Abstract
Dietary studies on generalist predators may provide valuable information on spatial or temporal changes in the structure of ecological communities. We initiated this study to provide baseline data and determine the utility of stable isotope analysis (SIA) to evaluate the foraging strategies of an opportunistic reptilian predator, the diamondback terrapin (Malaclemys terrapin), which specializes in salt marshes and mangrove estuaries along the Atlantic and Gulf coasts. We evaluated stable carbon (δ13C) and nitrogen (δ15N) isotope values of multiple tissues from terrapins inhabiting mainland and island mangrove habitats in south Florida and potential food sources to examine spatial and temporal variations in terrapin resource use. We fit linear regression models to determine the best predictors of isotopic values for both terrapins and their prey, and Stable Isotope Bayesian Ellipses in R (SIBER) analysis to examine terrapin isotopic niche space and overlap between groups. We identified differences in terrapin isotopic δ13C and δ15N values among all sites. Blood and scute tissues revealed different isotopic compositions and niche overlap between sites, suggesting diets or foraging locations may change over time, and amount of variation is site specific. Niche overlap between size classes was larger for blood (short term) versus scute (long term), suggesting greater variability in food habits or resource isotopes over the long term versus short term. These results demonstrate the usefulness of SIA in examining the spatial and temporal variability in diamondback terrapin resource use within estuary systems and further define their niche within these dynamic food webs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial and temporal variation in a species’ use of prey resources has the potential to influence ecological community structure and function. Individuals within a population may utilize diverse resources because they are of different sexes (McCullough et al. 1989), ages, or size classes (Reich et al. 2007) or inhabit different microhabitats (Durbec et al. 2010). Thus, investigations examining diet variation in natural populations are necessary to understand the plasticity of food webs. Dietary habits of reptile taxa have often been investigated by examining stomach or fecal contents (Erazmus 2012; Rosenblatt et al. 2015; Denton et al. 2016; Nifong 2016). An important underlying question in these studies is if using these “snap-shot” techniques represents long-term diet trends. If prey distributions and abundances are patchy and vary over time, the individuals’ stomach or fecal contents would only reflect their recent encounters, which may differ from their long-term food resource preferences (Araújo et al. 2007). Additionally, there are logistical difficulties that are both temporal (i.e., obtaining adequate data may require years of field investigation to recapture and sample the same individuals over time) and financial (i.e., many field surveys are relatively expensive) (Rugiero et al. 2012).
Stable isotope analysis (SIA) is commonly used by ecologists to evaluate spatial and temporal variation in dietary interactions and food web structure (Fry 2006; Layman et al. 2012). Stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) are commonly used in ecological studies to identify carbon sources in food web studies (δ13C values) and trophic position (δ15N values) (Fry 2006). Stable isotope analysis represents a complementary method to traditional methods used in ecology (i.e., stomach and fecal content analyses), providing a robust evaluation of diet and trophic niche (Newsome et al. 2012), and SIA can help account for diet items that fecal analysis may underestimate or fail to detect. Additionally, investigating stable isotope composition of multiple tissues with differing turnover times can provide temporal dietary information from a single sampling event. Animal tissues metabolize at different rates, and the isotopic composition generally reflects the diet of an animal at the time the consumer tissues were synthesized (Haramis et al. 2001; Rubenstein and Hobson 2004). As a result, each tissue records the consumer’s diet over different time scales (Fry 2006), and turnover rates can vary considerably among species and tissue types (Gannes et al. 1998; Post 2002). Isotope ratios in metabolically active tissues (e.g., blood, liver, or muscle) may represent dietary information spanning several days, weeks, or months. Metabolically inert tissues such as keratin (e.g., hair, hooves, scutes, scales) reflect food web conditions from the time and location the tissue was synthesized, locked into the keratin structure during synthesis (Hobson 1999). Analyzing these different tissues can help identify temporal variation in a species’ diet that may be due to inter-habitat movement patterns or inter-annual or climate-driven changes in resource availability (Ben-David et al. 1997; Rubenstein and Hobson 2004; Herzka 2005; Vander Zanden et al. 2010; Zbinden et al. 2011). Thus, stable isotope analysis represents an effective and minimally invasive approach to monitor changes in animal diets over time.
Diamondback terrapins (Malaclemys terrapin) are the only exclusively estuarine turtle species in the USA. They inhabit coastal salt marshes and mangrove estuaries along the Atlantic and Gulf coasts from Massachusetts to Texas. Terrapins are an important component of salt marsh systems helping to regulate the abundance of the dominant marsh grazer (the periwinkle, Littorina irrota), which when associated with drought-induced stress, can lead to cascading vegetation loss of ecologically important cordgrass (Spartina alterniflora) (Silliman and Bertness 2002; Silliman et al. 2005; Pfau and Roosenburg 2010). Terrapins may also serve as a major source of eelgrass (Zostera marina) inter-bed seed dispersal and genetic diversity (Sumoski and Orth 2012; Tulipani and Lipcius 2014). Furthermore, they are prey for many estuarine species, including bald eagles (Haliaeetus leucocephalus), herons and egrets (Ardeidae), river otters (Lontra canadensis), sharks (Elasmobranchii), American crocodiles (Crocodylus acutus), and crabs (Seigel 1980; Butler et al. 2006; Hart and McIvor 2008; Pfau and Roosenburg 2010). Additionally, terrapins represent important biomonitors of estuarine contamination (Blanvillain et al. 2007; Basile et al. 2011) because of their predatory foraging behavior, occurrence in a variety of estuarine habitats, long life-span (> 20 years, R. Wood, unpubl. data; Seigel 1984), and high site fidelity (Roosenburg et al. 1999; Gibbons et al. 2001; Butler 2002; Harden et al. 2007; Sheridan et al. 2010). Similarly, studies of terrapin diet could identify fluctuations in available resources within estuarine habitats over time.
Previous terrapin diet studies utilized fecal analysis, stomach flushing, or dissection (Tucker et al. 1995; Spivey 1998; Butler et al. 2012; Tulipani 2013; Denton et al. 2015, 2016; Alleman and Guillen 2017). Several of these studies have shown that both males and immature females of a similar size had similar diets, but both groups had diets that differed from larger mature females. These differences may be because adult females have more powerful jaws, enabling them to consume a wider diversity of prey (Tucker et al. 1995; Petrochic 2009; Butler et al. 2012; Tulipani 2013; Alleman and Guillen 2017). A seasonal shift in diet was also reported in female terrapins, coincident with movements between habitats (Butler et al. 2012; Alleman and Guillen 2017). Although these analyses provided evidence of terrapin resource use, they may be biased towards food items that are less easily digested such as crab carapaces (McGaw 2006), while simultaneously underestimating contributions of soft-bodied prey. Additionally, these studies provide only a snap-shot of an individual’s diet at any given time. For example, gut retention time for eelgrass seeds ingested by terrapins varied between 24 and 144 h (Ernst and Lovich 2009; Sumoski and Orth 2012). The previous examples of seed retention illustrate the passage time of indigestible materials through the terrapin digestion system; thus, fecal remains may represent what an individual has eaten only within a few days. Previous investigations into the diets of south Florida terrapin populations (Baldwin et al. 2005; Denton et al. 2015, 2016) have been limited to these methods, thus only providing a record of short-term diet. Currently, we lack the information required to distinguish between natural temporal diet shifts and changes due to shifts in available resources.
