Abstract
While multiple species of macroalgae and seagrass can benefit from elevated CO2 concentrations, competition between such organisms may influence their ultimate responses. This study reports on experiments performed with a Northwest Atlantic species of the macroalgae, Ulva, and the seagrass, Zostera marina, grown under ambient and elevated levels of pCO2, and subjected to competition with each other. When grown individually, elevated pCO2 significantly increased growth rates and productivity of Ulva and Zostera, respectively, beyond control treatments (by threefold and 27%, respectively). For both primary producers, significant declines in tissue δ13C signatures suggested that increased growth and productivity were associated with a shift from use of HCO3− toward CO2 use. When grown under higher pCO2, Zostera experienced significant increases in leaf and rhizome carbon content as well as significant increases in leaf carbon-to-nitrogen ratios, while sediments within which high CO2 Zostera were grown had a significantly higher organic carbon content. When grown in the presence of Ulva; however, above- and below-ground productivity and tissue nitrogen content of Zostera were significantly lower, revealing an antagonistic interaction between elevated CO2 and the presence of Ulva. The presence of Zostera had no significant effect on the growth of Ulva. Collectively, this study demonstrates that while Ulva and Zostera can each individually benefit from elevated pCO2 levels, the ability of Ulva to grow more rapidly and inhibit seagrass productivity under elevated pCO2, coupled with accumulation of organic C in sediments, may offset the potential benefits for Zostera within high CO2 environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shifts in carbonate chemistry due to the excessive diffusion of carbon dioxide (CO2) from fossil fuel combustion into surface oceans is expected to initiate shifts in the community structure of marine flora and fauna. While fossil fuel combustion is expected to increase CO2 levels 260% by 2100 (Meehl et al. 2007), coastal zones upwelling, riverine discharge, and eutrophication-enhanced microbial respiration can also significantly lower pH and increase pCO2 levels. Eutrophication-enhanced microbial respiration can cause the seasonal accumulation of respiratory CO2 that, in some cases, can exceed current pCO2 projections for the open ocean (> 1000 μatm) for the end of the century (Cai et al. 2011; Melzner et al. 2013; Wallace et al. 2014). While prior studies have demonstrated the negative implications of higher pCO2 and decreased CO32− availability on the growth of calcifying organisms (Gazeau et al. 2007; Talmage and Gobler 2010; Kroeker et al. 2013), other studies have shown that some, but not all, photosynthetic organisms can benefit from an increase in pCO2 (Palacios and Zimmerman 2007; Koch et al. 2013; Hattenrath-Lehmann et al. 2015; Young and Gobler 2016, 2017). Due to this, non-calcifying autotrophs may gain a competitive advantage over their calcifying counterparts under acidified conditions (Porzio et al. 2011). However, the extent to which individual, non-calcifying marine autotrophs will benefit from elevated CO2 concentrations will depend on competition (Young and Gobler 2017) and has yet to be fully explored.
The marine photosynthetic organisms that benefit from higher CO2 concentrations are generally non-calcifying autotrophs whose inorganic uptake is not substrate-saturated at current CO2 concentrations (Koch et al. 2013). Carbon acquisition in marine photosynthetic organisms involves the active transport of CO2 and bicarbonate (HCO3−) as well as the diffusive uptake of CO2 (Badger 2003). While CO2 is the preferred inorganic carbon source for many marine autotrophs, HCO3− is more abundant than CO2 in seawater at a pH of 8. As such, marine autotrophs require carbon concentrating mechanisms (CCM) and intracellular or extracellular carbonic anhydrase (CA) to convert HCO3− to CO2 to be used by RuBisCO (Badger 2003; Gao and McKinley 1994; Israel and Hophy 2002; Koch et al. 2013). Elevated CO2 has been shown to enhance the growth of marine macroalgae, including chlorophytes (Björk et al. 1993; Olischläger et al. 2013; Young and Gobler 2016), rhodophytes (Hofmann et al. 2012; Xu et al. 2010; Young and Gobler 2016), and phaeophytes (Hepburn et al. 2011). Chlorophytes, such as Ulva rigida, exposed to elevated CO2 concentrations may downregulate their CCMs, allowing more energy to be available for other biochemical processes such as vegetative growth (Koch et al. 2013; Young and Gobler 2016, 2017). Alternatively, the increased availability of CO2 in seawater may cause a shift toward the diffusive uptake of CO2 over use of CCM, thus relieving carbon limitation (Mercado et al. 1998; Young and Gobler 2016, 2017). Values of δ13C are often used to assess the types of carbon utilized by seagrasses and macroalgae with values of − 10‰ or higher in seagrasses and macroalgae being reflective of the sole use of HCO3− whereas macroalgae relying wholly on diffusion of CO2 for carbon attain a value of − 30‰ (Hepburn et al. 2011; Maberly et al. 1992; Raven et al. 2002). While δ13C values of − 30‰ or lower have not been observed in seagrasses, it is suggested that increased reliance on CO2 diffusion can significantly lower the δ13C of seagrasses (Vizzini et al. 2010).
Seagrasses are another group of autotrophs that have been shown to benefit from elevated CO2 concentrations. Most seagrass species are C3 plants capable of utilizing CO2 and HCO3− for photosynthesis, in which CCMs and external CA are used for the fixation of the carbon from HCO3− when CO2 diffusion is slow (Koch et al. 2013; Touchette and Burkholder 2000). As C3 plants, seagrasses are expected to benefit from increases in CO2 levels, as their initial carboxylating enzyme, RuBisCO, is not substrate-saturated at current CO2 concentrations (Koch et al. 2013). The seagrasses Zostera marina, Thalassia testudinum, and T. hemprichii exhibit increased photosynthetic rates, reproduction, below- and above-ground biomass, and production of non-structural carbohydrates in below- and above-ground structures when grown under high CO2 concentrations (Beer and Koch 1996; Campbell and Fourqurean 2013; Durako 1993; Jiang et al. 2010; Palacios and Zimmerman 2007; Zimmerman et al. 1995; Zimmerman et al. 1997).
Despite the benefits of elevated CO2 for seagrasses, the extent to which those benefits are realized in an ecosystem setting will partly depend on the outcome of competition with other estuarine autotrophs that may also benefit from such conditions (Young and Gobler 2017). Ephemeral macroalgae, such as Ulva, are well-known seagrass competitors (Hauxwell et al. 2001; McGlathery 2001; Valiela et al. 1997) that also benefit from elevated CO2 and can inhibit other autotrophs such as phytoplankton (Tang and Gobler 2011; Tang et al. 2015; Young and Gobler 2017). Being rooted in sediments, seagrasses are often more light-limited than nutrient-limited (Valiela et al. 1997). In temperate estuaries, seagrasses can persist in oligotrophic estuarine regions due to their ability to acquire nutrients from both the sediments and the water column, as well as their nutrient storage capabilities (Pedersen and Borum 1992; Short and McRoy 1984; Valiela et al. 1997). As nutrient loading increases, macroalgae gain a competitive advantage over seagrass due to higher rates of maximum nutrient uptake (Pedersen and Borum 1997; Valiela et al. 1997). In persistently eutrophic estuaries, ephemeral macroalgae often overgrow and shade-out seagrasses (McGlathery 2001; Valiela et al. 1997), as well as create unfavorable biogeochemical conditions such as anoxia and potentially toxic concentrations of ammonium (NH4+) (Hauxwell et al. 2001).
Recent studies have demonstrated that Ulva rigida, a dominant macroalgae within Northwest Atlantic coastal waters (Young and Gobler 2016, 2017), and Zostera marina, the primary seagrass of the same region, both grow more rapidly when exposed to elevated levels of CO2 (Palacios and Zimmerman 2007; Young and Gobler 2016, 2017; Zimmerman et al. 1995; Zimmerman et al. 1997). The objective of this study was to assess how elevated CO2 concentrations influence competition between these autotrophs. Both primary producers were grown with and without elevated levels of pCO2 as well as with and without the other primary producer. Growth and productivity responses, δ13C signatures, and elemental composition of the primary producers were evaluated at the start and end of experiments performed throughout the summer months in a Northwest Atlantic estuary.
