Abstract
Loss of estuarine and coastal habitats worldwide has reduced nursery habitat and function for diverse fishes, including juvenile Chinook salmon (Oncorhynchus tshawytscha). Underutilized off-channel habitats such as flooded rice fields and managed ponds present opportunities for improving rearing conditions and increasing habitat diversity along migratory corridors. While experiments in rice fields have shown enhanced growth rates of juvenile fishes, managed ponds are less studied. To evaluate the potential of these ponds as a nursery habitat, juvenile Chinook salmon (~ 2.8 g, 63 mm FL) were reared in cages in four contrasting locations within Suisun Marsh, a large wetland in the San Francisco Estuary. The locations included a natural tidal slough, a leveed tidal slough, and the inlet and outlet of a tidally muted managed pond established for waterfowl hunting. Fish growth rates differed significantly among locations, with the fastest growth occurring near the outlet in the managed pond. High zooplankton biomass at the managed pond outlet was the best correlate of salmon growth. Water temperatures in the managed pond were also cooler and less variable compared to sloughs, reducing thermal stress. The stress of low dissolved oxygen concentrations within the managed pond was likely mediated by high concentrations of zooplankton and favorable temperatures. Our findings suggest that muted tidal habitats in the San Francisco Estuary and elsewhere could be managed to promote growth and survival of juvenile salmon and other native fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries provide essential nursery habitat for diverse juvenile fishes, but many have been degraded by human activities (Rountree and Able 2007; Borja et al. 2010; Barbier et al. 2011). Tidal wetland habitat has decreased 25–50% globally due to land use conversion (Pendleton et al. 2012; Kirwan and Megonigal 2013) and other remaining habitats are critically vulnerable (Costanza et al. 1997; Edgar et al. 2000; Gedan et al. 2009). Habitat loss and fragmentation result in myriad negative effects on the structure, function, and productivity of nearshore marine ecosystems and their fisheries (Valentine-Rose et al. 2007; Rypel and Layman 2008). In severely human-dominated aquatic ecosystems, the ability to fully restore habitat can be limited by land ownership, funding, dams and other water infrastructure, flood control projects, water diversions, subsidence, and invasive species (Lund et al. 2007; Durand 2017). In these circumstances, ecological rehabilitation may be needed to help re-establish functional nursery habitats (Nagelkerken et al. 2015), but studies needed to inform rehabilitation efforts are often lacking.
The San Francisco Estuary (SFE) in California, USA, is highly altered, having lost approximately 80% of its historical tidal marsh habitat (Monroe et al. 1999; Brophy et al. 2019). This loss, coupled with dams, degradation of upstream habitat, overfishing, and altered hydrology, has contributed to long-term declines in Chinook salmon (Oncorhynchus tshawytscha) populations (Yoshiyama et al. 1998). Juvenile Chinook salmon use of estuarine tidal marsh habitats in the SFE is poorly understood compared to more northern estuaries (Nehlsen et al. 1991; Simenstad and Cordell 2000; Williams 2012), but tidal marsh is identified as critical habitat and an important component of salmon recovery plans. Limited studies suggest SFE brackish tidal marshes provide nursery habitats for Chinook salmon (Healey 1980; Levy and Northcote 1982) in which to forage and take refuge (Shreffler et al. 1992; Williams 2012; Enright et al. 2013). Juveniles which use both estuarine and freshwater rearing habitats typically have higher survivorship than those which rear primarily in one environment (Reimers 1971; Levy and Northcote 1982).
Increasing rearing habitat in tidal marshes can boost overall survivorship and population resilience when freshwater rearing habitats are unfavorable, such as during drought (Bottom et al. 2005; Hering et al. 2010; Volk et al. 2010). This “portfolio effect” is increasingly recognized as an important aspect of managing diverse fish populations, including salmonids (Schindler et al. 2010; Carlson and Satterthwaite 2011). Managing for a portfolio effect might include conservation of diversity in salmonid run timing (Satterthwaite and Carlson 2015), as well as habitat (Goertler et al. 2018; Herbold et al. 2018). For example, habitat diversity promotes diversification of life-history strategies, allowing for population asynchrony over space and time at evolutionary scales, but stability at higher levels of ecological organization (Rypel et al. 2012). Suisun Marsh, the largest contiguous tidal marsh remaining on the west coast of North America, may contribute to a diverse habitat portfolio. However, human use has fragmented portions of the marsh, limiting connectivity between tidal channels and the surrounding marsh plain.
Beginning in the late nineteenth century, much of Suisun Marsh’s historic wetlands were converted to managed ponds for hunting migratory waterfowl. Similar alterations along migratory corridors worldwide provide bird habitat for sport and conservation (Tamisier and Grillas 1994; Ma et al. 2010; Sass et al. 2017). These conversions move considerable tidal fish habitat into tidally muted, levee-lined ponds which limit fish access and reduce edge habitat. Most managed ponds in Suisun Marsh are inundated with shallow water from late fall through early spring to promote feeding and resting locations for hunted waterfowl (Moyle et al. 2014). Hydrologic connectivity between managed ponds and adjacent sloughs is controlled with a system of gates and levees, which mute the tidal range. Reduced connectivity between sloughs and marsh plains has decreased the extent and complexity of available habitat for native aquatic species, including salmon (Mount et al. 2012). Currently, 21,000 ha of habitat are managed in Suisun Marsh, compared with 2550 ha of unmanaged tidal habitat.
We designed this study to address two important questions surrounding the role of Suisun Marsh’s fragmented habitats in SFE fisheries: (1) Do Chinook salmon growth rates differ among natural tidal sloughs, tidal leveed sloughs, and muted tidal habitats (i.e., inlets and outlets of managed ponds) in Suisun Marsh? and (2) if growth differs, what environmental variables are responsible for the difference? To answer these questions, we held juvenile Chinook salmon in cages within each of these locations for 7 weeks from March to April of 2017. We then quantified and compared salmon growth within each habitat using mass growth rates, a metric commonly used to evaluate scope for fisheries productivity (Simenstad and Cordell 2000; Rypel and Layman 2008). We expected salmon to grow fastest in the natural tidal slough because it best represents historic salmon habitat in the marsh.
Methods
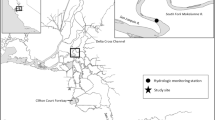
We conducted this study from 3/1/2017 to 4/21/2017 in Suisun Marsh, where juvenile salmon (30–75 mm FL) are generally found from January through May, with peak abundances in February and March (O’Rear et al. 2019). Our study locations, described below, were situated in the northwestern portion of the marsh, which includes managed ponds, preserved marsh, and a matrix of natural and leveed sloughs (Fig. 1).
First Mallard Slough (FM)
This natural, tidal slough in the Rush Ranch Ecological Reserve is relatively undisturbed and meanders through historic wetlands, terminating at an ephemeral creek (Fig. 1; Table 1). There are several small sloughs branching from this mainstem ranging from 0.1 to 0.5 km in length. Invasive common reed (Phragmites australis) and tules (Schoenoplectus spp.) line the slough (O’Rear and Moyle 2018) and the adjacent low-lying marsh plain overtops during ~ 15% of high tides (Enright et al. 2013).
