Abstract
Fjords and estuaries exchange large amounts of solutes, gases, and particulates between fluvial and marine systems. These exchanges and their relative distributions of compounds/particles are partially controlled by stratification and water circulation. The spatial and vertical distributions of N2O, an important greenhouse gas, along with other oceanographic variables, are analyzed from the Reloncaví estuary (RE) (~41° 30′ S) to the gulf of Corcovado in the interior sea of Chiloé (43° 45′ S) during the austral winter. Freshwater runoff into the estuary regulated salinity and stratification of the water column, clearly demarking the surface (<5 m depth) and subsurface layer (>5 m depth) and also separating estuarine and marine influenced areas. N2O levels varied between 8.3 and 21 nM (corresponding to 80 and 170 % saturation, respectively), being significantly lower (11.8 ± 1.70) at the surface than in subsurface waters in the Reloncaví estuary (14.5 ± 1.73). Low salinity and NO3 −, NO2 −, and PO4 3− levels, as well as high Si(OH)4 values were associated with low surface N2O levels. Remarkably, an accumulation of N2O was observed in the subsurface waters of the Reloncaví sound, associated with a relatively high consumption of O2. The sound is exposed to increasing anthropogenic impacts from aquaculture and urban discharge, occurring simultaneously with an internal recirculation, which leads to potential signals of early eutrophication. In contrast, within the interior sea of Chiloé (ISC), most of water column was quasi homohaline and occupied by modified subantarctic water (MSAAW), which was relatively rich in N2O (12.6 ± 2.36 nM) and NO3 − (18.3 ± 1.63 μM). The relationship between salinity, nutrients, and N2O revealed that water from the open ocean, entering into ISC (the Gulf of Corcovado) through the Guafo mouth, was the main source of N2O (up to 21 nM), as it gradually mixed with estuarine water. In addition, significant relationships between N2O excess vs. AOU and N2O excess vs. NO3 − suggest that part of N2O is also produced by nitrification. Our results show that the estuarine and marine waters can act as light source or sink of N2O to the atmosphere (air–sea N2O fluxes ranged from −1.57 to 5.75 μmol m−2 day−1), respectively; influxes seem to be associated to brackish water depleted in N2O that also caused a strong stratification, creating a barrier to gas exchange.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is an important trace atmospheric greenhouse gas, with a greater radiative efficiency than CO2 (298 times) (IPCC 2013), and a high capacity to weaken the ozone layer (Ravishankara et al. 2009). Since the industrial revolution, atmospheric N2O levels have increased from 270 to 320 ppb, at a ratio of 0.25–0.31 % year−1 (Battle et al. 1996; Thompson et al. 2004). Therefore, identifying the diverse natural sources of N2O is an important task in order to gain an understanding on their contribution to the overall global N2O budget.
N2O is mainly generated by nitrification through aerobic NH4 + oxidation (AAO) (Codispoti and Christensen 1985); moreover, certain species of nitrifying bacteria can produce N2O by means of NH4 + oxidation to NO2 −, which, in turn, is reduced to N2O by a process called nitrifier denitrification (Poth and Focht 1985). At low O2 concentrations, dissimilatory NO2 − reduction (called partial denitrification) to N2O (Codispoti and Christensen 1985) or dissimilatory NO3 − reduction to ammonium (DNRA) could be a relatively important N2O producing processes (Cole 1988). However, when O2 is low enough, a complete N oxide (e.g., NO3 −) reduction to N2 occurs, causing the consumption of N2O (Cohen and Gordon 1978; Codispoti and Christensen 1985). Both nitrification and denitrification processes demonstrate different responses to O2 concentration (Kock and Bange 2015) and depend, respectively, on NH4 + or organic matter as electron donors and O2 and NO3 − as electron acceptors. Subsequently, inconsistent levels of O2, NO3 −, and organic material (or ammonium as the degradation product), which are metabolized by nitrifying and/or denitrifying microorganisms, lead to spatially and temporally heterogeneous N2O production and emission across the air–sea interface.
Oceans are considered to be an important natural net source of atmospheric N2O and contribute to about 25 % of the total global emissions (Nevison et al. 1995; Bouwman et al. 1995; Zhang et al. 2010). Coastal zones, including estuaries and areas of coastal upwelling, are significant contributors to atmospheric N2O emissions (Cicerone and Orelamland 1998; Nevison et al. 1995; Musenze et al. 2014). Despite estuaries only representing 0.4 % of the overall area of the ocean, they contribute to approximately 33 % of the estimated oceanic N2O emissions (Bange et al. 1996; Seitzinger et al. 2000). However, it is necessary to validate these global estimates with local and regional measurements as estuarine and coastal waters are subjected to strong seasonal variations and intense eutrophication (Howarth et al. 1996; LaMontagne et al. 2003; Garnier et al. 2006).