Methods
Eelgrass and Macroalgae Collection and Preparation
Ulva rigida used for this study was collected from a shallow-water site in Shinnecock Bay, NY, USA (40.85° N, 72.50° W), while Zostera marina shoots were collected from eelgrass beds located 5 km east of the macroalgae (Fig. 1; Furman and Peterson 2015). Permission to access and collect the water, Z. marina, and U. rigida was received from the Southampton Town Trustees, Southampton, NY, USA, who hold jurisdiction over Shinnecock Bay. Large, well-pigmented fronds of U. rigida and ~ 20-cm rooted shoots of Z. marina were collected and transported to the Stony Brook Southampton Marine Science Center of Stony Brook University in seawater-filled containers within 15 min of collection. Our sequencing efforts and microscopy during this study affirmed that U. rigida was the species of Ulva present at the macroalgal sampling site (Young and Gobler 2016). We refer to the algae as Ulva due to the inconsistent macroalgal taxonomic nomenclature as well as the similarity of sequences of the internal transcribed spacer (ITS) region of the ribosome among Ulva species (Hofmann et al. 2010; Kirkendale et al. 2013). For the sake of consistency, Z. marina will be referred to as Zostera. Individual thalli of Ulva approximately 10 cm in length were cut from large thalli with care taken to avoid the potentially reproductive outer region of the organism and placed in a salad spinner to remove debris and epiphytes. Thalli were then extensively rinsed with filtered (0.2 μm) seawater before being spun again to further remove any debris, epiphytes, and excess seawater (Young and Gobler 2016). Additional samples of Ulva were cut, cleaned, rinsed, and spun as previously described, and frozen for further analyses (see below). Ulva samples were weighed on an A&D EJ300 digital balance (± 0.01 g) to obtain initial wet weight. Similarly, Zostera shoots were extensively rinsed with filtered seawater to remove debris and epiphytes. Four individual shoots (~ 20 cm) were placed in cylindrical, sand-filled 20 × 9-cm plastic planters. The sand used to fill the planters was collected from ocean-facing areas south of Shinnecock Bay to ensure low levels of organic carbon in the sand. At the beginning of experiments, an 18-gauge hypodermic needle was used to create a hole in the leaves of the short shoot just above the sheath for quantifying Zostera leaf growth (Wall et al. 2008; Zieman 1974).
Assessing the Effects of Elevated pCO2 and Competition on Zostera and Ulva
Four experiments were performed to assess the effects of competition and elevated pCO2 on the growth of Ulva and Zostera during May, June, July, and September. Polycarbonate containers (20 L) were acid-washed (10% HCl) and liberally rinsed with deionized water before being filled with filtered (0.2 μm) seawater. The containers were placed in outdoor water baths filled with seawater heated or cooled to temperatures consistent with ambient levels (~ 20–25 °C) via the temperature control system at the Stony Brook Southampton Marine Science Center. The containers were exposed to natural light intensity (~ 1000 μmol s−1 m−2) and duration, which was quantified via discrete and continuous measurements from a LI-COR LI-1500 light sensor logger and HOBO pendant light loggers, respectively. Light levels were measured just above the sediment surface where the Zostera shoots were planted, and did not differ across treatments in any experiment. For all experiments, nine containers were assigned to both ambient (~ 400 μatm) and elevated (~ 2000 μatm) concentrations of CO2, with the level in the elevated treatment representing both concentrations present within eutrophic estuaries (Baumann et al. 2015; Cai et al. 2017; Melzner et al. 2013; Wallace et al. 2014) as well as levels projected for world oceans in the twenty-second century (Caldeira and Wickett 2003, 2005; Foster et al. 2017). Three sets of containers, in triplicate, were established for experiments: One for only Zostera, one for only Ulva, and one with both Zostera and Ulva, resulting in 18 total experimental containers. For each experiment, all containers received nutrient additions (5 μM nitrate, 0.3 μM phosphate) every day for the duration of the experiments to mimic regional nutrient loading rates (~ 4 × 106 kg N year−1 for Great South Bay; Kinney and Valiela 2011), mimic levels seasonally present within collection sites (Young and Gobler 2016), and to ensure levels of nitrate were not toxic to Zostera (< 7 μM; Burkholder et al. 1992).
All containers were aerated via a 1.5″ × 0.5″ (~ 3.8 × 1.3 cm) air diffuser (Pentair) connected to a length of Tygon tubing that was inserted to the bottom of each container and connected to an air source. A gas proportionator (Cole Parmer® Flowmeter system, multitube frame) was used to mix ambient air with 5% CO2 gas (Talmage and Gobler 2010) to introduce the control (~ 400 μatm) and elevated (~ 2000 μatm) levels of pCO2 into the experimental containers. The gas mixtures were delivered at a net flow rate of 2500 ± 5 mL min−1 through a nine-way gang valve into the tubing that was placed through a small opening in the closed lid of the container, allowing for the gases to turn over the volume of the containers > 100 times daily (Talmage and Gobler 2010). Bubbling was initiated 2 days prior to the beginning of each experiment to allow CO2 concentrations and pH to reach a state of equilibrium. Each experiment persisted approximately 2 weeks, a duration consistent with prior studies that observed significant changes in the growth and productivity of Ulva and Zostera, respectively, in an experimental setting (Dennison and Alberte 1982; Wall et al. 2008; Young and Gobler 2016, 2017). Measurements of pH within containers were made daily using an Orion Star A321 Plus electrode (± 0.001) calibrated before each use with National Institute of Standards and Technology (NIST) traceable standards. Our prior research has found that this instrument provides pH measurements linearly consistent with measurements made spectrophotometrically and with ion-sensitive field-effect transistor-based pH meters (e.g., Durafet by Honeywell). Discrete water samples were collected at the beginning and conclusion of experiments to directly measure dissolved inorganic carbon (DIC; Wallace et al. 2014). The water samples were preserved using a saturated mercuric chloride (HgCl2) solution and stored at 4 °C until analyses. The samples were analyzed by a VINDTA 3D (Versatile Instrument for the Determination of Total inorganic carbon and titration Alkalinity) delivery system coupled with a UIC coulometer (model CM5017O). Levels of pCO2 (Table 1, Supplementary Tables S1) were calculated using measured levels of DIC, pH (NIST), temperature, and salinity, as well as the first and second dissociation constants of carbonic acid in seawater (Millero 2010) using the program CO2SYS (http://cdiac.ornl.gov/ftp/co2sys/). As a quality assurance measure, levels of DIC and pH of certified reference material (provided by Dr. Andrew Dickson of the University of California, San Diego, Scripps Institution of Oceanography; batch 158 = 2044 μmol DIC kg seawater−1) were measured during analyses of every set of samples. Further analysis of samples continued only after complete recovery (99.8 ± 0.2%) of certified reference material was attained. The delivery of air and CO2 resulted in actual pCO2 and pH values of ~ 400 and ~ 8.1 μatm, respectively, for ambient conditions and ~ 2000 and ~ 7.3 μatm, respectively, for the elevated CO2 conditions, mimicking the range found seasonally in estuarine environments (Baumann et al. 2015; Melzner et al. 2013; Wallace et al. 2014).
Experiments began with the introduction of Zostera, Ulva, and nutrients into the experimental containers, with discrete and continuous measurements of pH, temperature, and light made throughout the duration of experiments. At the end of experiments, final pH, temperature, and salinity measurements were made and final water samples for DIC analysis were collected and analyzed as described above. After DIC was measured, all Ulva samples were removed from their respective treatments, rinsed, spun, re-rinsed, re-spun, weighed as described above, and placed into small freezer bags for further analyses. Zostera shoots were removed from their respective treatments, with each leaf cut at the area just above the sheath. The rhizomes were measured for total length, length to the hole in the leaf created by the hypodermic needle (new growth), and width. Each leaf was cut where the hole was, separating the leaf into new and old growth, with all new and old growth from each container being dried to a constant weight at 60 °C for 24 h. For each experiment, seagrass productivity was calculated as areal productivity (cm2 m−2 day−1) and above-ground biomass production (g DW m−2 day−1) (Wall et al. 2008; Zieman 1974). Additionally, the number of new leaves produced within each treatment was determined. Weight-based growth rates for Ulva were determined using the relative growth formula (growth day−1) = (ln Wfinal – ln Winitial)/(Δt), where Wfinal and Winitial are the final and initial weights in grams and Δt is the number of days of the experiment. Our prior research has demonstrated that such weight-based growth rates are linearly consistent with areal-based growth of Ulva (Young and Gobler 2016, 2017). Significant differences in growth and productivity during each experiment were assessed using three-way ANOVA within SigmaPlot 11.0, where the main treatments were pCO2 (ambient or elevated), competition (Zostera or Ulva, alone or in the same container), and time of the experiment.
