of the Baltic Sea with the average depth of 26.2 m, water volume of 424 km3, and water residence time of 2 to 4 years (Yurkovskis et al. 1999; Purina et al. 2018). It is strongly influenced by freshwater runoff, since its drainage area (135,700 km2) significantly exceeds the Gulf of Riga surface area (16,330 km2). The average annual freshwater discharge (31 km3 year−1) comprises around 7% of the volume (424 km3) of the Gulf (Berzinsh 1995). The freshwater runoff is distributed unevenly with 86% of it being discharged in the south-eastern part of the Gulf of Riga, mainly from its largest tributaries, the rivers Daugava (20.4 km3 year−1), Lielupe (3.54 km3 year−1), and Gauja (2.33 km3 year−1) (Berzinsh 1995; FAO 2016). This creates an estuary-like transitional water area of up to 15 km radius from the Daugava river mouth. Water exchange with the Baltic Proper is facilitated through the Irbe Strait in the northwest and the Suur Strait in the north. That in combination with river discharge forms a pronounced latitudinal salinity gradient, most evident in spring, when salinities of 2 g kg−1 and below can be observed in south-eastern part of the Gulf and 6–7 g kg−1 in the Irbe Strait (Stipa et al. 1999; Skudra and Lips 2017). Furthermore, the southern part of the Gulf of Riga receives municipal waste water from the Riga city wastewater treatment plant. Approximately 350,000 m3 of treated waste water is discharged one kilometer from the shore daily (Aigars et al. 2017).
Sampling
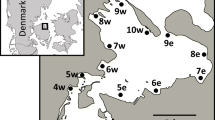
Sampling addressing interannual and spatial variability was done from May 2015 to May 2019 from the research vessel “Salme,” the LR navy hydrological vessel “Varonis,” and a multiple purpose ship “Mare.” Each year samples were collected in spring, summer, and autumn at 28 stations located in the Gulf of Riga and in the Daugava, Lielupe, and Gauja rivers’ delta areas (Fig. 1). Seasonal variation was assessed during 2017, when sampling at stations 101A, D, G, and L (Fig. 1) was done 1–3 times per month from March to November (except August, when sampling was done only at station 101A). SPM, primary production measurement, and phytoplankton samples in the Gulf of Riga were collected as an integrated (0–10 m) sample by a 10-m plastic hose (Ø 5 cm, collected sample volume 5 L) from the euphotic layer on the board of the research vessel. The method in detail, including quality assurance procedures, is described in HELCOM Guidelines for marine monitoring (HELCOM 2017). Water samples for nutrient concentrations were taken by Niskin type bathymeters at 0, 5, and 10 m depth horizons.
Surface layer SPM in the rivers was collected from the riverbank with a bucket attached to a telescopic shaft and filled in 5-L plastic cans, which were prewashed with 0.1 N HCl and rinsed with MilliQ grade deionized water, for short-term storage and transportation.
Water temperature and salinity vertical profiles were measured using CTD (conductivity, temperature, and depth) water probe (SBE 19plus Sea-Cat, Sea-Bird Scientific, USA) with vertical resolution of 0.5 m. Water transparency was measured with a Secchi disc.
Analytical Procedures
The concentration of chlorophyll a (Chl a) was measured according to HELCOM protocols (HELCOM 2017). Chl a was collected as SPM on glass microfiber filters (Whatman GF/C, equivalent to 1.2 µm pore size), and afterwards extracted in 96% ethanol for 24 h and further analyzed using a spectrophotometer (Cary 100 Conc UV–Visible Spectrophotometer, Varian, Australia).
Phytoplankton samples (300 mL) were fixed with acid Lugol’s solution. Subsamples of 10 and 25 mL of fixed samples were settled in a sedimentation chamber for 12 h and counted according to the Uthermöl technique using an inverted microscope (Leica DMI 3000, Leica Microsystems GmbH, Germany) at × 200 and × 400 magnification. The number of counted cells in all subsamples exceeded 500 (Utermöhl 1958; Olenina et al. 2006; HELCOM 2017). The carbon content (µg C m−3) was calculated according to Menden-Deuer and Lessard (2000).
Nutrient concentrations were determined according to Grasshoff et al. (1983). The concentrations of ammonium (NH4+) and phosphate (PO43−) were measured employing the indophenol blue and molybdenum blue methods, respectively. Nitrite (NO2−) and nitrate (NO3−), after reduction to nitrite in a copper coated cadmium column, were determined by nitrite reaction with an azo dye. Dissolved inorganic oxidized nitrogen (NO2+3) is the sum of nitrite and nitrate. The total nitrogen (Ntot) and total phosphorus (Ptot) were analyzed as nitrate and phosphate after wet digestion with persulfate. The dissolved silicates (DSi) were determined photometrically after silicate reaction with ammonium molybdate. Quality of the analyses was checked by inter-laboratory comparison exercises in the Quality Assurance of Information for Marine Environmental Monitoring in Europe Programme (QUASIMEME), from which our laboratory took part, with Z-scores in the range of [− 2;2].