Sheldrake Slough (SD)
This leveed, tidal slough is situated between two large managed wetlands (Fathen and Sheldrake duck hunting clubs), both of which exchange water with Sheldrake Slough from fall through spring (Fig. 1; Table 1). These exchanges occur by way of two major and one minor gated culverts. No side channels exist besides short extensions to gated culvert inlets/outlets. Vegetation on levee margins predominantly consists of up to a 1-m border of common reed and tules (O’Rear and Moyle 2018).
Wings Landing Duck Club (WL_IN and WL_OUT)
This managed, muted tidal wetland is located between Peytonia and Suisun Sloughs (Fig. 1; Table 1) and is slated for open tidal restoration. We used two locations located in the club’s perennially flooded broodstock pond, which is smaller and separate from the main pond and intended to provide waterfowl habitat when the main pond is drained. The broodstock pond maintained water levels high enough for fish survival throughout the study, without negatively impacting the duck clubs pond management timeline. The inlet is on the western side of the pond, 1.5 m deep, and relatively exposed. Water enters from Peytonia Slough over a flashboard gate during daily high tides. The outlet is on the eastern side of the pond, ~ 0.75 m deep, and partially shaded by emergent vegetation. Water moves from the inlet to the outlet via dendritic channels and small ponds and may concentrate near the outlet during the ebb flow before passing over another flashboard control structure back into Peytonia Slough.
Water at all locations is relatively fresh during the months of March and April, with Suisun Marsh 37-year averages of 1.8 and 2.0 ppt respectively (O’Rear et al. 2019). During 2017, a year with higher than average precipitation (Lund 2017), increased riverine outflows produced higher water levels and lower than average salinities across the marsh (1.0 ppt in March and 0.8 ppt in April).
We deployed cages to hold juvenile salmon at a fixed location with access to natural prey and exposure to ambient environmental conditions (Forrester et al. 2003). We constructed cylindrical cages out of 6.35 mm black extruded plastic mesh and floated them near the surface with floats providing approximately seven kilograms of buoyancy. Each cage measured 0.5 m in diameter and 0.75 m tall and was weighted on the bottom with a 19-mm diameter steel stock hoop. Cages attached to a 25.4-mm diameter Schedule 40 PVC pipe sleeve which slid over a 6-m piece of 12.7 mm diameter rebar driven into the marsh substrate. The assembly allowed cages to stay near the surface and move up and down the rebar support as the tide ebbed and flooded. To sample variation within First Mallard and Sheldrake sloughs, we placed cages in two clusters separated by about 0.8 km, each containing three replicate cages. We placed cages at the Wings Landing Duck Club in an inlet and an outlet location, which received three cages each. Multiple clusters at the inlet and outlet locations were not feasible due to space and depth limitations.
Fall-run juvenile Chinook salmon were obtained from Coleman National Fish Hatchery (Anderson, CA USA) and reared in tanks at the UC Davis Center for Aquatic Biology. They were fed 100% dry Skretting feed and maintained at 12 °C, consistent with concurrent water temperatures in Suisun Marsh. After 1 month, we transported 252 fish (mean ~ 2.8 g, 63 mm FL) to the experimental locations in an insulated tank of rearing water aerated with pure filtered oxygen. We measured, weighed, and placed 14 fish into each of the 18 cages. Because smaller fish more easily evaded collection during fish out-planting, those placed in the final clusters (Sheldrake Slough) had smaller initial sizes (mean ~ 2.7 g, 62 mm FL). Fourteen fish per cage created densities as low as possible while also allowing for three fish/cage to be sampled every other week for diet analysis. Increasing cage size to match natural densities would have posed significant impediments to boat traffic. High winds and rough water conditions made initial measurements of fish mass unreliable. These were corrected by length using linearly regressed log-transformed mass-length relationships collected within 24 h of outplant from the same tank and brood stock (n = 52, R2 = 0.9097) in which y is estimated weight and x is measured length,
We sampled cages every 2 weeks from 3/1/2017 to 4/21/2017. During sampling, all fish were netted and transferred to an aerated cooler, weighed (g) in a tared container of water with an Ohaus Scout Pro 200 mg scale, and measured to fork length (mm). We then haphazardly netted and euthanized three fishes per cage for later dissection and diet analysis (IACUC Protocol #19672). Euthanized fish were placed on ice and transferred to a − 18 °C freezer upon return from the field. This sampling reduced the total number of fish in each cage as the study progressed. While reduction in density confounds inter-week comparisons across site, it maintained relative weekly site comparison, and enabled us to collect valuable incremental diet data over the study period. Reducing density over time also balanced finite cage resources with increasing metabolic needs of growing fish. When caged fish occasionally died between sampling events, we removed the carcasses and euthanized fewer live fish in order to maintain consistent numbers of salmon in each cage and reduce the potential for density dependent effects between cages. We assessed cages twice per week for biofouling and cleaned as necessary to allow free water exchange.
All experimental fish that survived until euthanasia (n = 180) were examined for external abnormalities and internal organ condition. We measured stomach fullness on a scale of 0–5, with zero indicating an empty stomach and five a full stomach. Fullness estimates were made based on the ratio of stomach contents to stomach size to account for differences in fish size (Hyslop 1980). Individual prey items were counted and identified to order or genus for zooplankton, genus for amphipods, and order for less common terrestrial and aquatic insects.
We collected zooplankton once per week from March through April adjacent to each cage cluster and in the up-slough reaches of Sheldrake and First Mallard Sloughs. Zooplankton samples were collected using a SEA-GEAR conical 50 cm × 200 cm plankton net with 50 μm mesh with 1 L plastic codend and a General Oceanics flowmeter suspended from the mouth. We suspended the net below the surface of the water between a buoy and 57 g spherical lead weight and hand-towed it 20 m. On three occasions, we were unable to collect a sample due to low water levels. Tows at Wings Landing Inlet were hand-towed approximately 10 m on average due to pond width restrictions at this location. Samples were stored in 500 ml wide-mouth Mason jars, preserved with 5% formaldehyde and stained with 1% rose bengal. In the laboratory, we suspended zooplankton from each tow in a known volume of water (s), sub-sampled in 5 ml increments (a) and identified to order for juveniles and genus for adults. For each sample, all individuals were counted until 150 individuals from the most common taxa were reached. In the case of sparse samples, individuals present in 20% of the total sample volume were counted. The following equation was used to calculate density (d):
where d is the density of individuals per meter cubed, s is known volume of water in which the sample was suspended, a is the total volume of counted subsamples, c is the number of individuals counted, and v is the estimated water volume (m3) sampled by the tow. In cases of flow meter error, we calculated volume using averages of tows collected in similar flow conditions and locations (e.g., downstream slough, mid-slough, upstream slough, managed pond inlet, and managed pond outlet).