Furthermore, areas influenced by agriculture and industry may have a relatively higher estuarine and coastal water contribution compared to other areas, due to the use of fertilizers and diverse organic chemicals (Howarth and Paerl 2008). Some studies indicate that estuaries are significant sources of N2O (De Bie et al. 2002; Zhang et al. 2010). However, not much information is currently available about greenhouse gas distribution in estuaries, gulfs, and fjords in the Southern Hemisphere, particularly those located in Chilean Patagonia.
The estuarine waters of the Reloncaví estuary (RE) and the interior sea of Chiloé (ISC) are situated in a region with relatively low anthropogenic impacts, due to very low population density, and therefore, it is possible to use this zone to identify natural source and sink processes involved in N2O cycling. However, since the early 1980s, between 41.5° and 42° S, intensive fish (mainly salmon farms), shellfish, and seaweed productions have occurred (Subpesca 2016), with the Reloncaví fjord being one of the first fjords used for salmon aquaculture in Chile (Buschmann et al. 2009). Despite the frequent utilization of fjords in aquaculture, their biogeochemical dynamics remain poorly studied. The main objective of this study is to evaluate the N2O distribution and its flux across the air–sea interface and to determine N2O consumption/production processes. In addition, the relationships between environmental variables (temperature, density, salinity, nutrients) in the Reloncaví estuary–sound and adjacent sea, in Northern Chilean Patagonia, are investigated.
Methodology
Hydrographic Setting of the Study Site
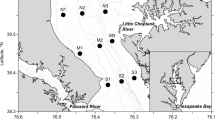
The study area is located between the Reloncaví estuary (41° 40′ S) and the Guafo mouth (43° 30′ S) located in the south of Chiloé Island (Northern Chilean Patagonian region). The sampling locations are shown in Fig. 1. The surrounding area has a complex topography and a coastline marked by canals, gulfs, and fjords. The area is sparsely populated; however, emerging aquaculture, fisheries, and tourism activities are taking place. The region is subject to a marked hydrographic variability, associated with regimes of high rainfall and temperature fluctuations (Castillo et al. 2016). The study area receives fresh water inputs from various rivers, including Puelo, Petrohué, and Cochamo (León-Muñoz et al. 2013).
The Reloncaví estuary has an overall length of 55 km and an average width of 2.8 km. The estuarine waters are connected directly to the Reloncaví Sound and indirectly to ISC. The ISC comprised the Gulf of Ancud, which is connected to the Pacific Ocean through the Chacao channel (to the north of Chiloe Island), and the Gulf of Corcovado, which is connected to the open ocean through the Guafo mouth (see Fig. 1). The fjord hydrography showed a relatively thin (<5 m depth), continuously stratified, buoyant layer with high stratification (Castillo et al. 2016). Below this thin layer, a relatively homogenous water of marine origin occupied the ISC. Estuarine circulation was represented by three-layer pattern currents; the thin upper outflow was relatively fast reaching 30 cm s−1 near the surface, below an intermediate inflow exists never exceeding 10 cm s−1, then the deep layer is thin and weak flowing at ∼1 cm s−1. This third layer (>80 m depth) is suggested to be a consequence of a tidal rectification of the flow (Valle-Levinson et al. 2007). This pattern may change seasonally, between two layers during winter to three layer during spring–summer. Estuarine waters gradually mix with marine waters, predominantly from subantarctic origin, described as modified subantarctic water (MSAAW), while subsurface equatorial water (ESSW) below 200 m depth influence the ISC (Sievers and Silva 2006).
Sampling
Sampling took place during the winter, from July 1 to July 20, 2013. The sampling was carried out on board the Chilean Navy oceanographic vessel AGS 61 Cabo de Hornos, as part of the multidisciplinary project CIMAR 19, under the supervision of the National Oceanographic Committee (Comité Oceanográfico Nacional [CONA]). Continuous profiles of temperature, salinity, and dissolved O2 were obtained using a CTD (SeaBird 25). Discrete water samples were collected in triplicate for N2O and nutrients (i.e., NO3 −, NO2 −, PO4 −3, Si(OH)4) using Niskin bottles mounted on an oceanographic rosette, over seven depths (0, 5, 10, 25, 50, 75, 100, and 200 m depth). The N2O samples were collected in 20-mL glass vials and preserved with 50 μL of saturated mercuric chloride (6 g L−1) and immediately sealed with hermetic stoppers and aluminum caps (Tilbrook and Karl 1995); this avoids the occurrence of gas bubbles and the contamination of vials by the atmosphere. N2O samples were stored at room temperature for subsequent analysis. In addition, water samples were collected for nutrient analysis in 15-mL falcon tubes, previously filtered through a 0.7-μm glass-fiber filter, and subsequently frozen until analysis.