Stable Isotope Analysis
For stable isotope analysis, the water sub-samples were vacuum filtered for at least 30 min on pre-combusted (at 450 °C for 2 h) 24-mm diameter glass microfiber filters (Whatman GF/F, equivalent to 0.7 µm pore size) to collect sufficient amount of material (160–2540 mL of sampled water until the filtering rate was 1 mL s−1). Once the samples were filtered, 5 mL of MilliQ grade water was added to wash off residual soluble salts. All filters were air dried at room temperature and thereafter lightly folded in aluminum foil cups for storage in desiccator until further preparation and analysis.
For gravimetric SPM mass determination, pre-weighted nitrocellulose membrane filters (Millipore, 45 mm diameter, 0.45 μm pore size) were used. After filtration (330–2080 mL of sampled water until the filtering rate was 1 mL s−1) and drying, the nitrocellulose filter weights were measured and mass concentration γf (mg L−1) and consequently mass of SPM on GF/F filter was calculated as follows:
where mf+s is filter + SPM mass, mf filter mass, and Vf filtered water volume through nitrocellulose filter. Due to restricted sampling (1 sample per station, no replicates) and non-homogenous distribution of SPM on GF/F filters (e.g., randomly distributed visible cyanobacteria filaments), acidification to remove inorganic carbonates was not performed as it has been reported to affect δ15N values (Kolasinski et al. 2008; Brodie et al. 2011). The carbonates in the Gulf of Riga form a very minor proportion of total carbon as demonstrated by Carman et al. (1996); therefore, it has been expected that carbonates would not have detectable impact on carbon isotopic ratio.
Prior to the stable isotope analyses, dried GF/F filters holding SPM were cut with a cross-section into 4 equal pieces (pseudo-subsamples). Thereafter, each pseudo-subsample was wrapped in a separate tin cup and analyzed in the Laboratory of Analytical Chemistry at Faculty of Chemistry, University of Latvia, using an elemental analyzer (EuroEA-3024, EuroVector S.p.A, Italy) coupled with a continuous flow stable isotope ratio mass spectrometer (Nu-HORIZON, Nu Instruments Ltd., UK). Isotope ratios were reported relative to Vienna Pee Dee Belemnite with a lithium carbonate anchor (VPDB-LSVEC) for δ13C and to atmospheric nitrogen (AIR) for δ15N as parts per thousands (‰):
where R = 13C/12C, 15 N/14 N. The average value of the 4 pseudo-subsamples was used to represent sample isotopic ratio. For stable isotope ratio measurement quality control, an internal standard sample of glutamic acid and international reference material L-glutamic acid USGS-40 (Reston Stable Isotope Laboratory of the US Geological Survey, Reston, Virginia, NIST®RM 8573) were used. Repeated measurements of 121 internal standard exhibited reproducibility of 0.14‰ for δ13C and 0.21‰ for δ15N. Reference material USG-40, where stable carbon isotopic and nitrogen isotopic compositions with combined uncertainties are δ13CVPDB-LSVEC = − 26.39 ± 0.04‰ and δ15NAIR = − 4.52 ± 0.06‰ (Qi et al. 2003), was used to check accuracy of the stable isotope ratio determination. Our results for reference standard USGS-40 were δ13C = − 26.38 (SD = ± 0.03‰, n = 20) and δ15N = − 4.54 (SD = ± 0.08‰, n = 20).
Primary Production Measurements
In order to evaluate the study area productivity, the light and dark bottle oxygen technique was used (Olesen et al. 1999). Water samples were filled in 18 transparent, calibrated glass bottles for oxygen measurements. Bottles were divided into 6 groups and wrapped in the plastic optical filters (GAMPRODUCTS) with 3 replicates to imitate the light conditions at specific depth of euphotic layer: 100%, 66%, 53%, 23%, 13%, and aluminum folium for 0% light transmittance. Initial oxygen concentrations were fixed with Winkler reagents (1 mL manganese chloride and 1 mL alkaline iodide) before incubation. The remaining samples were placed on a rotating wheel in the incubator with an electric thermometer that controls the water temperature close to natural conditions. At the end of incubation, samples were fixed with Winkler reagents. Oxygen concentrations were determined using the iodometric method—titration with standardized sodium thiosulfate in acid solution according to ISO 5813:1983. Oxygen consumption in the dark bottles (0% light transmittance) was used as a proxy of the phytoplankton community respiration, while the other five groups were used to evaluate the daily primary production rates of the water column.
Oxygen concentrations were converted to carbon units according to stoichiometry of the photosynthesis equation. Water elimination coefficient (k) was calculated for each sample from measured Secchi depth as follows:
where Ds is Secchi depth (m). The depth of specific light conditions (z; units expressed as meters below water surface) was calculated from:
where Iz is light intensity at specific depth (66%, 53%, 23%, 13%) and Io the light intensity below surface (100%). Daily water column net primary production (NPP, g C m−2 day−1) rates were estimated by trapezoidal integration of the data from the various light conditions. Gross primary production (GPP, g C m−2 day−1) was calculated by summing the NPP and the community respiration (R).