Above- and Below-Ground Tissue Analyses
For carbon (C), nitrogen (N), and stable carbon isotope (δ13C) analyses, frozen samples of Zostera and Ulva were dried at 60 °C for 48 h and then homogenized into a fine powder using a mortar and pestle. Total tissue C, N, and δ13C were analyzed using an elemental analyzer interfaced to a Europa 20-20 isotope ratio mass spectrometer at the UC Davis Stable Isotope Facility (Young and Gobler 2016). Three-way ANOVA within SigmaPlot 11.0 was used to assess significant differences in above-ground tissue (leaf) content for Zostera and Ulva for each experiment where the main treatment effects were pCO2 (ambient or elevated), the presence of Ulva when assessing Zostera or the presence of Zostera when assessing Ulva, and the time of the experiment.
The below-ground biomass production of Zostera rhizomes was determined by cutting the rhizome away from the meristem, liberally rinsing the rhizome with fresh water to remove sand and other debris, and carefully removing the roots. A fixed length (2 cm) was cut from each rhizome starting from where the rhizome was cut away from the meristem. The rhizomes and roots were then dried at 60 °C for 72 h and then weighed using a Mettler Toledo AB304-S/FACT analytical balance (± 0.0001). Below-ground production was calculated by dividing the dry weight by the total area of all rhizomes within each replicate per day (g cm−2 day−1). After being weighed, the rhizomes were homogenized into a fine powder using a mortar and pestle. Tissue C and N content of the homogenized samples were analyzed using a CE Instruments Flash EA 1112 elemental analyzer (Sharp 1974). Three-way ANOVA within SigmaPlot 11.0 were used to assess significant differences in below-ground production and tissue content of Zostera rhizomes during experiments, where the main treatment effects were pCO2 (ambient or elevated), the presence of Ulva, and the time of the experiment.
Isotopic mixing models were used to estimate the use of CO2 and HCO3− diluted by the introduction of the isotopically lighter 5% CO2 gas (Young and Gobler 2016). The model considered the δ13C and biomass of the tissue of Ulva and Zostera before and after experiments, the δ13C of the 5% CO2 gas used for the experiments (− 80‰), the δ13C of the marine CO2 and HCO3− pool (− 10 and 0‰, respectively; Maberly et al. 1992; Mook et al. 1974; Raven et al. 2002), C fractionation during the uptake of CO2 and HCO3− by Ulva and Zostera, which was found to be highly similar between both species and of a magnitude that did not significantly alter the results of the mixing models (− 20 and − 10‰ for both species, relative to pool, respectively; Hemminga and Mateo 1996; Maberly et al. 1992; Mook et al. 1974; Raven et al. 2002), C fractionation that occurs during the conversion of the tanked 5% CO2 gas bubbled within the experimental containers to HCO3− (+ 10‰; Maberly et al. 1992; Mook et al. 1974; Raven et al. 2002), and the DIC concentration with and without exposure to the 5% CO2 gas. The δ13C of the tanked gas was determined by syringe injection into a split/splitless inlet of a continuous flow gas chromatography combustion isotope ratio mass spectrometry (GC/C/IRMS; Young and Gobler 2016). Determination of the DIC concentration with and without the addition of the 5% CO2 gas indicated the contribution of the gas to the DIC concentration compared to ambient air. The model assumed that the 5% CO2 gas reached equilibrium with the total DIC pool, which was highly likely given the high turnover rate of seawater within the experimental containers by the bubbled CO2 mixture (> 100 times daily). It was further assumed that the tissue grown during the experiments took on a δ13C signature that was reflective of the DIC pool (Young and Gobler 2016). Lastly, separate calculations of the same mixing model were performed for Ulva and Zostera. The following equation was used as the hypothetical mixing model to estimate the δ13C signature of Ulva and Zostera in the high CO2 treatments had they grown exclusively using HCO3− or CO2, respectively:
where initial δ13C is the δ13C signature of the Ulva or Zostera at the start of experiments, ambient and elevated [DIC] are the concentrations of total dissolved inorganic carbon within ambient and elevated CO2 treatments, respectively, DIC and tank δ13C are the δ13C signatures found within the DIC pool for HCO3− (0‰) and CO2 (− 10‰), and the tanked 5% CO2 (− 80‰; measured via GC/C/IRMS), respectively, C fractionation is the biological fractionation by the autotrophs during the uptake of CO2 (− 20‰) or HCO3− (− 10‰), and initial and final DW denotes dry tissue weights of the autotrophs at the beginning and end of the experiments, respectively. For Zostera, initial dry weight was the dry weights of “old” growth, while final dry weight was the sum of “old” and “new” growth (see above). For Ulva, initial dry weight was determined by obtaining the dry weight of the additional Ulva samples created at the beginning of experiments (see above), while final dry weight was the dry weights of the final samples. A one-way ANOVA was used to assess the differences between actual δ13C signatures of Ulva and Zostera, and signatures calculated based on the exclusive use of HCO3− or CO2, with Tukey tests used to assess the differences between the individual groups.
Sediment and Dissolved Nutrient Analyses
The organic C content of sediments from the planters that held the Zostera shoots from each container at the end of experiments was analyzed. A small quantity of sediment (6–8 g) was removed, dried at 60 °C for 72 h, weighed, and combusted at 450 °C for 4 h, weighed again, and compared to the original dry weights to estimate the amount of organic C in the sediments.
To determine concentrations of nitrate (NO3−), phosphate (PO43−), and ammonium (NH4+) within experimental vessels, 20 mL of seawater was removed from each container and filtered by passing the seawater through pre-combusted (4 h at 450 °C) glass fiber filters (GF/F, 0.7 μm pore size). The filtrate was frozen in acid-washed scintillation vials for later analysis. The filtrate was analyzed colorimetrically for NO3−, PO43−, and NH3 by a QuikChem 8500 (Lachat Instruments) flow injection analysis system using methods for analysis of the nutrients highlighted by Parsons et al. (1984). Nutrients were measured at the beginning and at the end of the experiment. The average concentrations of NO3−, PO43−, and NH4+ at the end of experiments were 1.11 ± 0.03 μM, 0.94 ± 0.05 μM, and 6.94 ± 0.49 μM, respectively. Nutrient concentrations across all experiments and treatments are reported in Table 1 and Supplementary Tables S1.
Results
Zostera
Areal productivity, above-ground production, and below-ground production of Zostera were all highly sensitive to changes in pCO2 concentrations and varied seasonally (three-way ANOVA; p < 0.05 for time and CO2; Fig. 2; Supplementary Tables S2). Areal productivity under elevated CO2 was 31% higher than under ambient CO2. There was, however, an antagonistic interaction between elevated CO2 and the presence of Ulva, where despite the increased areal productivity under elevated CO2, the presence of Ulva suppressed productivity by 22% (three-way ANOVA; p < 0.05; Fig. 2a; Supplementary Tables S2). Elevated CO2 significantly increased the average above-ground production of Zostera by 33% compared to ambient CO2 treatments (three-way ANOVA; p < 0.05; Fig. 2b; Supplementary Tables S2). There was an antagonistic interaction between elevated CO2 and the presence of Ulva, in which above-ground production, under elevated CO2, was significantly reduced by competition with Ulva (p < 0.05; Fig. 2b). Lastly, the below-ground production of Zostera was significantly higher (35%) when exposed to elevated CO2 concentrations (Three-way ANOVA; p < 0.05; Fig. 2c; Supplementary Tables S2). In a manner similar to areal productivity and above-ground production, the below-ground production of Zostera, despite being significantly higher when exposed to elevated CO2, was reduced by 20% when competing with Ulva, demonstrating an antagonistic interaction between elevated CO2 and competition with Ulva (p < 0.05; Fig. 2c; Supplementary Tables S2).
a Areal productivity, b above-ground biomass production, and c below-ground biomass production of Zostera exposed to ambient and elevated CO2 concentrations, with and without competition from Ulva for experiments performed May through September. Growth measurements were taken at the end of experiments. Columns represent means ± standard error. Significant main treatment effects (CO2 and Ulva) appear on the top right of each figure
The δ13C content of Zostera varied throughout the summer and was significantly reduced by elevated CO2, with the average δ13C of the ambient and elevated CO2 treatments being about − 8 and − 19‰, respectively (three-way ANOVA; p < 0.05 for time and CO2; Fig. 3; Supplementary Tables S2–S3). The presence of Ulva had no significant effect on the δ13C content of Zostera (three-way ANOVA; p > 0.05; Fig. 3a; Supplementary Tables S2-S3). Isotopic mixing models demonstrated that Zostera, when exposed to elevated CO2 concentrations, had δ13C signatures (− 18.3‰) that were significantly lower than values expected if C was obtained exclusively from the use of HCO3− (− 13.8‰; Tukey test; p < 0.001; Supplementary Fig. S1 and Tables S2), but significantly higher than expected from the exclusive use of CO2 (− 21.9‰; Tukey test; p < 0.001; Supplementary Fig. S1 and Tables S2).