Concurrent with a diet study in which we examined fecal remains to determine the diets of south Florida terrapins (Denton et al. 2015, 2016), we initiated the first stable isotope investigation of diamondback terrapins in south Florida. We sampled terrapins inhabiting creeks within a densely forested mangrove complex and from island habitats with fringing, less dense mangrove forests. We collected blood and scute samples to answer several questions concerning the spatial and temporal foraging strategies of this exclusively estuarine reptile. Using SIA and subsequent comparisons of isotopic niche space, our study aimed to address the following questions on aspects of terrapin trophic ecology: (1) Are terrapins from the mainland creek complex isotopically distinct from those collected in the island habitats? (2) Does the overall isotopic niche of terrapins vary temporally? (3) Is there overlap in the isotopic niches between the different size classes of terrapins, and does it vary spatially or temporally? Results from these analyses will help us understand the foraging ecology of a proposed indicator species.
Methods
Study Sites
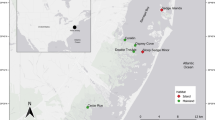
We sampled terrapins and their potential food resources in a forested mangrove creek system (Big Sable Creek [BSC]) in the western portion of Everglades National Park (ENP) and island habitats within Florida Bay (FB) in eastern ENP (Fig. 1). The BSC site is dominated by red and black mangroves (Rhizophora mangle and Avicennia germinans, respectively) with significant tidal fluctuations of 1.3 m, exposing large mudflats during low tide. The FB site consisted of two islands, both containing red and black mangrove swamps, fringed with mangrove forests. These islands have vast open spaces devoid of vegetation, often becoming inundated during the wet season (Enos 1989; Ross et al. 1992). We also sampled terrapins from an island in the Key West National Wildlife Refuge (KW) at the southernmost extent of the terrapins’ range (Fig. 1). The KW site is located 7 miles west of Key West, FL, on one of the mangrove dominated Mule Keys, which were formed from fossilized coral reef materials (Ross et al. 1992; McCarter 2012).
Location of the Big Sable Creek (BSC), Florida Bay (FB; comprised from FB1 and FB2), and Key West (KW) study sites in south Florida (source: Esri, DigitalGlobe, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, swisstopo, and the GIS User Community). All images are oriented north
Sample Collection and Processing
South Florida has a sub-tropical climate with a distinct annual pattern of wet (June–November) and dry (December–May) seasons. We captured and sampled terrapins from January 2012 through June 2013; during each sampling trip, we collected blood and scute tissues and subsequently marked, photographed, and catalogued each individual following Hart and McIvor (2008). In the southeast USA, female terrapins reach maturity at straight plastron lengths (SPL) between 135 and 143 mm, whereas males reach maturity between 90 and 100 mm SPL (Seigel 1984; Lovich and Gibbons 1990; Roosenburg 1991; Butler 2002). Based on these size ranges, we categorized all male and immature female terrapins with an (SPL < 135 mm) as small and adult females with an (SPL ≥ 135 mm) as large.
Terrapins were sampled for isotopes in the field except for those from the KW site, which were kept overnight and sampled at our field housing in Key West. All terrapins were released at their original capture location within 24 h. We collected approximately 1 ml of whole blood from each terrapin and kept sample vials on ice to be stored until freezing (− 20 °C). Due to constraints from sampling in the field, we were unable to centrifuge the blood into red blood and plasma fractions; thus, we performed our analysis on whole blood. We collected paired scute samples from the center of the left and right posterior costal scutes (Online Resource 1) using 6 mm biopsy punches and stored them in cryovials. Scutes are inert tissue for which no preservation method was necessary; thus, samples were collected and stored in cryovial boxes at ambient temperature until later processing in the lab. During each terrapin sampling event, we opportunistically collected potential prey (gastropods, crabs, barnacles, and fish), mangrove vegetation (detrital R. mangle and A. germinans), and seagrass (turtle grass, Thalassia testudinum) near terrapin capture sites (Table 2). These resource items were also kept on ice until freezing. In the lab, we thawed the blood samples and rinsed the scute samples with distilled water before drying. We dissected the muscle tissue from the potential prey items and rinsed the tissue with distilled water before drying. Vegetation samples were also cleaned with distilled water prior to drying. All samples were dried at 60 °C then ground into a homogenous powder. We pooled multiple (5–10) individuals of Balanus sp. from the FB site to meet minimum aliquots of 5 μg C and 10 μg N per sample.
Isotope Analysis
We sent samples to the Bioanalytical Laboratory at Washington State University to be analyzed for isotopic values of carbon (referenced to Vienna PeeDee Belemnite) and nitrogen (referenced to atmospheric N2; Peterson and Fry 1987). Analyses were run using an elemental analyzer connected to a Finnegan MAT Delta-S stable isotope ratio mass spectrometer via a Finnigan MAT ConFlo II interface. Reproducibility was monitored using organic reference standards, bovine liver (animal tissues) and apple leaves (primary producers). Typically, the influence of lipids on carbon’s isotopic value is consistent with absolute molar mass carbon and nitrogen, or C/N ratio > 3.5 for aquatic animals (Post et al. 2007). Several terrapin and prey samples had a C/N ratio > 3.5 (Tables 1 and 2); therefore, we lipid corrected the δ13C data for those samples following the method of Post et al. (2007). All data presented in the tables and figures represents the lipid-corrected δ13C values.
Discrimination factors between tissues have only been investigated for a few turtle species (Seminoff et al. 2006, 2007, 2009; Murray and Wolf 2012; Aresco et al. 2015), and those studies found them to be tissue and species specific. While discrimination factors have not been determined for terrapin tissues, if terrapin diets exhibited stable foraging patterns over time represented by the two tissues, we would expect the δ13C values of blood and scute tissue to be highly correlated. We followed similar methodology to Rosenblatt et al. (2015) to see if the correlation coefficient (r) and the coefficient of determination (R2) for each site were close to 1. If individual terrapins exhibited stable foraging patterns over the time periods represented by each tissue, we would expect the δ13C values of blood and scutes to be highly correlated, with linear regression best-fit lines characterized by high R2 values and slopes close to 1. Our data are available online from USGS ScienceBase (Denton et al. 2018).
Linear Modeling
We used linear regression to evaluate predictors of two isotope ratios (δ13C, δ15N) for both terrapins and their resources. We built a priori model sets consisting of all relevant single-covariate models (plus a null model) for each of the four response variables. We assessed eight covariates for terrapin δ13C and δ15N ratios: (1) season (wet vs. dry), (2) site (BSC, FB, or KW), (3) location (specific island or creek), (4) tissue type (blood vs. scute), (5) terrapin mass (g), (6) length (SPL, cm), (7) size class (small vs. large), and (8) sex. For δ13C and δ15N ratio resource models, in addition to (1) season, (2) site, and (3) location, we also evaluated (4) resource type (four animal classes [Actinopterygii, Gastropoda, Malacostraca, Maxillopoda], two plants [seagrass, mangrove]).