Statistical Analysis
The relationship between δ13C and δ15N and the distance from the mouth of the river Daugava (a proxy of runoff impacts) as well as salinity were analyzed by Pearson’s correlations. The analysis was done per sampling period (months) when data from at least 10 different stations were obtained.
K-means clustering algorithm was used to identify seasonal differences in δ13C and δ15N values (Hartigan and Wong 1979). The k-means algorithm establishes k centroids and clusters points by assigning them to the nearest centroid so that the total intra-cluster variation is minimized. The value of k was prespecified based on an exploratory analysis and, ultimately, it was set to 2.
Principal component analysis (PCA) was performed to classify the main sources of data spatial variability. Each cluster, defined by k-means algorithm, was analyzed independently to identify environmental pressures specific to the season. PCA was conducted on the correlation between the isotope values and environmental parameters (temperature, salinity, Chl a, concentration of nutrients ammonium, phosphate, nitrite + nitrate) and also abundant phytoplankton groups, separately. Statistical analysis and visualization of the results were done using software R 3.6.1 (Wickham 2009; R Core Team 2019).
Results
Physicochemical Parameters
The dynamics of temperature and nutrient concentrations in the Gulf of Riga (Online Resource 1) followed a typical seasonal pattern for temperate coastal waters. Low temperatures and high nutrient concentrations were observed during the autumn and winter months, whereas the summer months were characterized by high temperature and low nutrient concentrations. Salinity also followed a seasonal pattern with a slight decrease during the winter. Moreover, it significantly correlated (Table 1) with the distance from the Daugava river mouth in all studied periods (r = 0.61–0.87; p < 0.01), expressing persistent spatial gradient within the Gulf of Riga.
Spatial and Temporal Variation of δ13C and δ15N in SPM
δ13C and δ15N values varied from − 30.6 to − 18.5‰ and from − 0.6 to + 12.9‰, respectively, over the entire 2015–2019 study period in the Gulf of Riga. δ13C showed sporadic correlation with both salinity and the distance from the region of joint inflow of the main rivers (Table 1). δ15N demonstrated consistently negative correlation to the distance and salinity, though with varying significance levels (Table 1).
The carbon isotopes in SPM at station 101A appeared to reach an equilibrium with river SPM only during the convective water mixing periods from autumn to early spring coinciding with larger river runoffs and low phytoplankton GPP (Fig. 2; Fig. 3). In correspondence with the increase of phytoplankton biomass (Fig. 2), the values of δ13C in the SPM of the Gulf of Riga rapidly increased during April 2017 reaching maximum by April 25 (Fig. 3). Thereafter, the slow decline of δ13C was observed throughout May and the summer months. The δ13C values in riverine SPM followed an opposite pattern that coincided with runoff intensity by demonstrating decrease of values during spring and summer.
Temporal changes of phytoplankton carbon biomass by taxonomic classes and gross primary production (GPP) in the Gulf of Riga (station 101A) from March 2017 to November 2017. Group OTHERS includes classes Chlorophyceae, Cryptophyceae, Euglenophyceae, Ebriophyceae, Prasinophyceae, Prymnesiophyceae, and Incertae Sedis
δ15N values of SPM in the Gulf of Riga exhibited a bimodal distribution with two maxima in spring and autumn. The spring (Fig. 3) maxima was reached on May 12 after a rapid 15 N enrichment in April. Thereafter, unlike the δ13C, the δ15N values quickly decreased, reaching a summer minimum on June 28. The broader second δ15N maxima was observed during August and September (Fig. 3). Although the δ15N in riverine SPM exhibited general increase during spring and summer, the seasonal dynamic substantially differed from that observed in the Gulf of Riga. Nitrogen isotope ratio in 101A appeared to even out with river δ15N values over winter; however, equilibrium was reached a month later than for δ13C.
The k-means clustering algorithm revealed two seasonally distinct groups of SPM isotope ratios in the Gulf of Riga samples. Cluster I (Fig. 4) represents April and May (i.e., vernal period), when both δ13C and δ15N had more enriched values (from + 3.6 to + 11.1‰, mean + 7.6 ± 1.8‰, and from − 27.3 to − 18.5‰, mean − 22.6 ± 2.1‰ for δ15N and δ13C, respectively). During the rest of the year (cluster II; Fig. 4) isotope ratios were pooling at lower values (from 0 to + 6.5‰, mean + 4.0 ± 2.1‰, and from − 30.6 to − 25.4‰, mean − 27.5 ± 1.2‰ for δ15N and δ13C, respectively) that are closer to relative isotope ratios of freshwater SPM observed in the river stations (from − 0.7 to + 8.5‰ mean + 2.9 ± 2.8‰ and from − 33.0 to − 27.5‰ mean − 30.0 ± 1.8‰ for δ15N and δ13C, respectively).