Dry-to-ash weight differences found in the literature and from direct measurements of SFE organisms (Dumont et al. 1975; data: W. Kimmerer) were used to calculate the biomass of observed zooplankton. For unknown values, we used the biomass value of the most comparable known taxon. When no comparable taxa were available, known biomass values of zooplankton found in this study were averaged to create a conservative estimate applied in place of the unknown values. This estimate contributed to < 4% of total zooplankton biomass.
ONSET Hobo loggers continuously measured temperature (°C) at each location. Loggers were placed near the bottom of one cage at each location except for the downstream cluster in First Mallard slough, which used data from the National Estuarine Research Reserve System’s long-term water quality station. The station was located between the middle and most downstream cages in the cluster. We used a YSI Pro 2030 to collect salinity (psu), specific conductivity (μS), and dissolved oxygen (% saturation) measurements twice per week, during cage checks or fish sampling at each set of cages. We also took water grabs at these locations for measurement of pH, turbidity (ntu), total phosphorus (mg/L), total dissolved phosphorus (mg/L), ortho-phosphate (mg/L), total nitrogen (mg/L), total dissolved nitrogen (mg/L), ammonium nitrogen (mg/L), nitrate + nitrite nitrogen (mg/L), dissolved organic carbon (mg/L), chlorophyll a (ppb), phaeophytin a (ppb), total suspended solids (mg/L), and volatile suspended solids (mg/L) (R. Dahlgren Lab, UC Davis, CA USA). To account for daily temporal changes in water quality, we altered the order in which each location was visited and sampled throughout the study.
We used mass (g) to evaluate salmon growth rate for each location (Meeuwig et al. 2004; Lusardi et al. 2019). Mass is preferred over fork length as salmonids can maintain skeletal growth at the expense of tissue growth, leading to low body mass in relation to length (Nicieza and Metcalfe 1997). However, fork lengths are also reported in the discussion to allow comparison to other salmon growth studies, many of which report only fork length. Because fish were not individually marked, cages were treated as the experimental units. We calculated growth rate as:
where Y is the growth rate of a specific cage, F is the mass or fork length of individual fish in each cage, w is sampling week, wp is prior sampling week of interest, and d is number of days.
For statistical analyses, we used the program R (R Core Team 2020). Using the package lme4, we analyzed growth data as a two-way repeated measures analysis of variance (Bates et al. 2015). We modeled mass growth rate as the response variable, location and time (i.e., “sampling week”) as fixed variables, and cage as a random effect term. Shapiro-Wilk tests were used to test for normality, and Levene tests used to test for patterns of homoscedasticity. When model results were less than p = 0.05, we performed a Tukey HSD post hoc test to determine which locations differed. Bonferroni adjustments reduced potential type I errors due to multiple comparisons. Five of the 18 cages were compromised, resulting in loss of some or all experimental fish in affected cages (Table 2). Compromised cages were not included in the analysis.
To explore environmental drivers of salmon growth, we used a series of mixed effect growth models informed by principal components analysis (PCA). To reduce collinearity, we used PCA to develop a new set of synthetic and uncorrelated environmental variables or principal components (PCs) to aid in exploring key variables. Prior to running the PCA, we scaled environmental variables (using the base R ‘scale’ function) to achieve a similar magnitude among measurement values (Lusardi et al. 2019). The PCA produced three PCS that described 80% of environmental variation across habitat types (Table S1). Key PCs were identified with a scree plot and the top environmental variables that loaded on each of these key PCs identified as environmental variables of interest for our growth models (Gotelli and Ellison 2004). In this way, the list of candidate environmental driver variables was reduced from 21 to 3 variables. The environmental variables which explained the most variability in PC1, PC2, and PC3 were volatile suspended solids (10.7%), total phosphorus (13.6%), and nitrate + nitrite nitrogen (16.6%), respectively. We added to this list other key environmental variables known to directly influence salmon growth, including zooplankton biomass, temperature, and dissolved oxygen (Herrmann et al. 1962; Marine and Cech 2004; Jeffres et al. 2008; Katz et al. 2017). Salinity was not included due to how low salinity was in the marsh and the lack of substantial variability between sites and throughout the study period.
A series of mixed effect growth models were created with each of these scaled candidate environmental variables (Burnham et al. 2011; Lusardi et al. 2019). First, to determine which temperature metric to use, we compared maximum temperature, mean temperature, and temperature spread (daily maximum - daily minimum) models. Using the package lme4, we modeled mass growth rate as the response variable, an individual temperature metric and its interaction with time (i.e., “sampling week”) as the independent variables, and cage as a random effect term to account for cage effects within location. Normality of residuals was evaluated using QQ-plots and Akaike’s information criteria (adjusted for small sample size, AICc) was used to compare the models (Burnham et al. 2011). Relative model ranks (models considered dissimilar when displaying a delta AICc value > 2; Bolker 2008) and 95% confidence intervals were used to evaluate the effect of the explanatory variables on mass growth rate. The mean temperature model predicted growth the best and was therefore used in subsequent steps of analysis.
Next, using the same structure as the above, we compared a zooplankton biomass model, a dissolved oxygen model, a volatile suspended solids model, the mean temperature model, a total phosphorus model, a nitrate + nitrite nitrogen model, and an intercept model (Table S2). The model which fit the data best was then used as the basis for a new set of models in which the best fit model was edited to include additive and/or interactive effects of another environmental variable (Table S2). The interaction of time (i.e., “sampling week”) with each environmental variables was always included to account for repeated measures. The top model from this step was then similarly adjusted to include additive and/or interactive effects with a third environmental variable. Finally, models from each step which had an AICc value > 2 were compared to one another using AICc to see how these increasingly complex models related to simpler models from earlier steps.
We evaluated spatial and temporal variances in mean zooplankton biomass and mean stomach fullness using two-way repeated measures ANOVAs. We modeled zooplankton biomass using location and time (i.e., “sampling week”) as fixed variables. For stomach fullness, we modeled fullness as the response, with location and time as fixed variables and cage as a random effect term. We ran a Tukey HSD post hoc test when model results were less than p = 0.05 to explore which locations differed from one another. The normality of residuals was confirmed using Shapiro-Wilk tests and homoscedasticity checked using Levene tests.
Results
Fish at Wings Landing Outlet grew the most over the 7-week duration of the experiment (Fig. 2). Growth was affected by location (ANOVA, F(3, 44) = 5.36, p = 0.003), time (ANOVA, F(1, 44) = 12.01, p = 0.001), and the interaction of location and time (ANOVA, F(3, 44) = 9.58, p = 5.50e-05). Wings Landing Outlet had higher growth rates than the other three locations, while First Mallard Slough, Sheldrake Slough, and Wings Landing Inlet fish all had similar growth rates (Tukey HSD test, p < 0.05). Fish in Sheldrake Slough experienced the highest observed growth between week zero and two, followed by a sharp decline thereafter. Fish held at Wings Landing Outlet had the greatest survival between sampling weeks (100%; Table 3).