We fit all models using the lm() function in the base package of program R (v.3.2.4; R Development Core Team 2013). We compared models using corrected Akaike’s information criteria (AICC) values with the Multi-Model Inference package MuMIn (Barton 2016). We retained covariates from all models with more support than the null model, then built models with increasing complexity using those covariates. We ranked all new and retained models using AICC, assessed model fit using adjusted R2 values, and drew our final inferences from the best model in each set. We used AICC for model selection, and when the top models were < 2 ΔAICC, we chose the one with fewer parameters.
Isotope Niche Analysis
Isotopic diversity indices were calculated using SIBER—Stable Isotope Bayesian Ellipses in R (Jackson et al. 2011). We calculated the standard ellipse area (SEA), SEAC (corrected for small sample size), and the Bayesian estimation (SEAB). Overlap of SEAC among groups in isotopic niche space was calculated for each combination.
Results
We analyzed blood and scute samples from 99 terrapins across all study sites (BSC [52]; FB [24]; KW [23]; Table 1). Terrapins at all study sites exhibited high site fidelity (Wood 1981; Hart and McIvor 2008, B. Mealey personal comm, personal observation). We found that terrapins from BSC had the lowest δ13C values (overall mean = − 23.6‰ ± 1.1 SD), whereas KW terrapins had the highest (overall mean = − 17.1‰ ± 1.2 SD; Table 1). We found the lowest δ15N values in KW terrapins (overall mean = 4.6‰ ± 0.7 SD) and highest in FB terrapins (overall mean = 7.0‰ ± 0.8 SD; Table 1). Within the two islands comprising the FB site, there was no difference in the terrapin δ13C values (F4,43 = 2.67, p = 0.11), and while a difference was detected for δ15N values (F3,44 = 7.29, p < 0.01), the difference was < 1‰; thus, both islands remained pooled for comparisons against BSC (creek) and KW (island) sites. Within each site, correlation coefficients (r) of the terrapins’ blood and scute δ13C values were 0.65 (BSC), 0.42 (FB), and 0.51 (KW), with linear regression (R2) values of 0.38 (BSC), 0.13 (FB), and 0.23 (KW).
Resources from the BSC site had low δ13C values compared to those from FB or KW, which were similar in carbon isotope values (Table 2). The δ15N values varied by site, with similar resources being the highest at the FB site and lowest at the KW site (Table 2). Bivariate plots of terrapin and resource isotopic means (± SD) revealed variations in isotopic space at each site (Fig. 2), with wide ranges in δ13C values within prey classes. The mean δ13C values for potential prey (− 23.7 ± 1.3‰) were lower at the creek site (BSC), than either island site (δ13C FB, − 17.3 ± 3.3‰; KW, − 18.1 ± 1.5‰). The mean δ15N values for the two prey classes sampled at each site (gastropods and crabs) were highest in FB (7.1 ± 1.5‰) followed by BSC (2.9 ± 3.0‰) and KW (2.4 ± 1.5‰).
Linear Modeling Terrapins
The top performing model from our terrapin δ13C model set had an interaction of type, site, and size variables (Table 3), with an adjusted R2 = 0.85. This model received 74% of the model weight and Δ AICC > 3.61 over the next model, indicating clear separation (Burnham and Anderson 2002). The predictions from this model indicated that blood samples had consistently lower δ13C values compared to scutes and that small terrapins had consistently lower δ13C values compared to large terrapins, but that the magnitude of those variances among the sites differed (Fig. 3, Online Resource 2). Scute samples from large terrapins drove the size differences observed in δ13C values at both the BSC and FB sites.
The top model from our terrapin δ15N model set included type, plus an interaction of site and size variables (Table 4). It had an adjusted R2 = 0.49, indicating moderate model fit, and received 39% of the model weight. The next closest model in the set contained type plus an interaction between location and size and was competitive with the top model (Δ AICC = 0.34), receiving about 33% of the model weight and an adjusted R2 = 0.53. Both models indicated that blood samples were consistently higher in δ15N values relative to scutes (i.e., in both models, the blood parameter was larger than the scute parameter) and that this pattern was most parsimoniously explained by a single parameter across all sites (i.e., neither model contained an interaction between site and type, but rather an additive effect). Though not significantly different (i.e., 95% confidence intervals overlap, see Fig. 3, Online Resource 2), within FB large terrapins had higher δ15N values relative to small terrapins, while within BSC large terrapins had lower δ15N values relative to small terrapins. We were unable to sample small terrapins from KW; thus, no size comparisons could be made.
Linear Modeling Resources
The top model from our resource δ13C model set has an interaction of class and site variables (Online Resource 3). It has an adjusted R2 = 0.88, indicating good model fit, and it received 100% of the model weight, indicating it is the strongest model of the set. The top model indicates that prey items from creek site typically had more negative δ13C values, but that the magnitude of differences varies by prey type. The top model from our resource δ15N model set has an interaction of class and location variables (Online Resource 4). It has an adjusted R2 = 0.73, indicating moderate model fit, and it received 56% of the model weight, indicating it is the strongest model of the set. The next closest model was again competitive with the top model (ΔAICc = 0.52) which received 44% of the model weight with an adjusted R2 = 0.68 and included an interaction between class and site.
Isotope Niche Analysis
Niche overlap between tissue types was greater at KW than BSC and FB, while niche overlap between size classes at BSC and FB was greater for blood samples than scute samples (Table 5, Fig. 4). Small terrapin niche widths using SEAB were larger at the FB site than BSC but were similar between tissue types within each site (Fig. 5). Large terrapin niche widths (SEAB) showed greater variability between sites and tissue types, as does the niche widths (SEAB) between sites and tissue types for both size classes combined (Fig. 5).
Estimates of Bayesian standard ellipse areas (SEAB) for blood and scute samples at each site for only small terrapins (a), only large terrapins (b), and both size classes combined (c). Black dots correspond to the mean SEAB for each group, and shaded boxes represent the 50%, 75%, and 95% credible intervals from dark to light gray
Discussion
In this study, we answered several questions on the isotopic ecology of diamondback terrapins. Specifically, we determined (1) terrapins from the mainland creek complex are isotopically distinct from those in the island habitats; (2) through analysis of multiple tissues, we determined the isotopic niche of terrapins vary temporally; and (3) there is overlap in the isotopic niches between the two size classes of terrapins and the amount of overlap varies both spatially and temporally. We identified spatial differences in terrapin isotopic values for both δ13C and δ15N among all three sites for both blood and scute tissues, and these values followed isotopic trends in resources (i.e., prey) that corresponded to differences in the dominate pathways of primary production at each site (Table 1, Fig. 2). This was one of the first stable isotope studies performed on terrapins and first to estimate isotopic niche, with the only other known isotopic investigation on terrapin foraging being performed along the Georgia coast (Erickson et al. 2011). Both studies found stable isotope data to extend the interpretation of foraging strategies beyond the limits of more traditional diet studies.