The results of PCA showed that δ13C and δ15N values were affected by different abiotic parameters and gradients (Fig. 5). Salinity and nitrates + nitrites were the main environmental drivers (PC1; 34.4%) on overall data variability during the spring season (cluster I; Fig. 5; Online resource 2). Chl a, δ13C, and phosphates together contributed considerably to PC2 (cluster I; Fig. 5) implying δ13C relation to spring phytoplankton dynamics. The impact of seasonal changes on δ13C became more pronounced within cluster II data. They revealed temperature, phosphates, and δ13C as the main contributors to PC1 during the summer–winter period (cluster II; Fig. 5). δ15N had no influence on cluster I data variability, but together with the spatial (salinity) and temporal (chl a dynamics, nitrates + nitrites, ammonium) environmental gradients contributed to PC2 during the summer–winter period (cluster II; Fig. 5; Online resource 2).
The results of principal component analysis for cluster I (a, b) and cluster II (c, d) in combination with environmental parameters: temperature (temp), salinity (sal), concentration of chlorophyll a (chla), concentration of nutrients ammonium (NH4), phosphate (PO4), nitrite + nitrate (NO2 + 3). Variation explained (var.expl.) represented by eigenvalues of the axes (a, c) and biplot (b, d) of individual data scores—points—and loadings of explanatory variables—arrows
Impact of Seasonal Succession of Phytoplankton on Isotopic Composition of SPM
The spring bloom in 2017 had started by late March–early April reaching maximum biomass in late April (Fig. 2; Online Resource 3). The early spring bloom (March and April) was dominated by arctic and arctic-boreal diatoms (mainly Pauliella taeniata, Thalassiosira baltica, and Chaetoceros wighamii) while the late spring bloom (May, early June) was dominated by dinoflagellate Peridiniella catenata and mixotrophic ciliate Mesodinium rubrum. The gross primary production (GPP) (Fig. 2; Fig. S2 in Online Resource 3), mainly manifested as planktonic community respiration, started to increase with the development of phytoplankton spring bloom (April–May) reaching the first maximum in late May–early June.
The second maximum of GPP was observed in July–August, indicating intensive development of summer species. Summer phytoplankton from late June to September was characterized by a mix of different functional groups—cyanobacterium Aphanizomenon flosaquae, ciliates, chlorophytes, and cryptophytes (Fig. 2; Online Resource 3). During the autumn after convective mixing in October–November, diatoms became more abundant again, though decreasing GPP values indicated low activity of the phytoplankton community.
PCA conducted to identify impacts of biotic (phytoplankton succession) parameters on δ13C and δ15N affirms their relation (Fig. 6; Online resource 2). The δ13C, as one of the main contributors to PC1 in both seasonally distinct data pools (cluster I; cluster II), evidently is directly linked to processes in the phytoplankton community. This demonstrates a clear negative correlation with diatom biomass in the spring (cluster I) and shows a direct link to carbon mass of dinoflagellates and small-sized M. rubrum (cluster II). Although to a lesser degree, the δ15N shows clear correlation to specific taxa as well, contributing to PC2 for both data pools, diatoms during spring (cluster I), and large-sized M. rubrum and cyanobacteria during the summer–winter period (cluster II).
The results of principal component analysis for cluster I (a, b) and cluster II (c, d) in combination with carbon mass of abundant phytoplankton groups: diatoms (Diat), dinoflagellates (Dino), ciliates Mesodinium rubrum with cell size between 33 and 65 μm (Cili_33-65) and cell size between 16 and 33 μm (Cili_16-33), cyanobacteria (Cyano), and other taxa (other). Variation explained (var.expl.) represented by eigenvalues of the axes (a, c) and biplot (b, d) of individual data scores—points—and loadings of explanatory variables—arrows
Discussion
The spatial gradients of δ13C and δ15N have been previously documented in estuarine and continental shelf waters (McKinney et al. 2010, van de Merwe et al. 2016, Jędruch et al. 2017). Furthermore, it has been argued that observed seasonal variability of δ13C values of SPM in coastal lagoons is driven by the seasonal dynamic of riverine inputs (Remeikaite-Nikiene et al. 2017; Marcelina et al. 2018). However, in contrast with findings in these studies, we did not establish a clear spatial gradient of δ13C and δ15N values in the Gulf of Riga. The results strongly indicate that the cause of the variability in C and N isotope fractionation are biological processes. The co-variation of δ13C and δ15N with seasonally changing abiotic factors, like temperature and nutrient concentrations, establishes the seasonal nature of δ13C and δ15N values as observed by Savoye et al. (2003), reflecting the seasonal succession of phytoplankton species.