The top performing model included zooplankton biomass, dissolved oxygen, volatile suspended solids, and the interaction of each of these with time to explain mass growth rate over time (ΔAICc = 0, weight = 29%; Table 4). The second top performing model included zooplankton biomass, dissolved oxygen, mean temperature, and the interaction of each with time (ΔAICc = 0.5, weight = 22%; Table 4). The third best included zooplankton biomass and dissolved oxygen and the interaction of time with each (ΔAICc = 0.7, weight = 20%; Table 4). The remaining models each received < 20% weight. For the top-fitting model, the strongest positive relationship was between zooplankton biomass and growth rate with the strongest negative relationship between dissolved oxygen and growth rate; estimates for these parameters were positive and negative, respectively, and did not overlap zero (Table 5). There was a weak negative relationship between time and growth rate, volatile suspended solids and growth rate, and zooplankton*time with growth rate. These relationships were similar in the second and third models, with the second model also highlighting a slight negative relationship between temperature and growth rate (Table 5).
Zooplankton biomass (ugC/m3) differed across locations (ANOVA, F(3, 8) = 7.79, p = 0.009; Fig. 3) but not by sampling week (ANOVA, F(1, 8) = 1.36, p = 0.28). However, there was a strong interaction between location and sampling week on zooplankton biomass (ANOVA, F(3, 8) = 14.63, p = 0.001). First Mallard had slightly higher mean biomass in the beginning of the study, tapering by mid to late March. Zooplankton biomass at the Wings Landing Inlet remained consistently low throughout the study. Wings Landing Outlet had higher zooplankton biomass compared to First Mallard Slough and the Wings Landing Inlet (Tukey HSD test, p < 0.05), with mean biomass spiking between week three and four of the study and increasing through April (Fig. 3). Sheldrake Slough, which saw high zooplankton biomass in the beginning of the study but declining thereafter, fell statistically between the other locations (Tukey HSD test, p < 0.05).
The relative composition of taxa also differed among locations, with Daphnia being a large (84%) contributor to biomass at the Wings Landing Outlet while rarely (1%) contributing biomass at other locations (Fig. 4). Other Cladocera genera found in the tows included Bosmina, Chydorus, and Ceriodaphnia, which were distributed across locations, albeit in low numbers (< 1% total biomass). Copepods were found at all locations (53% total biomass) and were composed of the genera Acanthocyclops, Limnothona, Eurytemora, Pseudodiaptomus, and Sinocalanus. Other commonly found taxa included harpacticoid copepods and Eucypris ostracods.
Proportional taxa composition of zooplankton biomass collected via tows (a) compared to zooplankton biomass in the diets (b). Taxa found in the diet other than zooplankton are not included. “Other” categories include both taxa not covered in more specific groupings and those too deteriorated to identify further. All juvenile calanoid and cyclopoid copepods are included under “Other Copepoda” category while adults were differentiated further
Stomach fullness differed across locations (ANOVA, F(3, 44) = 7.66, p = 0.0003; Fig. 5). Fish held at the Wings Landing Outlet were fuller than fish from First Mallard and Sheldrake sloughs; Wings Landing Inlet were similar to those from other locations (Tukey HSD test, p < 0.05). Sampling week did not appear to affect stomach fullness by itself (ANOVA, F(1, 44) = 1.22, p = 0.28). However, stomach fullness was affected by the interaction between location and sampling week (ANOVA, F(3, 44) = 7.53, p = 0.0004). Proportions of prey items varied across locations but most commonly included cyclopoids, calanoids, gammarids, and corophiids. Generally, the zooplankton taxa with the highest biomass in the environment corresponded to taxa with the highest biomass in the diets (Fig. 4). Of all locations, stomach contents from Wings Landing Outlet contained the greatest zooplankton biomass. Wings Landing Outlet was also the only location where the zooplankter Daphnia was found consistently in stomachs. Fish stomachs from Sheldrake Slough contained high copepod biomass during the first weeks of the study, corresponding to a period of adjacent managed wetland exports into the slough and declining thereafter. Stomachs from Wings Landing Inlet contained more terrestrial insects versus other locations and, during the first sampling period, one fish notably consumed a large number of ostracods (Fig. 4). Other taxa consumed but rarely found included (in order of abundance): ostracods, chironomid larvae, harpacticoid copepods, isopods, and cumaceans.
Wings Landing Outlet had lower average daily mean and mean maximum temperatures compared to First Mallard Slough, Sheldrake Slough, and the Wings Landing Inlet (Table 6). Both the Wings Landing Outlet and Inlet displayed less diel fluctuation than Sheldrake and First Mallard sloughs. Additionally, bi-weekly discrete samples of dissolved oxygen concentrations were lower at Wings Landing Outlet than First Mallard Slough, Sheldrake Slough, and the Wings Landing Inlet (Table 6). While dissolved oxygen concentrations were uniformly low across all locations, Wings Landing Outlet had the lowest recorded value, at 2.0 mg/L, compared to minimum measured values of 4.8, 5.4, and 3.1 mg/L for First Mallard Slough, Sheldrake Slough, and Wings Landing Inlet, respectively. Mean volatile suspended solids were lowest in Wings Landing Outlet and highest in Sheldrake Slough (Table 6).
Discussion
This is the first study in the SFE to demonstrate that a productive tidally muted managed pond can benefit rearing salmon. Contrary to our expectation, salmon grew considerably faster in the managed pond outlet relative to the other locations, with observed growth rates that were comparable to other productive habitats (Table 7). Limited studies suggest that juvenile Chinook salmon in the SFE show less estuarine dependency than other populations, with shorter juvenile residence time and correspondingly slower growth rates (Kjelson et al. 1982; MacFarlane and Norton 2002). Whereas juvenile salmon in more northern populations use estuary rearing habitats into August, most salmon leave the SFE by June to avoid warming water temperatures in the estuary. Reduced estuarine dependence and growth rates in the SFE may be due to habitat loss, flow alterations, and changes to prey communities (Alpine and Cloern 1992; MacFarlane and Norton 2002; Jassby 2008; Kimmerer et al. 2012). However, growth rates observed at the managed wetland pond in this study generally fall within (and occasionally exceed) reported ranges from more northern estuaries (Healey 1980; Shreffler et al. 1992; Moore et al. 2016; Table 7).
Of the factors we measured, the high growth rates in the managed pond outlet were most attributable to considerably greater zooplankton biomass in the managed pond, relative to other locations. Fish held in the managed pond outlet were the fullest, with higher zooplankton biomass in their diets compared to other locations, verifying that the fish took advantage of the abundance of zooplankton in their environment. We speculate that volatile suspended solids (a measure of organic matter in the water) was included in the top performing model as it covaried with zooplankton biomass. The managed pond outlet, which had the highest zooplankton biomass, had the lowest amount of volatile suspended solids likely because these solids were being consumed or used by zooplankton or other higher trophic processes. We also saw 100% survival of fish between all sampling periods at the managed pond outlet location. While mortality between sampling events at other locations late in the study would presumably improve resource availability for the remaining fish, the managed pond outlet displayed the greatest growth even with the maximum amount of fish sharing the cage resources. We observed high growth rates in Sheldrake Slough in the first weeks of the study, when adjacent managed ponds were regularly releasing water into the slough to make room for water gained through precipitation. This corresponded to a spike in zooplankton biomass and fish growth in Sheldrake Slough which subsequently dissipated when inputs ceased.