The spatial differences identified in the isotopic values of terrapins among the sites (BSC, KW, FB) could be due to proportional contributions of isotopically distinct food resource use by terrapins among the three sites. Alternatively, baseline isotopic values for each site could differ, which would correspond to differences in the dominant pathways of primary production, hydrology, and nutrient cycling (Post 2002). If a single baseline for carbon was assumed, source studies of δ13C could be significantly biased (Barnes et al. 2009). Thus, we compared inferences drawn from the terrapin δ15N and δ13C models to the inferences drawn from the resource δ15N and δ13C models to determine whether isotopic variations in the prey items themselves were driving isotopic variations in the terrapins. Both δ13C and δ15N from the primary producers and associated prey (Table 2) indicated that prey baselines differed at each site. For example, gastropods are a primary consumer and their δ13C values varied between creek and island habitats (BSC < FB < KW), and we determined crabs’ δ15N values varied among all sites with FB > BSC > KW. Because the terrapins’ isotopic values mirrored these differences between sites, it is reasonable to conclude that the spatial variations in terrapins’ values are reflective of the different baselines at each site.
This isotopic pattern in δ13C baselines among sites follows a well-known pattern in primary producer isotope values (i.e., Mangrove δ13C < seagrass δ13C); thus, sites with less mangrove (FB and KW) and more seagrass (KW) were more positive. Grouping the two islands for the FB site contributed to the wider range in both δ13C and δ15N values for both terrestrial and marine vegetation, and prey isotope values followed patterns of dominant producers at each site (Table 2, Fig. 2). These prey have limited dispersal abilities as adults since a majority of dispersal occurs during the planktonic larval stages of these benthic prey communities (Grantham et al. 2003; Armitage and Fong 2004; Lundquist et al. 2004; Smith and Ruiz 2004; Kappes and Haase 2012). Thus, observed differences in the δ13C and δ15N values were likely driven by the environment in which they were sampled.
Spatial differences in δ15N values may be linked to 15N-enriched anthropogenic inputs derived from human sources (McClelland et al. 1997; Vizzini and Mazzola 2006), rather than being linked to variations in trophic structure and feeding habits of terrapins between sites. The FB site had the highest δ15N values, which could be due to closer proximity to runoff and nutrient loading (particularly nitrogen) from the Everglades and canals along the southeastern peninsula (Lapointe and Clark 1992; Rudnick et al. 1999). For example, Lapointe et al. (2004) found macroalgae in nearshore waters around Big Pine Key in southern FB had elevated δ15N values (~ + 4‰) which is characteristic of nitrogen enrichment from sewage inputs, with lower values (~ + 2‰) reported for macroalgae in upstream waters of western Florida Bay influenced by nitrogen rich Everglades runoff. Tracing sources of both natural and anthropogenic nitrogen within the system is necessary for mitigating contributions and maintaining healthy environmental conditions. Terrapin SIA can contribute to this understanding.
Terrapin tissues revealed a broad pattern where δ13C values of blood were more negative and δ15N values more positive relative to scutes, which agrees with our predicted parameter estimates (Table 1 and Online Resource 2); however, for KW, those differences are insignificant (within the analytical error). These differences could be due to variations in the isotopic discrimination between the tissues, variations in terrapin food resource or habitat use through the time recorded in the two tissues, or changes in resource isotopic values over time. Our results showed moderate correlation between blood and scute δ13C values from each site; however, lower R2 values from our linear regression suggest that tissue alone is not a strong predictor of δ13C values. Lastly, we compared the standard ellipses between blood and scute tissue, and we did not find a consistent, directional shift between sites (Fig. 4a). Thus, it is likely that the differences in δ13C values observed at each site represent variable foraging patterns over the time periods represented by the two tissues. During this study, we were unable to determine if there were temporal variations in the resources among sites in this study, and we recommend further investigation.
Within each of our sites, blood samples from both large and small terrapins had similar δ13C values indicating both were foraging in similar habitats in the weeks to months prior to capture. For large terrapins, however, δ13C values of scutes differed from blood (Figs. 3 and 4), potentially integrating δ13C values from nesting habitats. Stable isotopes of inert tissues (i.e., turtle scutes, mammal hair, feathers) retain a stable isotope record and have been a powerful tool providing insights on inaccessible life stages of a variety of fauna (Hobson and Stirling 1997; Hobson and Bairlein 2003; Cerling et al. 2006; Reich et al. 2007). Seminoff et al. (2007) determined nitrogen turnover in freshwater pond sliders (Trachemys scripta) to be 142 days (blood plasma) and 155 days (whole blood). Vander Zanden et al. (2010) microsampled loggerhead sea turtle (Caretta caretta) scutes into layers, determining each 50 μm layer represents 0.6 years of diet assimilation. Sea turtle scute samples ranged from 400 to 1100 μm, corresponding to (4) to (12) years of dietary information. If terrapin scutes represented a similar range, this could mean that scutes are recording the diet when the terrapin was smaller; possibly feeding at a lower trophic level, or potentially living and feeding in a different habitat, or both. Juveniles are rarely encountered at these sites, making it difficult to determine their diet and habitat use; thus, scutes can provide valuable dietary information during this cryptic life stage. Alternatively, isotope values of scute samples from large terrapins could represent mature females foraging in different habitats such as when traveling to nesting beaches. This was detected by Butler et al. (2012) when diets of mature females collected at the nesting beach in northeastern Florida differed significantly from both mature and immature females and males sampled within tidal creeks. Except for seven individuals from FB, our sampling occurred during the non-nesting season, and within BSC’s tidal creeks, there is little to no suitable nesting habitat; thus, females may experience a shift in prey’s availability or isotopic composition during similar forays to nesting beaches.
There are some limitations to the interpretation of our scute results due to the way they were processed. The layering of scutes is a very time-consuming, labor-intensive process and requires precise machinery which can be cost prohibitive. Thus, for this initial study, we homogenized the entire sample, which was composed of multiple layers. If terrapins lay down their scutes in a similar process to what Vander Zanden et al. (2010) determined for loggerhead sea turtles (Caretta caretta), each 50 μm layer could be representative of approximately 0.6 years. Since our homogenized scute samples had differing thicknesses between 100 and 450 μm, they could represent the terrapin assimilated diet between 1.2 and 5.4 years, depending on the thickness of each sample. Because our homogenized scutes included several growth layers, potentially representing multiple seasons or years, we were unable to test for temporal variations in isotopic values within the scutes. Even with these limitations, by examining the isotopic composition of multiple tissues, our results approximate the dietary niche of terrapins from months to ≥ 1 year in a single sampling, providing unprecedented information on their resource use over time.
Previous research has detected resource partitioning between large and small terrapins (Tucker et al. 1995; Petrochic 2009; Butler et al. 2012; Tulipani 2013; Alleman and Guillen 2017). In our study, the inclusion of size class in the top model helped to explain the variation in the magnitude of differences between isotope values from the tissue types and sites. The larger females were different from the smaller terrapins in their isotopic composition and niche space for both tissues, suggesting they may be feeding on a more variable diet over time (Figs. 4 and 5). Both size classes show a shift in niche space between tissues (Fig. 4), but there is a greater shift with less overlap between tissues for large terrapins than small. Bayesian ellipse areas SEAB also predict similar niche widths between tissues for small terrapins, while there is more variability in large terrapins’ estimated niche widths at each site (Fig. 5).