The dynamic of phytoplankton biomass and species succession in the Gulf of Riga followed the general pattern for cold temperate coastal waters (Olli and Heiskanen 1999; Yurkovskis et al. 1999; Jurgensone et al. 2011; Gustafsson et al. 2013; Purina et al. 2018). The pronounced phytoplankton bloom resulted in enrichment of 13C in SPM, similarly to that observed by Remeikaite-Nikiene et al. (2017), as well as in enrichment of 15 N. The δ15N curve observed in the river mouth stations (Fig. 3c) supports this statement as the phytoplankton biomass (expressed as chl a) in the river mouth reaches maximal values during summer months, as demonstrated by earlier study of Aigars et al. (2014), whereas the chl a values in the open part of the Gulf of Riga peak during the spring. The decrease of δ13C values in the riverine water during the summer were in clear contrast to the increase of δ13C values observed in the Gulf of Riga. This demonstrates the insignificance of allochthonous terrestrial material to phytoplankton in transitional waters of the Gulf during summer in contrast to other studies conducted in estuaries or river mouths of the Baltic Sea (Golubkov et al. 2017; Remeikaite-Nikiene et al. 2017; Marcelina et al. 2018). On the other hand, an increased proportion of allochthonous SPM in transitional waters was indicated during periods with low phytoplankton activity by analogous δ13C and δ15N values in pooled river SPM and transitional water SPM values in station 101A during winter (Fig. 3), and elevated C:N ratios before the spring bloom and in late autumn (Table S2 in Online Resource 1).
The PCA clearly indicates that capacity to affect seasonal fractionation of 13C varies among the phytoplankton groups during active biomass growth periods. Variation in δ13C values is mostly driven by diatoms in early spring and dinoflagellates and 33–65 μm sized M. rubrum in late spring, while other phytoplankton groups have minor or no effect. δ13C values in non-vernal periods are driven by dinoflagellate and 16–32 μm sized M. rubrum blooms. The relationship between δ15N and phytoplankton species is more complex than that of δ13C. The rapid increase in δ15N values in March–April can be explained by the development of the early spring bloom. The dominating phytoplankton species during this period were diatoms, known to prefer the more 15 N-enriched NO3− rather than NH4+ for nitrogen uptake during active growth phase (Domingues et al. 2011). After exhaustion of DSi pool (Table S2 in Online resource 1), diatoms were replaced by dinoflagellates and large-sized M. rubrum in late spring (May) further depleting the inorganic nitrogen winter pool by transferring the reminder of it from dissolved phase (majority of it in NO3− form) to particles (Savoye et al. 2003). The relations between δ15N, chl a, and salinity in the spring indicate enhanced growth in transitional waters. This may be due to increased agricultural runoffs being rapidly taken up by the fast-growing phytoplankton species before their dispersion across the Gulf of Riga.
The observed decrease of δ15N values in the summer was most likely caused by the development of the N2-fixing cyanobacterium A. flosaquae. In the Baltic Sea, this species has more N2-fixing heterocysts in June than in late summer (Klawonn et al. 2016). The observed difference in the number of heterocysts can explain 15 N enrichment in SPM after June, while A. flosaquae still maintains a significant proportion in phytoplankton biomass.
However, the negative correlation between cyanobacteria biomass and δ15N values is not obvious in summer according to PCA (Fig. 6, cluster II) most likely because the cyanobacteria were never the overwhelmingly dominating taxa in the non-vernal phytoplankton community during our study. δ15N values by the end of spring bloom in April only slightly exceeded the range of values that are typical for deep ocean inorganic nitrogen (Jędruch et al. 2017; Pantoja et al. 2002) or Baltic Proper surface SPM δ15N values (Winogradow et al. 2019) suggesting very limited impact of the river runoff, which delivers inorganic nitrogen of terrestrial and anthropogenic origin (see isoscapes of DIN, total nitrogen, and δ15N over the study period in Online resource 4, visualized with Ocean Data View v5.5.1. (Schlitzer 2021)). This is in line with the budget calculations presented by Müller-Karulis and Aigars (2011), which demonstrate that on average, 81% of annual nitrogen input was denitrified and 3% was buried. Therefore, only a minor fraction of annual input with clear anthropogenic δ15N signal is being retained in the water column.
Consequently, it could be expected that in the absence of a significant external nitrogen pool by the end of spring bloom, the δ15N value in SPM would be determined by the recycling of an already assimilated nitrogen pool. Contrary to this assumption, the highest δ15N value was observed in May, when no further phytoplankton carbon biomass increase could be detected (due to lack of dissolved phosphates and silicates; Table S2 in Online Resource 1). The most plausible explanation is the shift in phytoplankton species composition, e.g., successive diatom replacement by ciliates M. rubrum in May. It is highly possible that the fast vertical migration ability of this ciliate manifests δ15N value increase from utilizing the bottom layer nitrogen pool (Lips and Lips 2017), as can be observed in the δ15N values transitioning from April 25 to May 23. Furthermore, in early June, M. rubrum did not increase the bulk δ15N values despite its large abundance in the phytoplankton biomass, likely due to depletion of deeper dissolved nitrogen pools. Additionally, it is possible that unfiltered water, as sampled in our study, contains other taxa, e.g., small-sized zooplankton that could increase the overall δ15N values of bulk SPM as shown by Rolff (2000).