δ13C content of a Zostera and b Ulva exposed to ambient and elevated CO2 concentrations, with and without competition from the other primary producer for experiments performed May through September. Measurements were taken at the end of experiments. Columns represent means ± standard error. Significant main treatment effects (CO2 and Ulva) appear on the bottom left of each figure
Above-ground tissue (leaf) C of Zostera varied seasonally and was significantly higher when exposed to elevated CO2 concentrations (three-way ANOVA; p < 0.05 for time and CO2; Fig. 4a; Supplementary Tables S2 and S4) but was unaffected by the presence of Ulva (p > 0.05). Leaf N was significantly lower in the presence of Ulva and varied with time (three-way ANOVA; p < 0.05 for time and Ulva; Fig. 4b; Supplementary Tables S2 and S4) but was unaffected by exposure to elevated CO2 concentrations (p > 0.05). The leaf C:N ratio of Zostera was significantly higher when exposed to elevated CO2 concentrations and changed seasonally (three-way ANOVA; p < 0.05 for time and CO2; Fig. 4c; Supplementary Tables S2 and S4), but unaffected by the presence of Ulva (p > 0.05).
a Leaf C, b leaf N, and c leaf C:N content of Zostera exposed to ambient and elevated CO2 concentrations, with and without competition from Ulva for experiments performed May through September. Measurements were made at the end of experiments. Columns represent means ± standard error. Significant main treatment effects (CO2 and Ulva) appear on the top right of each figure
Below-ground tissue (rhizome) C was significantly higher when exposed to elevated CO2 concentrations compared to ambient concentrations and changed over the summer (three-way ANOVA; p < 0.05 for CO2 and time; Fig. 5a; Supplementary Tables S2 and S4) while the presence of Ulva had no effect (p > 0.05). The rhizome N content of Zostera was significantly lower in the presence of Ulva and changed through the summer (three-way ANOVA; p < 0.05 for Ulva and time; Fig. 5b; Supplementary Tables S2 and S4) but was unaffected by exposure to elevated CO2 concentrations (p > 0.05). Overall, the rhizome C:N was not significantly affected when exposed to elevated CO2 concentrations or in the presence of Ulva (three-way ANOVA; p > 0.05 for all; Fig. 5c; Supplementary Tables S2 and S4). The organic C content of sediments with Zostera shoots was significantly higher under elevated CO2 levels relative to ambient levels (three-way ANOVA; p < 0.05; Fig. 5d; Supplementary Tables S2 and S4). Organic C content of the sediments was not significantly affected by the presence of Ulva or time (three-way ANOVA; p > 0.05).
a Rhizome C, b rhizome N, c rhizome C:N content of Zostera, and d Zostera-containing sediment organic carbon exposed to ambient and elevated CO2 concentrations, with and without competition from Ulva for experiments performed May through September. Measurements were made at the end of experiments. Columns represent means ± standard error. Significant main treatment effects (CO2 and Ulva) appear on the top right of each figure
Ulva
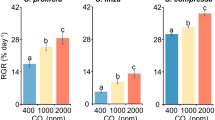
The growth of Ulva was highly sensitive to changes in CO2 concentrations and differed by season (three-way ANOVA; p < 0.05 for CO2 and time; Fig. 6; Supplementary Tables S2). Under elevated CO2 concentrations, growth was three-to-four times higher relative to growth under ambient concentrations (Fig. 6). Overall, Ulva growth was unaffected by the presence of Zostera (three-way ANOVA; p > 0.05; Fig. 6; Supplementary Tables S2). The δ13C content of Ulva was significantly lower when exposed to elevated CO2 concentrations, with the average δ13C of ambient and elevated CO2 treatments being approximately − 15 and − 28‰, respectively (three-way ANOVA; p < 0.001; Fig. 3b; Supplementary Tables S2-S3), while the presence of Zostera had no effect on the δ13C content of Ulva (p > 0.05). Isotopic mixing models demonstrated that when exposed to elevated CO2 concentrations Ulva yielded δ13C signatures (− 28‰) that were significantly lower than values expected if C was obtained exclusively from the use of HCO3− (− 17.9‰; Tukey test; p < 0.001; Supplementary Fig. S1 and Tables S2), but not significantly different than expected from the exclusive use of CO2 (− 28.9‰; Tukey test; p > 0.05; Supplementary Fig. S1 and Tables S2). Tissue C, N, and C:N of Ulva were unaffected by CO2 concentration or the presence of Zostera (three-way ANOVA; p > 0.05; Fig. 7; Supplementary Tables S2 and S4).
Growth rates of Ulva exposed to ambient and elevated CO2 concentrations, with and without competition from Zostera for experiments performed May through September. Growth measurements were made at the end of experiments. Columns represent means ± standard error. Significant main treatment effects (CO2 and Zostera) appear on the top right of each figure
Discussion
During this study, elevated CO2 concentrations significantly enhanced the growth of Ulva and the areal productivity and above- and below-ground biomass production of Zostera. For Zostera, areal productivity and above- and below-ground production were significantly repressed by the presence of Ulva. In contrast, Ulva was largely unaffected by Zostera. For both primary producers, tissue δ13C was significantly lowered by elevated CO2 concentrations. While the tissue C, N, and C:N of Ulva was unaffected by the presence of Zostera or elevated CO2 levels, Zostera experienced more complex responses. Zostera experienced increased leaf C and C:N ratios in response to elevated CO2, and reduced N content in the presence of Ulva. Elevated CO2 concentrations significantly increased Zostera rhizome C, while competition with Ulva significantly reduced the rhizome N of Zostera. Sediment organic C levels were significantly higher in treatments exposed to elevated CO2 but were not affected by Ulva. Together, these findings provide insight regarding how competition between seagrass and macroalgae may be altered by current and future high CO2 concentrations.
Time/season was a significant treatment effect for many of the parameters measured for Zostera and Ulva during this study, an outcome consistent with the time span during which experiments were performed (May through September). Over the course of experiments, temperature and photoperiods differed, two factors that likely drove seasonal changes in growth rates of Zostera (Bulthuis 1987; Hauxwell et al. 2006; Zimmerman et al. 1989) and Ulva (Henley 1992, 1993; Sand-Jensen 1988). Despite these seasonal changes, responses of Zostera and Ulva to elevated pCO2 and competition with each other were markedly consistent.