Blood δ13C values for both large and small at FB overlap (Fig. 4), but FB scute δ13C values from large > small. In the FB site, higher δ13C values of scutes from large terrapins could suggest they are not necessarily restricted to the mangroves as is suggested by the lower δ13C values of small terrapins. This could mean large terrapins foraged around the islands or on more marine prey while the smaller terrapins were more restricted to the interior or fringe of the islands. Additionally, terrapins from the BSC site did not fit our expected results based on previous literature. We expected larger mature female terrapins to have higher 15N values relative to smaller immature females or males since they could ingest a wider diversity of prey due to their larger jaw size (Tucker et al. 1995; Petrochic 2009; Butler et al. 2012; Tulipani 2013), yet we found the opposite to be true with small terrapins having higher δ15N values (Δ δ15N small − δ15N large = 0.36‰, Fig. 2). Denton et al. (2016) determined crabs were consumed by small terrapins more frequently than by large terrapins, which consumed a more diverse mixture of gastropod taxa including the larger mangrove periwinkle (Littoraria angulifera). While we were not able to determine the isotopic composition of several gastropod species due to the opportunistic sampling of potential prey during terrapin captures, periwinkles within these creeks were found to have much lower δ15N values than those of crabs. If the large females consume both low δ15N (gastropods) and high δ15N (crabs) prey, and since isotope values represent a mixture of assimilated food resources integrated over time, that could explain their lower δ15N values compared to small terrapins that selected less gastropods and more of the nitrogen-enriched crabs at the BSC site.
For each tissue type, the standard ellipse areas corrected for small sample size (SEAC) showed no overlap of isotopic niches between sites indicating that terrapin trophic niche is site specific within the time the tissues were synthesized. Within each site, there is overlap between the two tissues indicating that terrapins assimilate similar food resources over time. However, the shift in scute δ13C values that occurred at all three sites may represent a seasonal shift in terrapin diet corresponding to availability of prey species. A similar shift in terrapin diet was previously detected by Alleman and Guillen (2017), who suggested shifts corresponded to an increase in availability of fiddler crabs in the summer and juvenile blue crabs during fall seasons. Alternatively, the variation between tissues could suggest terrapins may be foraging in a different habitat throughout the time the tissues were synthesized. Further support for this comes from the size class comparisons for each of the tissues. Niche overlap is higher in the blood samples (Table 5, Fig. 4), which have a shorter turnover rate, while niche overlap is lower for scute samples, which represent diet assimilated over longer time scales. This indicates that both size classes utilize similar isotopic niches part of the time. The SEAB estimates between tissues for each size class indicate the large (female) terrapins are driving the separation by shifting their isotopic niche (Fig. 5), possibly reflecting their movements during nesting periods or changing food resource selection.
Mixing models are often used to analyze biological tracer data, such as stable isotopes, to characterize trophic links, and to estimate proportional contribution of resources in a consumer’s diet (Post 2002; Phillips 2012; Parnell et al. 2013). While based on simple concepts, they rely on several assumptions and can be misinterpreted. Mixing models may not be suitable for novel study systems where little is known about dietary preferences, there is little or too much isotopic variation among food sources, isotopic values of endmembers are not identified, turnover rates and discrimination factors are unknown, or any combination of the above (Parnell et al. 2010; Bond and Diamond 2011; Phillips et al. 2014). Based on Denton et al. (2016), there are additional gastropod and bivalve species known to be large contributors to their diet that during our opportunistic sampling we were unable to locate; therefore, we could not include them in our analysis. It was also evident that we were missing endmembers when we plotted the isotopic values of the terrapins and their prey (Fig. 2); thus, performing mixing models was determined to be premature. Additional sampling of terrapins and prey, including some of those “missing” food resources (e.g., Melampus coffeus, Polymesoda floridana; Denton et al. 2016) is recommended to allow mixing models to yield additional insight into specific resource selection and proportional contributions of differing prey. Additional sampling targeting small terrapins within FB would also increase the confidence of their calculated isotopic niche width which was limited due to the small sample size.
Conclusion
This study has provided the framework for future studies to investigate stable isotope values and foraging strategies throughout the range of this exclusively estuarine turtle. We detected differences in the isotopic values and niche space for terrapins by site, size class, and tissue types. Our results demonstrate how isotopic analysis from multiple tissues can be a powerful tool for understanding terrapins’ foraging ecology over hard to catch time periods. As ectotherms, terrapin tissue turnover rates span months to years, representing a history of the isotopic values from their local environment during the time the tissues were synthesized. Thus, this species could also be used as a biomonitor for assessing shifts in available resources in previously unstudied estuarine systems following disturbance events, such as hurricanes, sea level rise, or habitat degradation. Monitoring of terrapin isotopic values is recommended for ongoing conservation and management of M. terrapin throughout its range and to help managers understand the complex dynamics of estuarine food webs.
References
Alleman, Bryan J., and George J. Guillen. 2017. Prey availability and diet analysis of Texas diamond-backed terrapin (Malaclemys terrapin littoralis). Chelonian Conservation and Biology 16 (1): 52–61. https://doi.org/10.2744/CCB-1228.1.
Araújo, Márcio S., Daniel I. Bolnick, Glauco Machado, Ariovaldo A. Giaretta, and Sérgio F. Dos Reis. 2007. Using δ13C stable isotopes to quantify individual-level diet variation. Oecologia 152 (4): 643–654. https://doi.org/10.1007/s00442-007-0687-1.
Aresco, Matthew J., Joseph Travis, and Pamela S.D. MacRae. 2015. Trophic interactions of turtles in a north Florida lake food web: prevalence of omnivory. Copeia 103 (2): 343–356. https://doi.org/10.1643/CE-13-130.
Armitage, Anna R., and Peggy Fong. 2004. Gastropod colonization of a created coastal wetland: potential influences of habitat suitability and dispersal ability. Restoration Ecology 12 (3): 391–400. https://doi.org/10.1111/j.1061-2971.2004.00358.x.
Baldwin, John D., Lindsay A. Latino, Brian K. Mealey, Greta M. Parks, and Michael R.J. Forstner. 2005. The diamondback terrapin in Florida Bay and the Florida Keys: insights into turtle conservation and ecology. In Amphibians and reptiles status and conservation in Florida, ed. Walter E. Meshaka Jr. and Kimberly J. Babbitt, 180–186. Malabar: Krieger Publishing Company.
Barnes, Carolyn, Simon Jennings, and Jon T. Barry. 2009. Environmental correlates of large-scale spatial variation in the δ13C of marine animals. Estuarine, Coastal and Shelf Science 81 (3): 368–374. https://doi.org/10.1016/j.ecss.2008.11.011.
Barton, Kamil. 2016. MuMIn: multi-model inference. R package version 1.15.6. https://CRAN.R-project.org/package=MuMin.