Conclusions
The Gulf of Riga receives substantial riverine discharges, which are enriched by nitrogen originating from agricultural lands and municipal wastewater plants located in the drainage basin, as well as direct inputs from municipal wastewaters from Riga and Jurmala cities. The combined discharges form a strong and geographically distinctly located source of nitrogen and carbon of anthropogenic and terrestrial origin. Nevertheless, the δ13C or δ15N values of combined river SPM input signal can only be detected in the stations under direct impact of the river inflow over winter. Results of the present study demonstrate that neither terrestrial or anthropogenic sources or even a combination of them has sufficient strength of its δ13C or δ15N signal to create distinct spatial gradient of δ13C or δ15N values of SPM in the Gulf of Riga during the productive period (spring–autumn). We established that the main driver for δ13C or δ15N variability in SPM during this period is the succession of phytoplankton species. It evidently defines carbon and nitrogen isotopic ratios of SPM during the spring bloom and substantially affects isotope ratios until mid–autumn. Diatoms, dinoflagellates, and M. rubrum show the strongest positive relation to isotopic changes in the Gulf of Riga, whereas the diazotrophic cyanobacteria have an observable but a statistically insignificant negative effect on δ15N values. The results of this study emphasize the need to consider seasonal and spatial variations of the phytoplankton community, while assessing the spatial or seasonal variability of carbon and nitrogen isotope ratios of the SPM. In addition, the indirect evidence provided by this study let us hypothesize that the ciliate M. rubrum facilitates δ15N increase by incorporating near-bottom nitrogen before vertical migration to euphotic zone.
References
Aigars, J., I. Jurgensone, and M. Jansons. 2014. Dynamics of silica and phytoplankton population under altered conditions of river flow in the Daugava River, Latvia. Estonian Journal of Ecology. https://doi.org/10.3176/eco.2014.4.
Aigars, J., N. Suhareva, and R. Poikane. 2017. Distribution of polybrominated diphenyl ethers in sewage sludge, sediments, and fish from Latvia. Environments. https://doi.org/10.3390/environments4010012.
Berzinsh, V. 1995. Hydrology. In Ecosystem of the Gulf of Riga between 1920 and 1990, ed. O. Ojaveer, 7–32. Tallinn: Estonian Academy Publishers.
Brodie, C.R., M.J. Leng, J.S.L. Casford, C.P. Kendrik, J.M. Lloyd, Z. Yongqiang, and M.I. Bird. 2011. Evidence for bias in C and N concentrations and δ13C composition of terrestrial and aquatic organic materials due to pre-analysis acid preparation methods. Chemical Geology. https://doi.org/10.1016/j.chemgeo.2011.01.007.
Carman, R., J. Aigars and B. Larsen. 1996. Carbon and nutrient geochemistry of the surface sediments of the Gulf of Riga, Baltic Sea. Marine Geology. https://doi.org/10.1016/0025-3227(96)00033-3.
Cresson, P., S. Ruitton, M.-F. Fontaine, and M. Harmelin-Vivien. 2012. Spatio-temporal variation of suspended and sedimentary organic matter quality in the Bay of Marseilles (NW Mediterranean) assessed by biochemical and isotopic analyses. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2012.04.003.
Domingues, R.B., A.B. Barbosa, U. Sommer, and H.M. Galvão. 2011. Ammonium, nitrate and phytoplankton interactions in a freshwater tidal estuarine zone: Potential effects of cultural eutrophication. Aquatic Sciences. https://doi.org/10.1007/s00027-011-0180-0.
FAO. 2016. AQUASTAT country profile – Latvia. Food and Agriculture Organization of the United Nations (FAO). http://www.fao.org/nr/water/aquastat/countries_regions/Profile_segments/LVA-WR_eng.stm. Accessed 7 April, 2020.
Golubkov, S., M. Golubkov, A. Tiunov, and V. Nikolina. 2017. Long-term changes in primary production and mineralization of organic matter in the Neva Estuary (Baltic Sea). Journal of Marine Systems. https://doi.org/10.1016/j.jmarsys.2016.12.009.
Grasshoff, K., M. Ehrhardt and K. Kremling. Methods of seawater analysis, ed. K. Grasshoff, M. Ehrhardt, K. Kremling, 126-183. Weinheim; Deerfield Beach: Verlag chemie.
Gustafsson, Ö., J. Gelting, P. Andersson, U. Larsson, and P. Roos. 2013. An assessment of upper ocean carbon and nitrogen export fluxes on the boreal continental shelf: A 3-year study in the open Baltic Sea comparing sediment traps, 234Th proxy, nutrient, and oxygen budgets. Limnology and Oceanography. https://doi.org/10.4319/lom.2013.11.495.
Harmelin-Vivien, M., V. Loizeau, C. Mellon, B. Beker, D. Arlhac, X. Bodiguel, F. Ferraton, R. Hermand, X. Philippon, and C. Salen-Picard. 2008. Comparison of C and N stable isotope ratios between surface particulate organic matter and microphytoplankton in the Gulf of Lions (NW Mediterranean). Continental Shelf Research. https://doi.org/10.1016/j.csr.2008.03.002.