The observed zooplankton productivity at the managed pond outlet is consistent with the positive relationship between phytoplankton (a food source for zooplankton) and longer water residence time that has been observed in rivers, lakes, floodplains, estuaries, and lagoons when nutrients are not limited (Alpine and Cloern 1992; Lucas et al. 2009; Peierls et al. 2012). These habitats are engines of productivity that increase food production, allowing fish to feed successfully while minimizing energy expenditures on foraging (Corline et al. 2017). Freshwater floodplain habitats above the tidal excursion of the SFE have shown elevated levels of zooplankton biomass and correspondingly high Chinook salmon growth rates compared to local riverine habitats (Sommer et al. 2001; Jeffres et al. 2008; Katz et al. 2017). While these upriver floodplain habitats have been recognized for their benefits to salmon, wetland habitats in the tidal estuarine portion of this large deltaic ecosystem have received less attention. The few studies which have measured managed pond productivity in the SFE have found similar periods of high zooplankton abundance (Williamson, Durand, Phillips, and Tung, personal communication), suggesting that the pond in this study is likely not an outlier in the system. Additionally, as was observed in Sheldrake Slough, this study suggests that drainage of water from managed ponds, which overlaps with peak juvenile Chinook salmon outmigration (Kjelson et al. 1982), can export food resources into less productive surrounding waterways and benefit salmon indirectly. Similarities in ecological function among floodplains, tidal wetlands, and managed ponds indicate that productive managed ponds could provide rearing benefits for salmon.
Aside from food abundance, temperature and dissolved oxygen are important factors influencing growth and survival of juvenile salmon (Geist et al. 2006) and were included in the top performing growth rate models. The managed pond had lower mean temperatures and less diel temperature fluctuation relative to the tidal sloughs. This may be attributable to the combination of a relatively high ratio of emergent vegetation to channel width, inputs of slough water during high tides, and evaporative cooling at night (Heath et al. 1993; Enright et al. 2013; Hemes et al. 2018). Habitat features that produce thermal refugia are generally viewed as positive for coldwater fishes like salmonids (McCullough et al. 2009); thus, we believe these pond features provided additional benefits to salmon in this study.
While dissolved oxygen levels were low in the managed pond and reduced growth rate potential, the stress of low dissolved oxygen was likely offset by lower thermal stress and higher food abundance. This benefit of food abundance and lower thermal stress in the managed wetland outlet, which also had the lowest dissolved oxygen, is likely why we found a negative effect of increased dissolved oxygen on salmon growth in our mixed effect model. Dissolved oxygen was higher in the sloughs; however, both the leveed and natural slough experienced greater diel fluctuation and higher maximum temperatures, especially in late April. The spike in mortality at these locations during the final week of the study likely resulted from the physiological stress of temperature fluctuation in combination with too few prey resources to meet the increased metabolic needs resulting from warmer temperatures. Most fish that died during the study appeared emaciated.
While this field experiment suggests managed ponds may benefit rearing salmon, the use of cages limits full interpretation of growth rates and diet. For example, caging fish in the tidal sloughs may have subjected the juvenile salmon to water velocities from which they would otherwise seek refuge. Fish reared in the managed pond may have expended less energy as the tidal flow was muted by gates. Additionally, cage effects likely dampened true growth potential as study fish were held at higher densities than free-swimming fish would likely be found and were limited to consuming only what passed through the cages. This could have amplified competition for resources, magnified reliance on zooplankton, created an artificial structure for amphipods to colonize, and decreased access to other common prey items such as terrestrial insects (David et al. 2016). For example, First Mallard Slough has been recognized as valuable fish nursery habitat within Suisun Marsh (Colombano et al. 2020), although salmon in this study grew poorly there. Food resources at this location may be dominated by epibenthic invertebrates (O’Rear and Moyle 2018) rather than zooplankton; such invertebrates were less available in cages, especially cages in deeper locations which had less interaction with the benthos. The cages likely also limited access to insect fallout from overhanging vegetation due to the mesh material covering the top of the cages.
The uncertainties in this field experiment do not change the conclusion that it is possible for salmon to benefit from managed wetland zooplankton productivity. As a test of the comparative zooplankton resources available to juvenile salmon across different marsh locations, the study provided robust results that bear further investigation. While this study took place during a single year and differing environmental conditions may change the magnitude of zooplankton productivity and fish growth, these results uphold that there are conditions in which managed wetlands can provide valuable food resources. To reduce uncertainties, future studies should consider deploying instruments to measure water velocity as part of a bioenergetic model, find locations in which larger enclosures could be safely deployed, and study multiple years with varied environmental conditions. Additionally, the cage structure itself appeared to attract amphipods, which were found consistently in the diets across all sites. Future growth rate modeling would benefit from the inclusion of these prey resources, which will require new techniques for quantifying invertebrate cage colonization.
In altered estuaries, such as the SFE, it may not be possible to achieve conservation goals through tidal restoration alone, as the size of planned and feasible restoration is often limited (Moyle et al. 2018). Under these circumstances, exploring alternative options may be necessary to aid declining populations. The high fish growth rates in this study, resulting from muted tidal pond productivity, demonstrate the potential of an altered estuarine habitat to benefit rearing juvenile salmon. Managed wetlands could be used to supplement in-slough zooplankton during critical times, such as peak salmon outmigration. Additionally, productive managed ponds could be outfitted with water control structures to improve volitional passage of fish into these typically inaccessible habitats. Improvement to lateral movement of fishes from rivers and sloughs into floodplain and wetland habitats is an important element of restoration in impacted watersheds and connected river ecosystems (Junk et al. 1989; Jones and Stuart 2008; Baumgartner et al. 2014) and increase available rearing habitat. However, different salmon populations and run types have varied migration periods and life-history strategies that may complicate the utility of the managed habitats explored in this study. In addition, uncertainty about the ability of fish to navigate through these ponds, avoid avian predation, and find exits merits further research.
Actions that increase productivity and improve connectivity can increase the value of estuarine marshes for out-migrating juvenile salmon, especially in the SFE where salmon are in decline. Repeated studies of more northern Pacific Coast salmon populations demonstrate the value of estuaries as key stopover habitats along migratory routes and show that wetland restoration increases rearing opportunities (Bottom et al. 2005; Volk et al. 2010; Moore et al. 2016). Increasing habitat and ecosystem function in estuaries can expand rearing opportunities for out-migrating salmon and encourage diversification of life-history strategies. When restoration is not possible, taking advantage of underappreciated productive habitats could provide an opportunity to manage for multiple ecosystem services (Needles et al. 2015) and create a desirable mosaic of habitat (Ritter et al. 2008) for varied foraging opportunities. Novel or managed habitats in other coastal and estuarine ecosystems may have similar capacities to provide nursery benefits to juvenile fishes (Jude and Pappas 1992) and these possibilities should be further explored.
Change history
10 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12237-021-01013-1
References
Alpine, A.E., and J.E. Cloern. 1992. Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnology and Oceanography 37 (5): 946–955.