Basile, Emily R., Harold W. Avery, Walter F. Bien, and Jennifer M. Keller. 2011. Diamondback terrapins as indicator species of persistent organic pollutants: using Barnegat Bay, New Jersey as a case study. Chemosphere 82 (1): 137–144. https://doi.org/10.1016/j.chemosphere.2010.09.009.
Ben-David, M., R.W. Flynn, and D.M. Schell. 1997. Annual and seasonal changes in diets of martens: evidence from stable isotope analysis. Oecologia 111 (2): 280–291. https://doi.org/10.1007/s004420050236.
Blanvillain, Gaelle, Jeffrey A. Schwenter, Rusty D. Day, David Point, Steven J. Christopher, William A. Roumillat, and David W.M. Owens. 2007. Diamondback terrapins, Malaclemys terrapin, as a sentinel species for monitoring mercury pollution of estuarine systems. Environmental Toxicology and Chemistry 26: 1441–1450.
Bond, Alexander L., and Antony W. Diamond. 2011. Recent Bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecological Applications 21 (4): 1017–1023. https://doi.org/10.1890/09-2409.1.
Burnham, Kenneth P., and David R. Anderson. 2002. Model selection and multimodel inference: a practical information-theoretic approach, Ecological modelling. Vol. 172. 2nd ed. New York: Springer. https://doi.org/10.1016/j.ecolmodel.2003.11.004.
Butler, Joseph A. 2002. Population ecology, home range, and seasonal movements of the Carolina diamondback terrapin, Malaclemys terrapin centrata, in northeastern Florida. Final report. Tallahassee: Florida Fish and Wildlife Conservation Commission, 65 pp.
Butler, Joseph A., George L. Heinrich, and Richard a. Seigel. 2006. Third workshop on the ecology, status, and conservation of diamondback terrapins (Malaclemys terrapin): results and recommendations. Chelonian Conservation and Biology. 5 (2): 331–334. https://doi.org/10.2744/1071-8443(2006)5[331:TWOTES]2.0.CO;2.
Butler, Joseph A., George L. Heinrich, and Melinda L. Mitchell. 2012. Diet of the Carolina diamondback terrapin (Malaclemys terrapin centrata) in northeastern Florida. Chelonian Conservation and Biology 11 (1): 124–128.
Cerling, Thure E., George Wittemyer, Henrik B. Rasmussen, Fritz Vollrath, Claire E. Cerling, Todd J. Robinson, and Iain Douglas-Hamilton. 2006. Stable isotopes in elephant hair document migration patterns and diet changes. Proceedings of the National Academy of Sciences of the United States of America 103 (2): 371–373. https://doi.org/10.1073/pnas.0509606102.
Denton, Mathew J., Kristen M. Hart, Anton Oleinik, Roger Wood, and John D. Baldwin. 2015. Malaclemys terrapin rhizophorarum (mangrove diamondback terrapin). Herpetological Review 46: 426–427.
Denton, Mathew J., Kristen M. Hart, Amanda W.J. Demopoulos, Anton Oleinik, and John D. Baldwin. 2016. Diet of diamondback terrapins (Malaclemys terrapin) in subtropical mangrove habitats in south Florida. Chelonian Conservation and Biology 15 (1): 54–61. https://doi.org/10.2744/CCB-1187.1.
Denton, Mathew J., Kristen M. Hart, Amanda W.J. Demopoulos, John D. Baldwin, and Brian J. Smith. 2018. Diamondback terrapin foraging ecology in south Florida utilizing stable isotopes, 2012-2014. USGS Science Base. https://doi.org/10.5066/F7Z89BK7.
Durbec, M., L. Cavalli, J. Grey, R. Chappaz, and B. Nguyen The. 2010. The use of stable isotopes to trace small-scale movements by small fish species. Hydrobiologia 641 (1): 23–31. https://doi.org/10.1007/s10750-009-0051-z.
Enos, Paul, ed. 1989. Islands in the bay—a key habitat of Florida Bay. Bulletin of Marine Science 44: 365–386.
Erazmus, Kayleigh R. 2012. Diet and prey choice of female diamondbacked terrapins (Malaclemys terrapin) in Jamaica Bay, New York. Hempstead: Hofstra University.
Erickson, Matthew R., Grosse, A., and John C. Maerz. 2011. Dietary analysis of the diamondback terrapin (Malaclemys terrapin) along the Georgia coast. In The Society for Integrative and Comparitive Biolody (SICB) annual meeting, Abs. P3.66.
Ernst, Carl H., and Jeffrey E. Lovich. 2009. Turtles of the United States and Canada. 2nd ed. Baltimore: Johns Hopkins University Press.
Fry, Brian. 2006. Stable isotope ecology. Stable isotope ecology. 1st ed. New York: Springer-Verlag. https://doi.org/10.1007/0-387-33745-8.
Gannes, Leonard Z., Carlos Martınez del Rio, and Paul Koch. 1998. Natural abundance variations in stable isotopes and their potential uses in animal physiology ecology. Comparative Biochemistry and Physiology 119A: 725–737.
Gibbons, J. Whitfield, Jeffrey E. Lovich, Anton D. Tucker, Nancy N. Fitzsimmons, and Judith L. Greene. 2001. Demographic and ecological factors affecting conservation and management of the diamondback terrapin (Malaclemys terrapin) in South Carolina. Chelonian Conservation and Biology 4: 66–74.
Grantham, Brian A., Ginny L. Eckert, and Alan L. Shanks. 2003. Dispersal potential of marine invertebrates in diverse habitats. Ecological Applications 13: S108–S116.
Haramis, G. Michael, L. Dennis G. Jorde, A. Macko, Jane L. Walker, and Holly Drive. 2001. Stable-isotope analysis of canvasback winter diet in upper Chesapeake Bay. The Auk 118 (4): 1008–1017. https://doi.org/10.1642/0004-8038(2001)118[1008:SIAOCW]2.0.CO;2.
Harden, Leigh Anne, Nicholas A. DiLuzio, J. Whitfield Gibbons, and Michael E. Dorcas. 2007. Spatial and thermal ecology of diamondback terrapins (Malaclemys terrapin) in a South Carolina salt marsh. Journal of the North Carolina Academy of Science 123: 154–162.
Hart, Kristen M., and Carole C. McIvor. 2008. Demography and ecology of mangrove diamondback terrapins in a wilderness area of Everglades National Park, Florida, USA. Copeia 2008 (1): 200–208. https://doi.org/10.1643/CE-06-161.
Herzka, Sharon Z. 2005. Assessing connectivity of estuarine fishes based on stable isotope ratio analysis. Estuarine, Coastal and Shelf Science 64 (1): 58–69. https://doi.org/10.1016/j.ecss.2005.02.006.
Hobson, Keith A. 1999. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120 (3): 314–326. https://doi.org/10.1007/s004420050865.
Hobson, Keith A., and Franz Bairlein. 2003. Isotopic fractionation and turnover in captive garden warblers (Sylvia borin): implications for delineating dietary and migratory associations in wild passerines. Canadian Journal of Zoology 81 (9): 1630–1635. https://doi.org/10.1139/z03-140.