Hartigan, J., and M. Wong. 1979. Algorithm AS 136: A K-means clustering Algorithm. Journal of the Royal Statistical Society: Series C (applied Statistics). https://doi.org/10.2307/2346830.
HELCOM. 2017. Guidelines for monitoring of phytoplankton species composition, abundance and biomass. HELCOM Monitoring Manual. http://www.helcom.fi/Lists/Publications/Guidelines%20for%20monitoring%20phytoplankton%20species%20composition,%20abundance%20and%20biomass.pdf. Accessed 21 October, 2019.
Jędruch, A., U. Kwasigroch, M. Bełdowska, and K. Kuliński. 2017. Mercury in suspended matter of the Gulf of Gdańsk: Origin, distribution and transport at the land–sea interface. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2017.03.019.
Jurgensone, I., J. Carstensen, A. Ikauniece, and B. Kalveka. 2011. Long-term changes and controlling factors of phytoplankton community in the Gulf of Riga (Baltic Sea). Estuaries and Coasts. https://doi.org/10.1007/s12237-011-9402-x.
Karlson, A.M.L., J. Duberg, N.H. Motwani, H. Hogfors, I. Klawonn, H. Ploug, J. Barthel Svedén, A. Garbaras, B. Sundelin, S. Hajdu, U. Larsson, R. Elmgren, and E. Gorokhova. 2015. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio. https://doi.org/10.1007/s13280-015-0660-x.
Klawonn, I., N. Nahar, J. Walve, B. Andersson, M. Olofsson, J.B. Svedén, S. Littman, M.J. Whitehouse, M.M.M. Kuypers, and H. Ploug. 2016. Cell-specific nitrogen- and carbon-fixation of cyanobacteria in a temperate marine system (Baltic Sea). Environmental Microbiology. https://doi.org/10.1111/1462-2920.13557.
Kolasinski, J., K. Rogers, and P. Frouin. 2008. Effects of acidification on carbon and nitrogen stable isotopes of benthic macrofauna from a tropical coral reef. Rapid Communications in Mass Spectrometry. https://doi.org/10.1002/rcm.3694.
Landrum, J.P., M.A. Altabet, and J.P. Montoya. 2011. Basin-scale distributions of stable nitrogen isotopes in the subtropical North Atlantic Ocean: Contribution of diazotroph nitrogen to particulate organic matter and mesozooplankton. Deep Sea Research Part i: Oceanographic Research Papers. https://doi.org/10.1016/j.dsr.2011.01.012.
Lips, I., and Lips, U. 2017. The importance of Mesodinium rubrum at post-spring bloom nutrient and phytoplankton dynamics in the vertically stratified Baltic Sea. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2017.00407.
Marcelina, Z., S. Adam, and R. Pierre. 2018. Spatial and temporal variability of organic matter sources and food web structure across benthic habitats in a low diversity system (southern Baltic Sea). Journal of Sea Research. https://doi.org/10.1016/j.seares.2018.05.007.
McKinney, R.A., A.J. Oczkowski, J. Prezioso, and K.J.W. Hyde. 2010. Spatial variability of nitrogen isotope ratios of particulate material from Northwest Atlantic continental shelf waters. Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2010.08.004.
Menden-Deuer, S., and E.J. Lessard. 2000. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography. https://doi.org/10.4319/lo.2000.45.3.0569.
Montoya, J.P., E.J. Carpenter, and D.G. Capone. 2002. Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnology and Oceanography. https://doi.org/10.4319/lo.2002.47.6.1617.
Müller-Karulis, B., and J. Aigars. 2011. Modeling the long-term dynamics of nutrients and phytoplankton in the Gulf of Riga. Journal of Marine Systems. https://doi.org/10.1016/j.jmarsys.2011.03.006.
Olenina, I., S. Hajdu, A. Andersson, L. Edler, N. Wasmund, S. Busch, J. Göbel, S. Gromisz, S. Huseby, M. Huttunen, A. Jaanus, P. Kokkonen, I. Ledaine, and E. Niemkiewicz. 2006. Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Baltic Sea Environment Proceedings 106: 144.
Olesen, M., C. Lundsgaard, and A. Andrushaitis. 1999. Influence of nutrients and mixing on the primary production and community respiration in the Gulf of Riga. Journal of Marine Systems. https://doi.org/10.1016/S0924-7963(99)00054-8.
Olli, K., and A.-S. Heiskanen. 1999. Seasonal stages of phytoplankton community structure and sinking loss in the Gulf of Riga. Journal of Marine Systems. https://doi.org/10.1016/S0924-7963(99)00056-1.
Pantoja, S., D.J. Repeta, J.P. Sachs, and D.M. Sigman. 2002. Stable isotope constraints on the nitrogen cycle of the Mediterranean Sea water column. Deep Sea Research Part i: Oceanographic Research Papers. https://doi.org/10.1016/S0967-0637(02)00066-3.