The physiological response of macroalgae and seagrass to elevated CO2 concentrations depends on their mode of carbon acquisition as well as if the inorganic carbon uptake of the organism is substrate-saturated at present CO2 concentrations (Badger 2003; Koch et al. 2013). Prior studies have shown that elevated CO2 concentrations may cause macroalgae to downregulate their CCMs that convert HCO3− to CO2 which may, in turn, allow more energy to be available for other processes such as vegetative growth (Björk et al. 1993; Cornwall et al. 2012; Koch et al. 2013). Numerous species of seagrass, such as Z. marina, have been shown to possess C3 photosynthetic pathways along with CCMs and external CA to utilize HCO3− for photosynthesis (Beer and Rehnberg 1997; Beer and Wetzel 1982; Invers et al. 1999) that may downregulate under high CO2. The δ13C signatures of Ulva and Zostera during this study decreased significantly when exposed to higher CO2 and isotopic mixing models suggest that these autotrophs switched from primarily HCO3− use to primarily CO2 use and potentially downregulated their CCMs, although further study is needed to definitively affirm this. The precise change in tissue δ13C at the beginning of experiments (− 9.8 and − 15.7‰ for Zostera and Ulva, respectively) to the conclusion of experiments (− 18.3 and − 28‰ for Zostera and Ulva, respectively), indicate a greater reliance on the diffusive uptake of CO2 (Hepburn et al. 2011; Maberly et al. 1992; Raven et al. 2011). Another possibility is that elevated CO2 levels may alleviate inorganic C limitation that may occur during photosynthesis, allowing for enhanced growth and productivity. Ulva rigida and U. compressa (formerly Enteromorpha) utilize CCMs, as they do not receive enough CO2 through diffusive uptake alone at present CO2 concentrations (Mercado et al. 1998). Similarly, for many seagrasses, the diffusive supply of CO2 to leaves is slow and inefficient, causing them to rely on the active transport of HCO3−, along with CCMs and external CA (Beer 1989; Beer and Koch 1996; Koch et al. 2013). Thus, it is plausible that enhanced growth and productivity of Ulva and Zostera was the result of elevated CO2 levels alleviating inorganic C limitation.
The benefits of elevated CO2 levels for the growth of Ulva and productivity of Zostera in the present study are consistent with prior studies of this algae (Björk et al. 1993; Olischläger et al. 2013; Young and Gobler 2016) and seagrass (Beer and Koch 1996; Palacios and Zimmerman 2007; Zimmerman et al. 1997). Direct competition between these primary producers under high CO2 has not been previously explored and may, however, offset some of the benefits of elevated CO2 for Zostera. For example, there existed an antagonistic interaction between elevated CO2 and the presence of Ulva for the areal productivity and above- and below-ground production of Zostera, whereby the benefit of high CO2 was partly or largely negated by Ulva. The overgrowth of macroalgae can decrease water clarity, shade seagrass, and reduce productivity (Valiela et al. 1997). In this study, Ulva outgrew Zostera by 21 and 5% under elevated and ambient CO2 concentrations, respectively, but light levels reaching Zostera shoots did not differ across treatments, making light limitation unlikely to have altered Zostera productivity in these experiments.
Macroalgae may also directly compete with Zostera for nutrients which can limit seagrass growth (Duarte 1995). The decline in leaf and rhizome N and increase in leaf C:N for Zostera in the presence of Ulva is consistent with the findings of Davis and Fourqurean (2001), suggesting that the rapid use of N by Ulva deprived Zostera of an adequate N supply. This hypothesis is further supported by significantly lower concentrations of nitrate and ammonium in the presence of Ulva (three-way ANOVA; p < 0.05; Supplementary Tables S1-S2). In a natural setting, excessive nutrient loading favors the growth of fast-growing, ephemeral macroalgae such as Ulva due to the ability to rapidly assimilate and store nitrogenous nutrients (Fan et al. 2014; Liu et al. 2009; Naldi and Wheeler 1999; Pedersen and Borum 1997). In contrast, seagrasses generally dominate more oligotrophic estuaries due to their ability to acquire nutrients through their roots and to store N in their leaves, stems, and rhizomes for use (Pedersen and Borum 1992; Short and McRoy 1984; Valiela et al. 1997).
There was likely increased nutrient competition in the combined Ulva-Zostera treatment under high CO2 given the higher levels of total autotrophic biomass in this treatment and the faster growth rates for both species within this treatment. There were some signs of N-stress in Zostera in the combined Ulva-Zostera treatment under high CO2, as productivity and leaf and root N content were significantly reduced. Prior research has indicated that maximum growth of Zostera occurs at ammonium and nitrate concentrations of ~ 2 and ~ 3–4 μM, respectively (Zimmerman et al. 1987). During experiments, 5 μM of nitrate was added to each container daily and the final concentrations of ammonium in all vessels were consistently above 5 μM, data suggesting that the growth of Zostera was not fully N-limited. While it is plausible that the nitrate concentrations were rapidly depleted to < 2 μM due to uptake by Ulva each day and that the ammonium concentrations only rose to above 5 μM at the end of experiments, we do not have measurements to support such hypotheses. We emphasize that, in an ecosystem setting, when Ulva and Zostera compete under high CO2, nutrient-loading rates will likely not change and thus any nutrient competition that may have emerged within the Ulva-Zostera treatment under high CO2 would be likely to occur in estuaries as well. Nutrient levels added to each experimental vessel here were carefully chosen to be environmentally realistic and consistent with regional N loading rates (Kinney and Valiela 2011) but also to ensure N levels were not toxic (< 7 μM nitrate per day; Burkholder et al. 1992). Future studies with Ulva and Zostera that examine both changing N levels and changing CO2 levels will be able to provide a clearer sense of the extent to which Zostera inhibition by Ulva under high CO2 is caused by nutrient competition, among other factors.
Macroalgae can indirectly inhibit the productivity of seagrass through changes in the biogeochemical environment (Hauxwell et al. 2001). While not measured in the present study, the accumulation of sulfides in sediments as the result of anoxia caused by the decomposition of macroalgal mats can decrease seagrass productivity and cause mortality (Koch et al. 2007). Additionally, as nutrient concentrations increase, the reduced dissolved O2 concentrations as a result of increased respiratory demands of rapidly growing macroalgae (i.e., Ulva) may increase energy costs for seagrass translocating oxygen between the above-ground structures and the roots (Cabello-Pasini et al. 2011; Hauxwell et al. 2001; Pregnall et al. 1984). While excessive NH4+ concentrations in the water column can also lower seagrass productivity, NH4+ concentrations rarely exceeded 10 μM during the present study, making it unlikely that macroalgae-driven NH4+ toxicity played a role in decreased Zostera productivity in the presence of Ulva (McGlathery et al. 1997; Valiela et al. 1997). Finally, given that Ulva spp. have been shown to produce allelochemicals that inhibit the growth of microalgae (Nan et al. 2004; Tang and Gobler 2011), it is plausible that the inhibition of Zostera by Ulva was mechanistically facilitated via allelopathy, which has been reported in a recent study by Alexandre et al. (2017). Further, given the stronger inhibitory effects of Ulva on Zostera under elevated pCO2, it is possible that allelochemical production was strengthened under high pCO2 perhaps because the active allelochemicals are C-rich compounds (Fajer et al. 1992; Hattenrath-Lehmann et al. 2015).
Consistent with prior studies of macroalgae, the tissue C and N content of Ulva was mostly unaffected by changes in CO2 concentration (Gordillo et al. 2001; Young and Gobler 2016, 2017) nor was it affected by competition with Zostera. However, the leaf and rhizome C of Zostera was responsive to changes in CO2 concentration. On average, leaf and rhizome C was significantly increased when exposed to elevated CO2 concentrations. This increase in leaf and rhizome C content may be due to an increase in non-structural carbohydrates, which is consistent with other studies on the response of seagrasses to elevated CO2 levels (Jiang et al. 2010; Zimmerman et al. 1995; Zimmerman et al. 1997). Seagrasses, like many terrestrial C3 plants, can store carbohydrates when supply exceeds demand (Campbell and Fourqurean 2013), and use the carbohydrates for various functions, such as growth, lost tissue replacement, and for defensive compounds (Campbell and Fourqurean 2013; Chapin III et al. 1990; Dawes and Lawrence 1979). In the context of the present study, increased leaf and rhizome C may have been a consequence of increased areal productivity and above- and below-ground production from elevated CO2 concentrations. These trends also account for the significantly higher leaf C:N was under elevated CO2 levels. These complex changes in the macroelemental content of Zostera when exposed to elevated CO2 concentrations could have important implications for their palatability to herbivores (Arnold et al. 2012; Stiling and Cornelissen 2007) as many anti-feeding compounds are C-rich compounds (Fajer et al. 1992).