Hobson, Keith A., and Ian Stirling. 1997. Low variation in blood δ13C among Hudson Bay polar bears: implications for metabolism and tracing terrestial feeding. Marine Mammal Science 13 (3): 359–367.
Jackson, Andrew L., Richard Inger, Andrew C. Parnell, and Stuart Bearhop. 2011. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80 (3): 595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x.
Kappes, Heike, and Peter Haase. 2012. Slow, but steady: dispersal of freshwater molluscs. Aquatic Sciences 74 (1): 1–14. https://doi.org/10.1007/s00027-011-0187-6.
Lapointe, Brian E., and Mark W. Clark. 1992. Nutrient inputs from the watershed and coastal eutrophication in the Florida Keys. Estuaries 15 (4): 465–476. https://doi.org/10.2307/1352391.
Lapointe, Brian E., Peter J. Barile, and William R. Matzie. 2004. Anthropogenic nutrient enrichment of seagrass and coral reef communities in the Lower Florida Keys: discrimination of local versus regional nitrogen sources. Journal of Experimental Marine Biology and Ecology. 308 (1): 23–58. https://doi.org/10.1016/j.jembe.2004.01.019.
Layman, Craig A., Marcio S. Araujo, Ross Boucek, Caroline M. Hammerschlag-Peyer, Elizabeth Harrison, Zachary R. Jud, Philip Matich, et al. 2012. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biological Reviews: 542–562.
Lovich, Jeffrey E., and J. Whitfield Gibbons. 1990. Age at maturity influences adult sex ratio in the turtle Malaclemys terrapin. Oikos 59 (1): 126–134.
Lundquist, Carolyn J., Simon F. Thrush, John W. Oldman, and Alastair K. Senior. 2004. Limited transport and recolonization potential in shallow tidal estuaries. Limnology and Oceanography 49 (2): 386–395. https://doi.org/10.4319/lo.2004.49.2.0386.
McCarter, Molly. 2012. Florida keys wilderness: a report on wilderness character monitoring. U.S. Fish and Wildlife Service. 114 pp.
McClelland, James W., Ivan Valiela, and Robert H. Michener. 1997. Nitrogen-stable isotope signatures in estuarine food webs: a record of increasing urbanization in coastal watersheds. Limnology and Oceanography 42 (5): 930–937. https://doi.org/10.4319/lo.1997.42.5.0930.
McCullough, Dale R., David H. Hirth, and Stephen J. Newhouse. 1989. Resource partitioning between sexes in white-tailed deer. The Journal of Wildlife Management 53 (2): 277–283. https://doi.org/10.2307/3801123.
McGaw, Iain J. 2006. Feeding and digestion in low salinity in an osmoconforming crab, Cancer gracilis II. Gastric evacuation and motility. Journal of Experimental Biology 209 (19): 3777–3785. https://doi.org/10.1242/jeb.02442.
Murray, Ian W., and Blair O. Wolf. 2012. Tissue carbon incorporation rates and diet-to-tissue discrimination in ectotherms: tortoises are really slow. Physiological and Biochemical Zoology 85 (1): 96–105. https://doi.org/10.1086/663867.
Newsome, Seth D., Justin D. Yeakel, Patrick V. Wheatley, and M. Tim Tinker. 2012. Tools for quantifying isotopic niche space and dietary variation at the individual and population level. Journal of Mammalogy 93 (2): 329–341. https://doi.org/10.1644/11-MAMM-S-187.1.
Nifong, James C. 2016. Living on the edge: trophic ecology of Alligator mississippiensis (American alligator) with access to a shallow estuarine impoundment. Bulletin of the Florida Museum of Natural History 54: 13–49.
Parnell, Andrew C., Richard Inger, Stuart Bearhop, and Andrew L. Jackson. 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS One 5 (3): e9672. https://doi.org/10.1371/journal.pone.0009672.
Parnell, Andrew C., Donald L. Phillips, Stuart Bearhop, Brice X. Semmens, Eric J. Ward, Jonathan W. Moore, Andrew L. Jackson, Jonathan Grey, David J. Kelly, and Richard Inger. 2013. Bayesian stable isotope mixing models. Environmetrics 24: 387–399. https://doi.org/10.1002/env.2221.
Peterson, Bruce J., and Brian Fry. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18 (1): 293–320. https://doi.org/10.1146/annurev.es.18.110187.001453.
Petrochic, Sara L. 2009. Feeding ecology of the northern diamondback terrapin, Malaclemys terrapin terrapin. Thesis. Long Island University.
Pfau, Beate, and Willem M. Roosenburg. 2010. Diamondback terrapins in Maryland: research and conservation. Radiata 19: 2–34.
Phillips, Donald L. 2012. Converting isotope values to diet composition: the use of mixing models. Journal of Mammalogy 93 (2): 342–352. https://doi.org/10.1644/11-MAMM-S-158.1.
Phillips, Donald L., Richard Inger, Stuart Bearhop, Andrew L. Jackson, Jonathan W. Moore, Andrew C. Parnell, Brice X. Semmens, and Eric J. Ward. 2014. Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology 92 (10): 823–835. https://doi.org/10.1139/cjz-2014-0127.
Post, David M. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83 (3): 703–718. https://doi.org/10.2307/3071875.
Post, David M., Craig A. Layman, D. Albrey Arrington, Gaku Takimoto, John Quattrochi, and Carman G. Montaña. 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152 (1): 179–189. https://doi.org/10.1007/s00442-006-0630-x.
R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing www.r-project.org.
Reich, Kimberly J., Karen A. Bjorndal, and Alan B. Bolten. 2007. The “lost years” of green turtles: using stable isotopes to study cryptic lifestages. Biology Letters 3 (6): 712–714. https://doi.org/10.1098/rsbl.2007.0394.
Roosenburg, Willem M. 1991. The diamondback terrapin: population dynamics, habitat requirements, and opportunities for conservation. In New perspectives in the Chesapeake System: a research and management partnership, 227–234.
Roosenburg, Willem M., Katherin L. Hayley, and Scott McGuire. 1999. Habitat selection and movements of diamondback terrapins, Malaclemys terrapin, in a Maryland estuary. Chelonian Conservation and Biology 3: 425–429.
Rosenblatt, Adam E., James C. Nifong, Michael R. Heithaus, Frank J. Mazzotti, Michael S. Cherkiss, Brian M. Jeffery, Ruth M. Elsey, et al. 2015. Factors affecting individual foraging specialization and temporal diet stability across the range of a large “generalist” apex predator. Oecologia 178: 5–16. https://doi.org/10.1007/s00442-014-3201-6.
Ross, Michael S., Joseph J. O’Brien, and Laura J. Flynn. 1992. Ecological site classification of Florida Keys terrestrial habitats. Biotropica 24 (4): 488–502.
Rubenstein, Dustin R., and Keith A. Hobson. 2004. From birds to butterflies: animal movement patterns and stable isotopes. Trends in Ecology & Evolution 19 (5): 256–263. https://doi.org/10.1016/j.tree.2004.03.017.