Barbier, E.B., S.D. Hacker, C. Kennedy, E.W. Koch, A.C. Stier, and B.R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81 (2): 169–193.
Bates, D., M. Mächler, B. Bolker, and S. Walker. 2015. Fitting linear mixed effects models using lme4. Journal of Statistical Software 67 (1): 1–48.
Baumgartner, L., B. Zampatti, M. Jones, I. Stuart, and M. Mallen-Cooper. 2014. Fish passage in the Murray-Darling Basin, Australia: Not just an upstream battle. Ecological Management & Restoration 15: 28–39.
Bolker, B.M. 2008. Ecological models and data in R. Princeton University Press.
Borja, Á., D.M. Dauer, M. Elliott, and C.A. Simenstad. 2010. Medium-and long-term recovery of estuarine and coastal ecosystems: Patterns, rates and restoration effectiveness. Estuaries and Coasts 33 (6): 1249–1260.
Bottom, D.L., K.K. Jones, T.J. Cornwell, A. Gray, and C.A. Simenstad. 2005. Patterns of Chinook salmon migration and residency in the Salmon River estuary (Oregon). Estuarine, Coastal and Shelf Science 64 (1): 79–93.
Brophy, L.S., C.M. Greene, V.C. Hare, B. Holycross, A. Lanier, W.N. Heady, K. O’Connor, H. Imaki, T. Haddad, and R. Davis. 2019. Insights into estuary habitat loss in the western United States using a new method for mapping maximum extent of tidal wetlands. PLoS One 14 (8): e0218558. https://doi.org/10.1371/journal.pone.0218558.
Burnham, K.P., D.R. Anderson, and K.P. Huyvaert. 2011. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65 (1): 23–35.
Carlson, S.M., and W.H. Satterthwaite. 2011. Weakened portfolio effect in a collapsed salmon population complex. Canadian Journal of Fisheries and Aquatic Sciences 68 (9): 1579–1589.
Colombano, D.D., A.D. Manfree, T.A. O’Rear, J.R. Durand, and P.B. Moyle. 2020. Estuarine-terrestrial habitat gradients enhance nursery function for resident and transient fishes in the San Francisco Estuary. Marine Ecology Progress Series 637: 141–157.
Corline, N.J., T. Sommer, C.A. Jeffres, and J. Katz. 2017. Zooplankton ecology and trophic resources for rearing native fish on an agricultural floodplain in the Yolo bypass California, USA. Wetlands Ecology and Management 25 (5): 533–545.
Costanza, R., R. d'Arge, R. De Groot, S. Farber, M. Grasso, B. Hannon, K. Limburg, et al. 1997. The value of the world’s ecosystem services and natural capital. Nature 387 (6630): 253–260.
David, A.T., C.A. Simenstad, J.R. Cordell, J.D. Toft, C.S. Ellings, A. Gray, and H.B. Berge. 2016. Wetland loss, juvenile salmon foraging performance, and density dependence in Pacific Northwest estuaries. Estuaries and Coasts 39 (3): 767–780.
Dumont, H.J., I. Van de Velde, and S. Dumont. 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19 (1): 75–97.
Durand, J.R. 2017. Evaluating the aquatic habitat potential of flooded polders in the Sacramento-San Joaquin Delta. San Francisco Estuary and Watershed Science 15 (4). https://doi.org/10.15447/sfews.2017v15iss4art4.
Edgar, G.J., N.S. Barrett, D.J. Graddon, and P.R. Last. 2000. The conservation significance of estuaries: A classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study. Biological Conservation 92 (3): 383–397.
Enright, C., S.D. Culberson, and J.R. Burau. 2013. Broad timescale forcing and geomorphic mediation of tidal marsh flow and temperature dynamics. Estuaries and Coasts 36 (6): 1319–1339.
Forrester, G.E., B.I. Fredericks, D. Gerdeman, B. Evans, M.A. Steele, K. Zayed, L.E. Schweitzer, I.H. Suffet, R.R. Vance, and R.F. Ambrose. 2003. Growth of estuarine fish is associated with the combined concentration of sediment contaminants and shows no adaptation or acclimation to past conditions. Marine Environmental Research 56 (3): 423–442.
Gedan, K.B., B.R. Silliman, and M.D. Bertness. 2009. Centuries of human-driven change in salt marsh ecosystems. Annual Review of Marine Science 1 (1): 117–141.
Geist, D.R., C.S. Abernethy, K.D. Hand, V.I. Cullinan, J.A. Chandler, and P.A. Groves. 2006. Survival, development, and growth of fall Chinook salmon embryos, alevins, and fry exposed to variable thermal and dissolved oxygen regimes. Transactions of the American Fisheries Society 135 (6): 1462–1477.
Goertler, P.A.L., T.R. Sommer, W.H. Satterthwaite, and B.M. Schreier. 2018. Seasonal floodplain-tidal slough complex supports size variation for juvenile Chinook salmon (Oncorhynchus tshawytscha). Ecology of Freshwater Fish 27 (2): 580–593.
Gotelli, N.J., and A.M. Ellison. 2004. A primer of ecological statistics. Sunderland: Sinauer Associates, Inc..
Healey, M. 1980. Utilization of the Nanaimo River estuary by juvenile Chinook salmon, Oncorhynchus tshawytscha. Fishery Bulletin 77 (3): 653–668.
Heath, A.G., B.J. Turner, and W.P. Davis. 1993. Temperature preferences and tolerances of three fish species inhabiting hyperthermal ponds on mangrove islands. Hydrobiologia 259 (1): 47–55.
Hemes, K.S., E. Eichelmann, S. Chamberlain, S.H. Knox, P.Y. Oikawa, C. Sturtevant, J. Verfaillie, D. Szutu, and D.D. Baldocchi. 2018. A unique combination of aerodynamic and surface properties contribute to surface cooling in restored wetlands of the Sacramento-San Joaquin Delta, California. Journal of Geophysical Research – Biogeosciences 123 (7): 2072–2090.
Herbold, B., S.M. Carlson, R. Henery, R.C. Johnson, N. Mantua, M. McClure, P.B. Moyle, and T. Sommer. 2018. Managing for Salmon resilience in California’s variable and changing climate. San Francisco Estuary and Watershed Science 16 (2). https://doi.org/10.15447/sfews.2018v16iss2art3.
Hering, D.K., D.L. Bottom, E.F. Prentice, K.K. Jones, and I.A. Fleming. 2010. Tidal movements and residency of subyearling Chinook salmon (Oncorhynchus tshawytscha) in an Oregon salt marsh channel. Canadian Journal of Fisheries and Aquatic Sciences 67 (3): 524–533.
Herrmann, R.B., C.E. Warren, and P. Doudoroff. 1962. Influence of oxygen concentration on the growth of juvenile coho salmon. Transactions of the American Fisheries Society 91 (2): 155–167.
Hyslop, E.J. 1980. Stomach contents analysis—A review of methods and their application. Journal of Fish Biology 17 (4): 411–429.