The ~ 20% increase in the organic C of the Zostera-bearing sediments when exposed to elevated CO2 concentrations has not been previously reported but has a series of important implications. The present study, along with prior studies (Jiang et al. 2010; Palacios and Zimmerman 2007), have shown that elevated CO2 increases the below-ground biomass production of seagrasses as well as rhizome C content when exposed to elevated CO2 concentrations. Organic C released by seagrass production, as well as the leakage of photosynthates by the rhizomes can influence sediment sulfate reduction, N fixation, and bacterial activity (Hansen et al. 2000; Pollard and Moriarty 1991; Welsh 2000). The accelerated consumption of oxygen by organic carbon-fueled microbial respiration can create an anaerobic environment within sediments which, when coupled with the high concentration of sulfate in seawater, provides a more suitable environment for sulfate-reducing bacteria, which account for more than 50% of organic C fixation in marine sediments (Moriarty et al. 1985; Pollard and Moriarty 1991; Welsh 2000). While sulfate-reducing bacteria play an important role in maintaining a suitable biogeochemical environment in seagrass-inhabited sediments (Pollard and Moriarty 1991), continued accumulation of sulfides in the sediments can harm seagrasses. Holmer et al. (2005) found that sulfides intruding into the below-ground structures of Z. marina can be re-oxidized into elemental sulfur which, after continued accumulation, can degrade seagrass meristems and cause mortality. Additionally, Goodman et al. (1995) found that increased sulfides in sediments containing Z. marina significantly reduced Pmax, increased the light requirement for photosynthesis to equal respiration, and decreased the initial slope of the PI curve. Hence, elevated CO2 concentrations that increase below-ground production significantly increase sediment organic C, which could ultimately stunt the growth and photosynthetic abilities of seagrass meadows. The relative effect of excess organic matter production within sediments on seagrass exposed to elevated CO2 will likely be influenced by the initial sediment composition as well as the amount of oxygen transported to seagrass rhizomes that could offset some of the negative impacts. Regardless, it is possible that prior studies that have assessed the effects of elevated CO2 concentrations on Zostera, but not sediments, may have overestimated the long-term benefits by not considering changes to sediment biogeochemistry.
The overgrowth of seagrass beds by macroalgae in high CO2 settings can have a variety of ecosystem-wide consequences. Temperate seagrass meadows have high species richness, host a high abundance of invertebrate species, are used as nurseries by numerous species of juvenile shellfish (Heck Jr. et al. 1995) and crustaceans (Heck and Thoman 1984; Perkins-Visser et al. 1996), and often serve as a habitat for demersal fish to brood or produce eggs (Blanc and Daguzan 1998; Francour 1997). The overgrowth of macroalgae associated with rising levels of CO2 can decrease seagrass shoot density, recruitment, and growth (Hauxwell et al. 2001) as well as result in shifts in trophic interactions, including the loss of invertebrates and fish that rely on seagrass for food, cover, and as nurseries (McGlathery 2001). Secondary metabolites released by Ulva can also directly cause mortality in some invertebrates (Johnson and Welsh 1985; Magre 1974; Nelson et al. 2003) and larval fish (Johnson 1980). The extent to which the synthesis of such compounds may be altered via exposure to excessive CO2 has yet to be determined.
In conclusion, while both Ulva and Zostera may experience growth benefits when exposed to high, but realistic, levels of pCO2, such benefits for Zostera may be offset by Ulva both directly (shading: Valiela et al. 1997; competition for N: Duarte 1995; allelopathy: Alexandre et al. 2017) and indirectly (changing the biogeochemical environment: Hauxwell et al. 2001). Elevated CO2 increased below-ground production and rhizome C content of Zostera, but such increased below-ground production may cause long-term harm for Zostera as increases in sediment organic C may promote sulfide toxicity. Finally, despite the benefits that Zostera gains from elevated CO2, rapidly growing macroalgae such as Ulva have a growth advantage in eutrophic estuaries and, as the results of the present study suggest, may lower the productivity of Zostera. To date, shifts in the dominance of macroalgae over seagrasses in estuaries have been primarily attributed to nutrient overloading and light limitation. This study demonstrates that in estuaries where Ulva and Zostera co-exist and compete, climate change and eutrophication-driven increases in pCO2 are likely to be important in promoting the dominance of Ulva over Zostera.
References
Alexandre, A., A. Baeta, A.H. Engelen, and R. Santos. 2017. Interactions between seagrasses and seaweeds during surge nitrogen acquisition determine interspecific competition. Scientific Reports 7 (1): 13651.
Arnold, T., C. Mealy, H. Leahey, A.W. Miller, J.M. Hall-Spencer, M. Milazzo, and K. Maers. 2012. Ocean acidification and the loss of phenolic substances in marine plants. PLoS One 7 (4): e35107.
Badger, M. 2003. The role of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynthesis Research 77: 83–94.
Baumann, H., R.B. Wallace, T. Tagliaferri, and C.J. Gobler. 2015. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries and Coasts 38 (1): 220–231.
Beer, S. 1989. Photosynthesis and photorespiration of marine angiosperms. Aquatic Botany 34: 153–166.
Beer, S., and E. Koch. 1996. Photosynthesis of marine macroalgae and seagrasses in globally changing CO2 environments. Marine Ecology Progress Series 141: 199–204.
Beer, S., and J. Rehnberg. 1997. The acquisition of inorganic carbon by the seagrass Zostera marina. Aquatic Botany 56: 277–283.
Beer, S., and R.G. Wetzel. 1982. Photosynthetic carbon fixation pathways in Zostera marina and three Florida seagrasses. Aquatic Botany 13: 141–146.
Björk, M., K. Haglund, Z. Ramazanov, and M. Pedersén. 1993. Inducible mechanisms for HCO3 − utilization and repression of photorespiration in protoplasts and thalli of three species of Ulva (Chlorophyta). Journal of Phycology 29: 166–173.
Blanc, A., and J. Daguzan. 1998. Artificial surfaces for cuttlefish eggs (Sepia officinalis L.) in Morbihan Bay, France. Fisheries Research 38 (3): 225–231.
Bulthuis, D.A. 1987. Effects of temperature on photosynthesis and growth of seagrasses. Aquatic Botany 27 (1): 27–40.
Burkholder, J.M., K.M. Mason, and H.B. Glasgow Jr. 1992. Water-column nitrate enrichment promotes decline of eelgrass Zostera marina: Evidence from seasonal mesocosm experiments. Marine Ecology Progress Series 81: 163–178.
Cabello-Pasini, A., V. Macías-Carranza, R. Abdala, N. Korbee, and F.L. Figueroa. 2011. Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida (Chlorophyta). Journal of Applied Phycology 23: 363–369.
Cai, W.-J., X. Hu, W.-J. Huang, M.C. Murrell, J.C. Lehrter, S.E. Lohrenz, W.-C. Chou, et al. 2011. Acidification of subsurface coastal waters enhanced by eutrophication. Nature Geoscience 4: 766–770.
Cai, W.-J., W.-J. Huang, G.W. Luther, D. Pierrot, M. Li, J. Testa, M. Xue, et al. 2017. Redox reactions and weak buffering capacity lead to acidification in the Chesapeake Bay. Nature Communications 8 (1): 369.
Caldeira, K., and M.E. Wickett. 2003. Oceanography: Anthropogenic carbon and ocean pH. Nature 425: 365.
Caldeira, K., and M. E. Wickett. 2005. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. Journal of Geophysical Research: Oceans 110:C09S04.
Campbell, J.E., and J.W. Fourqurean. 2013. Effects of in situ CO2 enrichment on the structural and chemical characteristics of the seagrass Thalassia testudinum. Marine Biology 160: 1465–1475.
Chapin, F.S., III, E.-D. Schulze, and H.A. Mooney. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447.
Cornwall, C.E., C.D. Hepburn, D. Pritchard, K.I. Currie, C.M. McGraw, K.A. Hunter, and C.L. Hurd. 2012. Carbon-use strategies in macroalgae: Differential responses to lowered pH and implications for ocean acidification. Journal of Phycology 48 (1): 137–144.
Davis, B.C., and J.W. Fourqurean. 2001. Competition between the tropical alga, Halimeda incrassat, and the seagrass, Thalassia testudinum. Aquatic Botany 71: 217–232.
Dawes, C.J., and J.M. Lawrence. 1979. Effects of blade removal on the proximate composition of the rhizome of the seagrass Thalassia testudinum banks ex könig. Aquatic Botany 7: 255–266.
Dennison, W.C., and R.S. Alberte. 1982. Photosynthetic responses of Zostera marina L. (eelgrass) to in situ manipulations of light intensity. Oecologia 55: 137–144.
Duarte, C.M. 1995. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41 (1): 87–112.