Rudnick, David T., Zhenquan Chen, D.L. Childers, J.N. Boyer, and T.D. Fontaine. 1999. Phosphorus and nitrogen inputs to Florida Bay: The importance of the everglades watershed. Estuaries 22 (2): 398–416. https://doi.org/10.2307/1353207.
Rugiero, Lorenzo, Giuliano Milana, Massimo Capula, Giovanni Amori, and Luca Luiselli. 2012. Long term variations in small mammal composition of a snake diet do not mirror climate change trends. Acta Oecologica 43: 158–164. https://doi.org/10.1016/j.actao.2012.07.002.
Seigel, Richard A. 1980. Predation by raccoons on diamondback terrapins, Malaclemys terrapin tequesta. Society for the Study of Amphibians and Reptiles 14: 87–89.
Seigel, Richard A. 1984. Parameters of two populations of diamondback terrapins (Malaclemys terrapin) on the Atlantic coast of Florida. In: Seigel, R.A., Hunt, L.E., Knight, J.L., Malaret, L., and Zuschlag, N.L. (Eds.). Vertebrate ecology and systematics. A tribute to Henry S. Fitch. Univ. Kansas Mus. Nat. Hist. Spec. Publ.
Seminoff, Jeffrey A., T. Todd Jones, Tomoharu Eguchi, David R. Jones, and Peter H. Dutton. 2006. Stable isotope discrimination (δ13C and δ15N) between soft tissues of the green sea turtle Chelonia mydas and its diet. Marine Ecology Progress Series 308: 271–278. https://doi.org/10.3354/meps308271.
Seminoff, Jeffrey A., Karen A. Bjorndal, and Alan B. Bolten. 2007. Stable carbon and nitrogen isotope discrimination and turnover in pond sliders Trachemys scripta: insights for trophic study of freshwater turtles. Copeia 2007 (3): 534–542.
Seminoff, Jeffrey A., T. Todd Jones, Tomoharu Eguchi, Mervin Hastings, and David R. Jones. 2009. Stable carbon and nitrogen isotope discrimination in soft tissues of the leatherback turtle (Dermochelys coriacea): insights for trophic studies of marine turtles. Journal of Experimental Marine Biology and Ecology 381 (1): 33–41. https://doi.org/10.1016/j.jembe.2009.08.018.
Sheridan, Claire M., James R. Spotila, Walter F. Bien, and Harold W. Avery. 2010. Sex-biased dispersal and natal philopatry in the diamondback terrapin, Malaclemys terrapin. Molecular Ecology 19 (24): 5497–5510. https://doi.org/10.1111/j.1365-294X.2010.04876.x.
Silliman, Brian R., and Mark D. Bertness. 2002. A trophic cascade regulates salt marsh primary production. Proceedings of the National Academy of Sciences of the United States of America 99 (16): 10500–10505. https://doi.org/10.1073/pnas.162366599.
Silliman, Brian R., Johan Van De Koppel, Mark D. Bertness, Lee E. Stanton, and Irving A. Mendelssohn. 2005. Drought, snails, and large-scale die-off of southern U.S. salt marshes 310: 1803–1806. doi:https://doi.org/10.1126/science.1118229.
Smith, Nancy F., and Gregory M. Ruiz. 2004. Phenotypic plasticity in the life history of the mangrove snail Cerithidea scalariformis. Marine Ecology Progress Series 284: 195–209. https://doi.org/10.3354/meps284195.
Spivey, Philip B. 1998. Home range, habitat selection, and diet of the diamondback terrapin (Malaclemys terrapin) in a North Carolina estuary. University of Georgia.
Sumoski, Sarah E., and Robert J. Orth. 2012. Biotic dispersal in eelgrass Zostera marina. Marine Ecology Progress Series 471: 1–10. https://doi.org/10.3354/meps1014.
Tucker, Anton D., Nancy N. Fitzsimmons, and J. Whitfield Gibbons. 1995. Resource partitioning by the estuarine turtle Malaclemys terrapin: trophic, spatial, and temporal foraging constraints. Herpetologica 51: 167–181.
Tulipani, Diane C. 2013. Foraging ecology and habitat use of the northern diamondback terrapin (Malaclemys terrapin terrapin) in southern Chesapeake Bay. The College of William and Mary in Virginia.
Tulipani, Diane C., and Romuald N. Lipcius. 2014. Evidence of eelgrass (Zostera marina) seed dispersal by northern diamondback terrapin (Malaclemys terrapin terrapin) in lower Chesapeake Bay. PloS One 9: e103346. https://doi.org/10.1371/journal.pone.0103346.
Vander Zanden, Hannah B., Karen A. Bjorndal, Kimberly J. Reich, and Alan B. Bolten. 2010. Individual specialists in a generalist population: results from a long-term stable isotope series. Biology Letters 6 (5): 711–714. https://doi.org/10.1098/rsbl.2010.0124.
Vizzini, Salvatrice, and Antonio Mazzola. 2006. The effects of anthropogenic organic matter inputs on stable carbon and nitrogen isotopes in organisms from different trophic levels in a southern Mediterranean coastal area. Science of the Total Environment 368 (2-3): 723–731. https://doi.org/10.1016/j.scitotenv.2006.02.001.
Wood, Roger. 1981. The mysterious mangrove terrapin. The Florida Naturalist 6–7
Zbinden, Judith A., Stuart Bearhop, Philip Bradshaw, Brownen Gill, Dimitris Margaritoulis, Jason Newton, and Brendan J. Godley. 2011. Migratory dichotomy and associated phenotypic variation in marine turtles revealed by satellite tracking and stable isotope analysis. Marine Ecology Progress Series 421: 291–302. https://doi.org/10.3354/meps08871.
Acknowledgments
We thank the many people who assisted in the field including M. Cherkiss, T. Selby, A. Crowder, D. Nemire-Pepe, H. Crowell, A. Daniels, S. Sisk, J. Beauchamp, and C. Denton. We also thank S. Kudman for her assistance in processing samples, J. McClain-Counts for her expertise and assistance with sample analysis, and all reviewers for suggestions and comments that improved the manuscript.
Funding
This study was supported by the US Geological Survey (USGS), the US National Park Service (Permit # EVER-2013-SCI-0060), US Fish and Wildlife Service (Permit # 2013-007), and USGS Institutional Animal Care Protocol USGS-SESC-IACUC2011-05 and funded through the USGS Priority Ecosystem Science Program (PESFY2012-2014; K. Hart, principle investigator) and the Diamondback Terrapin Working Group Research Grant (2012-Denton). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marianne Holmer
Electronic Supplementary Material
Online Resource 1
(PDF 250 kb)
Online Resource 2
(PDF 265 kb)
Online Resource 3
(PDF 344 kb)
Online Resource 4
(PDF 344 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Denton, M.J., Demopoulos, A.W.J., Baldwin, J.D. et al. Stable Isotope Analysis Enhances Our Understanding of Diamondback Terrapin (Malaclemys terrapin) Foraging Ecology. Estuaries and Coasts 42, 596–611 (2019). https://doi.org/10.1007/s12237-018-0476-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0476-6