Jassby, A. 2008. Phytoplankton in the upper San Francisco Estuary: Recent biomass trends, their causes, and their trophic significance. San Francisco Estuary and Watershed Science 6 (1). https://doi.org/10.15447/sfews.2008v6iss1art2.
Jeffres, C.A., J.J. Opperman, and P.B. Moyle. 2008. Ephemeral floodplain habitats provide best growth conditions for juvenile Chinook salmon in a California river. Environmental Biology of Fishes 83 (4): 449–458.
Jones, M.J., and I.G. Stuart. 2008. Regulated floodplains—a trap for unwary fish. Fisheries Management and Ecology 15 (1): 71–79.
Jude, D.J., and J. Pappas. 1992. Fish utilization of Great Lakes coastal wetlands. Journal of Great Lakes Research 18 (4): 651–672.
Junk, W. J., P. B. Bayley, and R. E. Sparks. 1989. The flood-pulse concept in river-floodplain systems. In: Dodge, D.P., Ed., Proceedings of the international large river symposium (LARS), Canadian Journal of Fisheries and Aquatic Sciences special publication 106, NRC research press, Ottawa, 110–127.
Katz, J.V.E., C. Jeffres, L. Conrad, T.R. Sommer, J. Martinez, S. Brumbaugh, N. Corline, and P.B. Moyle. 2017. Floodplain farm fields provide novel rearing habitat for Chinook salmon. PLoS One 12 (6): e0177409. https://doi.org/10.1371/journal.pone.0177409.
Kimmerer, W.J., A.E. Parker, U.E. Lidström, and E.J. Carpenter. 2012. Short-term and interannual variability in primary production in the low-salinity zone of the San Francisco Estuary. Estuaries and Coasts 35 (4): 913–929.
Kirwan, M.L., and J.P. Megonigal. 2013. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504 (7478): 53–60.
Kjelson, M.A., P.F. Raquel, and F.W. Fisher. 1982. Life history of fall-run juvenile Chinook salmon, Oncorhynchus tshawytscha, in the Sacramento-San Joaquin Estuary, California. In Estuarine comparisons, ed. V.S. Kennedy, 393–411. New York: Academic Press.
Levy, D.A., and T.G. Northcote. 1982. Juvenile salmon residency in a marsh area of the Fraser River estuary. Canadian Journal of Fisheries and Aquatic Sciences 39 (2): 270–276.
Lucas, L.V., J.K. Thompson, and L.R. Brown. 2009. Why are diverse relationships observed between phytoplankton biomass and transport time? Limnology and Oceanography 54 (1): 381–390.
Lund, J. 2017. After drought, California urgently needs to focus on big picture of water management. Sacramento Bee, Op-Ed. https://sacbee.relaymedia.com/amp/opinion/op-ed/soapbox/article129221644.html. Accessed 20 August 2018.
Lund, J. R., E. Hanak, W. Fleenor, R. Howitt, J. Mount, and P. Moyle. 2007. Envisioning futures for the Sacramento-San Joaquin Delta. San Francisco, CA: Public Policy Institute of California. http://www.ppic.org/main/publication.asp?i=671. Accessed 22 August 2020.
Lusardi, R.A., B.G. Hammock, C.A. Jeffres, R.A. Dahlgren, and J.D. Kiernan. 2019. Oversummer growth and survival of juvenile coho salmon (Oncorhynchus kisutch) across a natural gradient of stream water temperature and prey availability: An in situ enclosure experiment. Canadian Journal of Fisheries and Aquatic Sciences 77 (2): 413–424. https://doi.org/10.1139/cjfas-2018-0484.
Ma, Z., Y. Cai, B. Li, and J. Chen. 2010. Managing wetland habitats for waterbirds: An international perspective. Wetlands 30 (1): 15–27.
MacFarlane, R.B., and E.C. Norton. 2002. Physiological ecology of juvenile Chinook salmon (Oncorhynchus tshawytscha) at the southern end of their distribution, the San Francisco Estuary and Gulf of the Farallones, California. Fishery Bulletin 100 (2): 244–257.
Marine, K.R., and J.J. Cech Jr. 2004. Effects of high water temperature on growth, smoltification, and predator avoidance in juvenile Sacramento River Chinook Salmon. North American Journal of Fisheries Management 24 (1): 198–210.
McCullough, D.A., J.M. Bartholow, H.I. Jager, R.L. Beschta, E.F. Cheslak, M.L. Deas, J.L. Ebersole, J.S. Foott, S.L. Johnson, K.R. Marine, M.G. Mesa, J.H. Petersen, Y. Souchon, K.F. Tiffan, and W.A. Wurtsbaugh. 2009. Research in thermal biology: Burning questions for coldwater stream fishes. Reviews in Fisheries Science 17 (1): 90–115.
Meeuwig, M.H., J.B. Dunham, J.P. Hayes, and G.L. Vinyard. 2004. Effects of constant and cyclical thermal regimes on growth and feeding of juvenile cutthroat trout of variable sizes. Ecology of Freshwater Fish 13 (3): 208–216.
Monroe, M., P. R. Olofson, J. N. Collins, R. M. Grossinger, J. Haltiner, and C. Wilcox. 1999. Baylands ecosystem habitat goals; San Francisco Bay Area Wetlands Ecosystem Goals Project. U.S. Environmental Protection Agency, San Francisco, California/S.F. Bay Regional Water Quality Control Board, Oakland, California. https://sfestuary.org/wp-content/uploads/2012/12/1Habitat_Goals.pdf. Accessed 25 November 2019.
Moore, J.W., J. Gordon, C. Carr-Harris, A.S. Gottesfeld, S.M. Wilson, and J. Harvey Russell. 2016. Assessing estuaries as stopover habitats for juvenile Pacific salmon. Marine Ecology Progress Series 559: 201–215.
Mount, J., W. Bennett, J. Durand, W. Fleenor, E. Hanak, J. Lund, and P. Moyle. 2012. Aquatic ecosystem stressors in the Sacramento-san Joaquin Delta. Public Policy Institute of California. https://www.ppic.org/content/pubs/report/R_612JMR.pdf. Accessed 24 November 2019.
Moyle, P.B., A.D. Manfree, and P.L. Fiedler, eds. 2014. Suisun marsh: Ecological history and possible futures. Berkeley: Univ of California Press.
Moyle, P.B., J. Durand, and C. Jeffres. 2018. Making the delta a better place for native fishes. Orange County Coast Keeper. https://www.coastkeeper.org/wp-content/uploads/2018/03/Delta-White-Paper_completed-3.6.pdf. Accessed 23 January 2020.
Nagelkerken, I., M. Sheaves, R. Baker, and R.M. Connolly. 2015. The seascape nursery: A novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish and Fisheries 16 (2): 362–371.
Needles, L.A., S.E. Lester, R. Ambrose, A. Andren, M. Beyeler, M. Connor, J. Eckman, B. Costa-Pierce, S.D. Gaines, K. Lafferty, H. Lenihan, J. Parrish, M.S. Peterson, A. Scaroni, J. Weis, and D.E. Wendt. 2015. Managing bay and estuarine ecosystems for multiple services. Estuaries and Coasts 38 (Supplemental 1): S35–S48. https://doi.org/10.1007/s12237-013-9602-7.