Ploug, H., N. Musat, B. Adam, C. L. Moraru, G. Lavik, T. Vagner, B. Bergman, and M. M. M. Kuypers. 2010. Carbon and nitrogen fluxes associated with the cyanobacterium Aphanizomenon sp. in the Baltic Sea. The ISME Journal. https://doi.org/10.1038/ismej.2010.53.
Purina, I., A. Labucis, I. Barda, I. Jurgensone, and J. Aigars. 2018. Primary productivity in the Gulf of Riga (Baltic Sea) in relation to phytoplankton species and nutrient variability. Oceanologia. https://doi.org/10.1016/j.oceano.2018.04.005.
Qi, H., T.B. Coplen, H. Geilmann, W.A. Brand, and J.K. Böhlke. 2003. Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Communications in Mass Spectrometry. https://doi.org/10.1002/rcm.1219.
R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Remeikaite-Nikiene, N., G. Lujaniene, V. Malejevas, R. Barisevičiūte, M. Zilius, I. Vybernaite-Lubiene, G. Garnaga-Budre, and A. Stankevičius. 2017. Assessing nature and dynamics of POM in transitional environment (the Curonian Lagoon, SE Baltic Sea) using a stable isotope approach. Ecological Indicators. https://doi.org/10.1016/j.ecolind.2017.06.035.
Rolff, C. 2000. Seasonal variation in δ13C and δ15N of size-fractionated plankton at a coastal station in the northern Baltic proper. Marine Ecology Progress Series. https://doi.org/10.3354/meps203047.
Savoye, N., A. Aminot, P. Tréguer, M. Fontugne, N. Naulet and R. Kérouel. 2003. Dynamics of particulate organic matter δ15N and δ13C during spring phytoplankton blooms in a macrotidal ecosystem (Bay of Seine, France). Marine Ecology Progress Series. https://doi.org/10.3354/meps255027.
Schlitzer, R. 2021. Ocean Data View. https://odv.awi.de.
Skudra, M., and U. Lips. 2017. Characteristics and inter-annual changes in temperature, salinity and density distribution in the Gulf of Riga. Oceanologia. https://doi.org/10.1016/j.oceano.2016.07.001.
Stipa, T., T. Tamminen, and J. Seppälä. 1999. On the creation and maintenance of stratification in the Gulf of Riga. Journal of Marine Systems. https://doi.org/10.1016/S0924-7963(99)00049-4.
Utermöhl, H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Verhandlungen Der Internationalen Vereinigung Für Theoretische Und Angewandte Limnologie 9: 1–38.
van de Merwe, J.P., S.Y. Lee, R.M. Connolly, K.A. Pitt, and A.D.L. Steven. 2016. Assessing temporal and spatial trends in estuarine nutrient dynamics using a multi-species stable isotope approach. Ecological Indicators. https://doi.org/10.1016/j.ecolind.2016.02.058.
Wickham, H. 2009. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag.
Wimmer, B., M. Hrad, M. Huber-Humer, A. Watzinger, S. Wyhlidal, and T.G. Reichenauer. 2013. Stable isotope signatures for characterising the biological stability of landfilled municipal solid waste. Waste Management. https://doi.org/10.1016/j.wasman.2013.02.017.
Winogradow, A., A. Mackiewicz, and J. Pempkowiak. 2019. Seasonal changes in particulate organic matter (POM) concentrations and properties measured from deep areas of the Baltic Sea. Oceanologia. https://doi.org/10.1016/j.oceano.2019.05.004.
Xu, J., H. Lyu, X. Xu, Y. Li, Z. Li, S. Lei, S. Bi, M. Mu, C. Du, and S. Zeng. 2019. Dual stable isotope tracing the source and composition of POM during algae blooms in a large and shallow eutrophic lake: All contributions from algae? Ecological Indicators. https://doi.org/10.1016/j.ecolind.2019.03.014.
Yurkovskis, A., E. Kostrichkina, and A. Ikauniece. 1999. Seasonal succession and growth in the plankton communities of the Gulf of Riga in relation to long-term nutrient dynamics. Hydrobiologia. https://doi.org/10.1023/A:1003574706608.
Acknowledgements
We would like to express our sincerest gratitude to Māris Bērtiņš from the University of Latvia for conducting the mass spectrometric analysis. Further thanks to our colleagues at the Latvian Institute of Aquatic Ecology for the field work and maintenance of a long-term database, as well as the advice, and many lively discussions during the preparation of this article.
Funding
This research was supported by the state research program “The value and dynamic of Latvia’s ecosystems under changing climate – EVIDEnT,” Administration of Latvian Environmental Protection Fund (project no. 1–08/145/2017), and the Ministry of Environmental Protection and Regional Development (project Nr. IL/106/2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Charles Simenstad
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tunēns, J., Aigars, J., Poikāne, R. et al. Stable Carbon and Nitrogen Isotope Composition in Suspended Particulate Matter Reflects Seasonal Dynamics of Phytoplankton Assemblages in the Gulf of Riga, Baltic Sea. Estuaries and Coasts 45, 2112–2123 (2022). https://doi.org/10.1007/s12237-022-01071-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01071-z