Durako, M.J. 1993. Photosynthetic utilization of CO2 (aq) and HCO3 in Thalassia testudinium (Hydrocharitacae). Marine Biology 115 (3): 373–380.
Fajer, E.D., M.D. Bowers, and F.A. Bazzaz. 1992. The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. The American Naturalist 140 (4): 707–723.
Fan, X., D. Xu, Y. Wang, X. Zhang, S. Cao, S. Mou, and N. Ye. 2014. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: Implications for the explosion in green tides. Journal of Applied Phycology 26: 537–544.
Foster, G.L., D.L. Royer, and D.J. Lunt. 2017. Future climate forcing potentially without precedent in the last 420 million years. Nature Communications 8: 14845.
Francour, P. 1997. Fish assemblages of Posidonia oceanica beds at port-Cros (France, NW Mediterranean): Assessment of composition and long-term fluctuations by visual census. Marine Ecology 18 (2): 157–173.
Furman, B.T., and B.J. Peterson. 2015. Sexual recruitment in Zostera marina: Progress toward a predictive model. PLoS One 10 (9): e0138206.
Gao, K., and K.R. McKinley. 1994. Use of macroalgae for marine biomass production and CO2 remediation: A review. Journal of Applied Phycology 6: 45–60.
Gazeau, F., C. Quiblier, J.M. Jansen, J.-P. Gattuso, J.J. Middelburg, and C.H.R. Heip. 2007. Impact of elevated CO2 on shellfish calcification. Geophysical Research Letters 34: L07603.
Goodman, J.L., K.A. Moore, and W.C. Dennison. 1995. Photosynthetic responses of eelgrass (Zostera marina) to light and sediment sulfide in a shallow barrier island lagoon. Aquatic Botany 50: 37–47.
Gordillo, F.J.L., F.X. Niella, and F.L. Figueroa. 2001. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213: 64–70.
Hansen, J.W., J.W. Udy, C.J. Perry, W.C. Dennison, and B.A. Lomstein. 2000. Effect of the seagrass Zostera capricorni on sediment microbial processes. Marine Ecology Progress Series 199: 83–96.
Hattenrath-Lehmann, T.K., J.L. Smith, R.B. Wallace, L.R. Merlo, F. Koch, H. Mittelsdorf, J.A. Goleski, D.M. Anderson, and C.J. Gobler. 2015. The effects of elevated CO2 on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. Limnology and Oceanography 60: 198–214.
Hauxwell, J., J. Cebrian, C. Furlong, and I. Valiela. 2001. Macroalgal canopies contribute to eelgrass (Zostera marina) decline in temperate estuarine ecosystems. Ecology 82 (4): 1007–1022.
Hauxwell, J., J. Cebrian, and I. Valiela. 2006. Light dependence of Zostera marina annual growth dynamics in estuaries subject to different degrees of eutrophication. Aquatic Botany 84 (1): 17–25.
Heck, K.L., Jr., K.W. Able, C.T. Roman, and M.P. Fahay. 1995. Composition, abundance, biomass, and production of macrofauna in a New England estuary: Comparisons among eelgrass meadows and other nursery habitats. Estuaries 18 (2): 379–389.
Heck, K.L., and T.A. Thoman. 1984. The nursery role of seagrass meadows in the upper and lower reaches of the Chesapeake Bay. Coastal and Estuarine Research Federation 7 (1): 70–92.
Hemminga, M. A., and M. A. Mateo. 1996. 3. Marine Ecology Progress Series 140:285–298.
Henley, W.J. 1992. Growth and photosynthesis of Ulva rotundata (Chlorophyta) as a function of temperature and square wave irradiance in indoor culture. Journal of Phycology 28 (5): 625–634.
Henley, W.J. 1993. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. Journal of Phycology 29 (6): 729–739.
Hepburn, C.D., D.W. Pritchard, C.E. Cornwall, R.J. McLeod, J. Beardall, and J.A. Raven. 2011. Diversity of carbon use strategies in a kelp forest community: Implications for a high CO2 ocean. Global Change Biology 17 (7): 2488–2497.
Hofmann, L.C., J.C. Nettleton, C.D. Neefus, and A.C. Mathieson. 2010. Cryptic diversity of Ulva (Ulvales, Chlorophyta) in the Great Bay estuarine system (Atlantic USA): Introduced and indigenous distromatic species. European Journal of Phycology 45 (3): 230–239.
Hofmann, L.C., S. Straub, and K. Bischof. 2012. Competition between calcifying and noncalcifying temperate marine macroalgae under elevated CO2 levels. Marine Ecology Progress Series 464: 89–105.
Holmer, M., M.S. Frederiksen, and H. Møllegaard. 2005. Sulfur accumulation in eelgrass (Zostera marina) and effect of sulfur on eelgrass growth. Aquatic Botany 81: 367–379.
Invers, O., M. Pérez, and J. Romero. 1999. Bicarbonate utilization in seagrass photosynthesis: Role of carbonic anhydrase in Posidonia oceanica (L.) Delile and Cymodocea nodosa (Ucria) Ascherson. Journal of Experimental Marine Biology and Ecology 235 (1): 125–133.
Israel, A., and M. Hophy. 2002. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biology 8: 831–840.
Jiang, Z.J., X.-P. Huang, and J.-P. Zhang. 2010. Effects of CO2 enrichment on photosynthesis, growth, and biochemical composition of seagrass Thalassia hemprichii (Ehrenb.) aschers. Journal of Integrative Plant Biology 52 (10): 904–913.
Johnson, D. A. 1980. Effects of phytoplankton and macroalgae on larval and juvenile winter flounder culture. University of Rhode Island, Kingston, Rhode Island, U.S.A.
Johnson, D.A., and B.L. Welsh. 1985. Detrimental effects of Ulva lactuca (L.) exudates and low oxygen on estuarine crab larvae. Journal of Experimental Marine Biology and Ecology 86 (1): 73–83.
Kinney, E.L., and I. Valiela. 2011. Nitrogen loading to great South Bay: Land use, sources, retention, and transport from land to bay. Journal of Coastal Research 27 (4): 672–686.
Kirkendale, L., G.W. Saunders, and P. Winberg. 2013. A molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. Journal of Phycology 49 (1): 69–81.
Koch, M., G. Bowes, C. Ross, and X.-H. Zhang. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology 19: 103–132.
Koch, M.S., S. Schopmeyer, C. Kyhn-Hansen, and C.J. Madden. 2007. Synergistic effects of high temperature and sulfide on tropical seagrasses. Journal of Experimental Marine Biology and Ecology 341: 91–101.
Kroeker, K.J., F. Micheli, and M.C. Gambi. 2013. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nature Climate Change 3: 156–159.
Liu, D., J.K. Keesing, Q. Xing, and P. Shi. 2009. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Marine Pollution Bulletin 58 (6): 888–895.
Maberly, S.C., J.A. Raven, and A.M. Johnston. 1992. Discrimination between 12C and 13C by marine plants. Oecologia 91 (4): 481–492.
Magre, E.J. 1974. Ulva lactuca L. negatively affects Balanus balanoides (L.) (Cirripedia Thoracica) in tidepools. Crustaceana 27 (3): 231–234.
McGlathery, K.J. 2001. Macroalgal blooms contribute to the decline of seagrass in nutrient-enriched coastal waters. Journal of Phycology 37 (4): 453–456.
McGlathery, K.J., D. Krause-Jensen, S. Rysgaard, and P.B. Christensen. 1997. Patterns of ammonium uptake within dense mats of the filamentous macroalga Chaetomorpha linum. Aquatic Botany 59: 99–115.
Meehl, G.A., T.F. Stocker, W.D. Collins, P. Friedlingstein, A.T. Gaye, J.M. Gregory, A. Kitoh, et al. 2007. Global climate projections. In Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, ed. S.D. Solomon, Manning M. Qin, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor, and H.L. Miller. Cambridge: Cambridge University Press.
Melzner, F., T. Jörn, W. Koeve, A. Oschlies, M.A. Gutowska, H.W. Bange, H.P. Hansen, and A. Körtzinger. 2013. Future ocean acidification will be amplified by hypoxia in coastal habitats. Marine Biology 160: 1875–1888.