Nehlsen, W., J.E. Williams, and J.A. Lichatowich. 1991. Pacific salmon at the crossroads: Stocks at risk from California, Oregon, Idaho, and Washington. Fisheries 16 (2): 4–21.
Nicieza, A.G., and N.B. Metcalfe. 1997. Growth compensation in juvenile Atlantic salmon: Responses to depressed temperature and food availability. Ecology 78 (8): 2385–2400.
O’Rear, T. A., and P. B. Moyle. 2018. Suisun marsh fish study: Trends in fish and invertebrate populations of Suisun marsh. Center for Watershed Sciences, University of California, Davis. https://watershed.ucdavis.edu/files/biblio/Suisun Marsh Fish Report 2016 Final.pdf. Accessed 25 November 2019.
O’Rear, T. A., P. B. Moyle, and J. R. Durand. 2019. Trends in fish and invertebrate populations of Suisun marsh. Center for Watershed Sciences, University of California, Davis. https://watershed.ucdavis.edu/files/biblio/Suisun Marsh Fish Report 2017 Final No Appendix (low res).pdf. Accessed 25 November 2019.
Peierls, B.L., N.S. Hall, and H.W. Paerl. 2012. Non-monotonic responses of phytoplankton biomass accumulation to hydrologic variability: A comparison of two coastal plain North Carolina estuaries. Estuaries and Coasts 35 (6): 1376–1392.
Pendleton, L., D.C. Donato, B.C. Murray, S. Crooks, W.A. Jenkins, S. Sifleet, C. Craft, J.W. Fourqurean, J.B. Kauffman, N. Marbà, P. Megonigal, E. Pidgeon, D. Herr, D. Gordon, and A. Baldera. 2012. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One 7 (9): e43542. https://doi.org/10.1371/journal.pone.0043542.
R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Reimers, P. E. 1971. The length of residence of juvenile fall Chinook salmon in Sixes River, Oregon. Dissertation, Oregon State University. http://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/9z903242m. Accessed 21 August 2018.
Ritter, A.F., K. Wasson, S.I. Lonhart, R.K. Preisler, A. Woolfolk, K.A. Griffith, S. Connors, and K.W. Heiman. 2008. Ecological signatures of anthropogenically altered tidal exchange in estuarine ecosystems. Estuaries and Coasts 31 (3): 554–571.
Rountree, R.A., and K.W. Able. 2007. Spatial and temporal habitat use patterns for salt marsh nekton: Implications for ecological functions. Aquatic Ecology 41 (1): 25–45.
Rypel, A.L., and C.A. Layman. 2008. Degree of aquatic ecosystem fragmentation predicts population characteristics of gray snapper (Lutjanus griseus) in Caribbean tidal creeks. Canadian Journal of Fisheries and Aquatic Sciences 65 (3): 335–339.
Rypel, A.L., K.M. Pounds, and R.H. Findlay. 2012. Spatial and temporal trade-offs by bluegills in floodplain river ecosystems. Ecosystems 15 (4): 555–563.
Sass, G.G., A.L. Rypel, and J.D. Stafford. 2017. Inland fisheries habitat management: Lessons learned from wildlife ecology and a proposal for change. Fisheries 42 (4): 197–209.
Satterthwaite, W.H., and S.M. Carlson. 2015. Weakening portfolio effect strength in a hatchery-supplemented Chinook salmon population complex. Canadian Journal of Fisheries and Aquatic Sciences 72 (12): 1860–1875.
Schindler, D.E., R. Hilborn, B. Chasco, C.P. Boatright, T.P. Quinn, L.A. Rogers, and M.S. Webster. 2010. Population diversity and the portfolio effect in an exploited species. Nature 465 (7298): 609–612.
Shreffler, D.K., C.A. Simenstad, and R.M. Thom. 1992. Foraging by juvenile salmon in a restored estuarine wetland. Estuaries 15 (2): 204–213.
Simenstad, C.A., and J.R. Cordell. 2000. Ecological assessment criteria for restoring anadromous salmonid habitat in Pacific Northwest estuaries. Ecological Engineering 15 (3–4): 283–302.
Sommer, T.R., M.L. Nobriga, W.C. Harrell, W. Batham, and W.J. Kimmerer. 2001. Floodplain rearing of juvenile Chinook salmon: Evidence of enhanced growth and survival. Canadian Journal of Fisheries and Aquatic Sciences 58 (2): 325–333.
Tamisier, A., and P. Grillas. 1994. A review of habitat changes in the Camargue: An assessment of the effects of the loss of biological diversity on the wintering waterfowl community. Biological Conservation 70 (1): 39–47.
Valentine-Rose, L., C.A. Layman, D.A. Arrington, and A.L. Rypel. 2007. Habitat fragmentation decreases fish secondary production in Bahamian tidal creeks. Bulletin of Marine Science 80 (3): 863–877.
Volk, E.C., D.L. Bottom, K.K. Jones, and C.A. Simenstad. 2010. Reconstructing juvenile Chinook salmon life history in the Salmon River estuary, Oregon, using otolith microchemistry and microstructure. Transactions of the American Fisheries Society 139 (2): 535–549.
Williams, J.G. 2012. Juvenile Chinook salmon (Oncorhynchus tshawytscha) in and around the San Francisco estuary. San Francisco Estuary and Watershed Science 10 (3). https://doi.org/10.15447/sfews.2012v10iss3art2.
Yoshiyama, R.M., F.W. Fisher, and P.B. Moyle. 1998. Historical abundance and decline of Chinook Salmon in the Central Valley region of California. North American Journal of Fisheries Management 18 (3): 487–521.
Acknowledgments
Jennica Moffat, D. Hammond, S. Carlson, C. Jasper, N. Ekasumara, L. Floyd, T. O’Rear, D. Cocherell, D. Colombano, R. Dahlgren, and X. Wang provided invaluable project assistance. Forest Halford and M. Ferner provided property and reserve access. Tom Taylor of ESA Consulting (Sacramento, CA USA) loaned us experimental cages. Finally, we thank the anonymous reviewers whose valuable comments considerably improved this manuscript.
Funding
This research was supported by funding from the California Department of Water Resources (grant 4600011551) and California Department of Fish and Wildlife (Delta Water Quality and Ecosystem Restoration Grant No P1696010). This work was also supported by the California Agricultural Experimental Station of the University of California Davis [grant numbers CA-D-ASC-2098 to NAF and CA-D-WFB-2467-H to ALR]. ALR was also supported by the California Trout and Peter B. Moyle Endowment in Coldwater Fish Ecology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Laure Carassou
The original online version of this article was revised: Table 7 was corrected.
Supplementary Information
ESM 1
(DOCX 43 kb).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aha, N.M., Moyle, P.B., Fangue, N.A. et al. Managed Wetlands Can Benefit Juvenile Chinook Salmon in a Tidal Marsh. Estuaries and Coasts 44, 1440–1453 (2021). https://doi.org/10.1007/s12237-020-00880-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-020-00880-4