Mercado, J.M., F.J.L. Gordillo, F.X. Niella, and F.L. Figueroa. 1998. External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. Journal of Experimental Marine Biology and Ecology 221: 209–220.
Millero, F.J. 2010. History of the equation of state of seawater. Oceanography 23 (3): 18–33.
Mook, W.G., J.C. Bommerson, and W.H. Staverman. 1974. Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth and Planetary Science Letters 22: 169–176.
Moriarty, D.J.W., P.I. Boon, J.A. hansen, and D.C. White. 1985. Microbial biomass and productivity in seagrass beds. Geomicrobiology 4 (1): 21–51.
Naldi, M., and P.A. Wheeler. 1999. Changes in nitrogen pools in Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta) under nitrate and ammonium enrichment. Journal of Phycology 35 (1): 70–77.
Nan, C., H. Zhang, and G. Zhao. 2004. Allelopathic interactions between the macroalga Ulva pertusa and eight microalgal species. Journal of Sea Research 52 (4): 259–268.
Nelson, T.A., D.J. Lee, and B.C. Smith. 2003. Are “green tides” harmful algal blooms? Toxic properties of water-soluble extracts from two bloom-forming macroalgae, Ulva fenestrate and Ulvaria obscura (Ulvophyceae). Journal of Phycology 39: 874–879.
Olischläger, M., I. Bartsch, L. Gutow, and C. Wiencke. 2013. Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycological Research 61 (3): 180–190.
Palacios, S.L., and R.C. Zimmerman. 2007. Response of eelgrass Zostera marina to CO2 enrichment: Possible impacts of climate change and potential for remediation of coastal habitats. Marine Ecology Progress Series 344: 1–13.
Parsons, T.R., Y. Maita, and C.M. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. Oxford: Pergamon Press.
Pedersen, M.F., and J. Borum. 1992. Nitrogen dynamics of eelgrass Zostera marina during a late summer period of high growth and low nutrient availability. Marine Ecology Progress Series 80: 65–73.
Pedersen, M.F., and J. Borum. 1997. Nutrient control of estuarine macroalgae: Growth strategy and the balance between nitrogen requirements and uptake. Marine Ecology Progress Series 161: 155–163.
Perkins-Visser, E., T.G. Wolcott, and D.L. Wolcott. 1996. Nursery role of seagrass beds: Enhanced growth of juvenile blue crabs (Callinectes sapidus Rathbun). Journal of Experimental Marine Biology and Ecology 198 (2): 155–173.
Pollard, P.C., and D.J.W. Moriarty. 1991. Organic carbon decomposition, primary and bacterial productivity, and sulphate reduction, in tropical seagrass beds of the Gulf of Carpentaria, Australia. Marine Ecology Progress Series 69: 149–159.
Porzio, L., M.C. Buia, and J.M. Hall-Spencer. 2011. Effects of ocean acidification on macroalgal communities. Journal of Experimental Marine Biology and Ecology 400 (1): 278–287.
Pregnall, A.M., R.D. Smith, T.A. Kursar, and R.S. Alberte. 1984. Metabolic adaptation of Zostera marina (eelgrass) to diurnal periods of root anoxia. Marine Biology 83 (2): 141–147.
Raven, J.A., M. Giordano, J. Beardall, and S.C. Maberly. 2011. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynthesis Research 109 (1): 281–296.
Raven, J.A., A.M. Johnston, J.E. Kübler, R. Korb, S.G. McInroy, L.L. Handley, C.M. Scrimgeour, et al. 2002. Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Functional Plant Biology 29 (3): 355–378.
Sand-Jensen, K. 1988. Minimum light requirements for growth in Ulva lactuca. Marine Ecology Progress Series 50: 187–193.
Sharp, J.H. 1974. Improved analysis for particulate organic carbon and nitrogen from seawater. Limnology and Oceanography 19: 984–989.
Short, F.T., and P.C. McRoy. 1984. Nitrogen uptake by leaves and roots of the seagrass Zostera marina L. Botanica Marina 27 (12): 547–556.
Stiling, P., and T. Cornelissen. 2007. How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Global Change Biology 13 (9): 1823–1842.
Talmage, S.C., and C.J. Gobler. 2010. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proceedings of the National Academy of Sciences of the United States of America 107 (40): 17246–17251.
Tang, Y.Z., and C.J. Gobler. 2011. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 10 (5): 480–488.
Tang, Y.Z., Y. Kang, D. Berry, and C.J. Gobler. 2015. The ability of the red macroalga, Porphyra purpurea (Rhodophyceae) to inhibit the proliferation of seven common harmful microalgae. Journal of Applied Phycology 27 (1): 531–544.
Touchette, B.W., and J.M. Burkholder. 2000. Overview of the physiological ecology of carbon metabolism in seagrasses. Journal of Experimental Marine Biology and Ecology 250: 169–205.
Valiela, I., J. McClelland, J. Hauxwell, P.J. Behr, D. Hersh, and K. Foreman. 1997. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42: 1105–1118.
Vizzini, S., A. Tomasello, G.D. Maida, M. Pirrotta, A. Mazzola, and S. Calvo. 2010. Effect of explosive shallow hydrothermal vents on δ13C and growth performance in the seagrass Posidonia oceanica. Journal of Ecology 98: 1284–1291.
Wall, C.C., B.J. Peterson, and C.J. Gobler. 2008. Facilitation of seagrass Zostera marina productivity by suspension-feeding bivalves. Marine Ecology Progress Series 357: 165–174.
Wallace, R.B., H. Baumann, J.S. Grear, R.C. Aller, and C.J. Gobler. 2014. Coastal Ocean acidification: The other eutrophication problem. Estuarine, Coastal and Shelf Science 148: 1–13.
Welsh, D.T. 2000. Nitrogen fixation in seagrass meadows: Regulation, plant-bacteria interactions and significance to primary productivity. Ecology Letters 3: 58–71.
Xu, Z., D. Zou, and K. Gao. 2010. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Botanica Marina 53 (2): 123–129.
Young, C.S., and C.J. Gobler. 2016. Ocean acidification accelerates the growth of two bloom-forming, estuarine macroalgae. PLoS One 11 (5): e0155152.
Young, C.S., and C.J. Gobler. 2017. The organizing effects of elevated CO2 on competition among estuarine primary producers. Scientific Reports 7: 7667.
Zieman, J.C. 1974. Methods for the study of the growth and production of turtle grass, Thalassia testudinum König. Aquaculture 4: 139–143.
Zimmerman, R.C., D.G. Kohrs, D.L. Steller, and R. Alberte. 1995. Sucrose partitioning in Zostera marina L. in relation to photosynthesis and the daily light-dark cycle. Plant Physiology 108: 1665–1671.
Zimmerman, R.C., D.G. Kohrs, D.L. Steller, and R.S. Alberte. 1997. Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant Physiology 115: 599–607.
Zimmerman, R.C., R.D. Smith, and R.S. Alberte. 1987. Is growth of eelgrass nitrogen limited? A numerical simulation of the effects of light and nitrogen on the growth dynamics of Zostera marina. Marine Ecology Progress Series 41: 167–176.
Zimmerman, R.C., R.D. Smith, and R.S. Alberte. 1989. Thermal acclimation and whole-plant carbon balance in Zostera marina L. (eelgrass). Journal of Experimental Marine Biology and Ecology 130 (2): 93–109.
Acknowledgements
We gratefully acknowledge the assistance of Jennifer Goleski during the analysis of dissolved nutrients during the study, and of members of Bradley Peterson’s laboratory in the retrieval of the Zostera shoots. We thank David Hirschberg of the School of Marine and Atmospheric Sciences (Stony Brook) for the elemental composition analysis of rhizomes. We are appreciative of the logistical support provided by the Stony Brook Southampton Marine Science Center staff throughout this study.
Funding
Funding for these experiments was obtained from the New York Sea Grant (award #R-FMB-38), the Laurie Landeau Foundation, the Simmons Foundation, and the Chicago Community Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ken Dunton
Electronic supplementary material
ESM1
(DOCX 108 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Young, C.S., Peterson, B.J. & Gobler, C.J. The Bloom-Forming Macroalgae, Ulva, Outcompetes the Seagrass, Zostera marina, Under High CO2 Conditions. Estuaries and Coasts 41, 2340–2355 (2018). https://doi.org/10.1007/s12237-018-0437-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0437-0