Chemical Analysis
N2O was analyzed via the generation of a 5-mL ultra-pure helium headspace into the vial, using a gas-tight syringe, and then equilibrating the gas and liquid phases at 40 °C within the vial. N2O samples were analyzed via the headspace equilibrium method, with He in the vial (McAuliffe 1971), in a SHIMADZU GC-17A gas chromatograph equipped with an electron capture detector (ECD). Ar/CH4 gas mix was used as a carrier gas, with a flux of 6.5 mL min−1 and a temperature of 300 °C. The nutrient analysis was performed using colorimetric techniques, following the method in Grasshoff et al. (1983), with an autoanalyzer SEAL analytical (AA3).
Data Analysis
Taking into consideration that the winds (>10 m s−1) in the fjord valley mainly blow down fjord during the winter, reinforcing the upper layer outflow, and that wind velocities are higher than surface water current velocities or surface outflow (which is 5 m s−1, even at maximum tidal currents; Valle-Levinson et al. 2007), we did not include the latter in the estimation of gas transfer velocity (k w), but it may lead an underestimation of N2O fluxes in the fiord. Discrete data for N2O taking in the brackish layer in the RE and in the mixed layer of the ISC were used to determine gas fluxes at the air–sea interface (μmol day−1 m−2), using the following relationship:
where k w(m s−1) is the gas transfer velocity calculated from wind speed, cw is the concentration of dissolved gas in the water, and c sat is the atmosphere gas concentration. Water temperature and salinity in the upper layer in the cases of RE or in the mixed layer in the cases of ISC was used to calculate gas saturation concentrations. The calculation of k w was made using the parameterization of Nightingale et al. (2000):
where u is the wind speed (m s−1), and Sc is the Schmidt number (Wanninkhof 1992). Wind speed and direction data were obtained from the onboard meteorological station. A temperature-based criterion was used to determine the mixed layer depth from vertical profiles of temperature measured every 1 dbar using the CTD sensor. In the RE, temperature-based criterion also was used to determine the mixed layer for then estimate average N2O levels in the same (determined based on umbral method; Price et al. 1986; Peters et al. 1988; Lukas and Lindstrom 1991). With these calculations, it is possible to estimate N2O fluxes.
Saturation percentages of N2O were calculated using the measured N2O concentrations and those estimated to be in equilibrium with the current gas concentrations according to the atmosphere register (NOAA 2011). N2O solubility was estimated according to Weiss and Price (1980) based on in situ temperature and salinity. Delta or excess N2O, used to determine the apparent N2O production in water masses (Nevison et al. 1995, 2003), was estimated as:
where [N2O]in situ is the measured concentration of N2O, and [N2O]eq is the N2O levels in equilibrium with the atmosphere at the time of the last contact with the atmosphere.
The apparent oxygen utilization (AOU) was calculated from the O2 concentrations [O2]w, using the data collected during the cruise to calculate O2 saturation [O2]eq:
where [O2]w is the measured concentration of O2, and [O2]eq is the O2 levels in equilibrium.
Statistical analysis to check normality of the data was carried out using the Kolmogorov–Smirnov test. A non-parametric analysis was performed to examine the degree of association between variables. Spearman’s (rho) test was used to analyze correlations. Linear regressions were used to evaluate the relationships among ΔN2O, AOU, and NO3 −. Data were processed using R program. Spatial data were plotted using Ocean Data View.
Results
Distribution of Environmental Variables in the Water Column
A vertical cross section of temperature, salinity, Sigma-t, and O2 is shown in Fig. 2. Physical variables, mainly salinity, clearly separated the study area into two sections; those influenced by river discharge, including the Reloncaví estuary (RE) and sound (Sts. 7b, 7c, 6, 5, 4, 3, 8, and 9), and the marine area associated with the ISC, including the Gulf of Ancud and Gulf of Corcovado (Sts. 14, 16, 20, 21, 33, 36, and 38). Surface temperature fluctuated between 9 and 11.5 °C, with the lowest values in the RE and the highest in the ISC (Fig. 2a). In the RE, the vertical distribution of salinity demarked the presence of two layers, separated by a marked halocline, which produced a highly stratified system. A surface layer to 5 m and a subsurface layer from 5 to 200 m were identified (Fig. 2b). Surface salinity widely fluctuated between 2.53 and 32.2, with the lowest values at the surface of the Reloncaví estuary (<5 m depth), due to the predominant influence of freshwater from the Puelo River. In contrast, stations within the Gulf of Ancud and Corcovado (Sts. 16, 20, 21, 33, 36, 38) showed a quasi homohaline and homo density water column, extending from the surface to the bottom waters (Fig. 2b, c), with salinity values reaching 33. Remarkably, Sts. 16, 20, and 21 had a light diminishing in salinity in a surface think layer probably due to the influence of river discharge from the continent. The dissolved O2 fluctuated between 128 and 379 μM with the lowest concentrations in the RE and gradually increasing toward the ISC (Gulf of Corcovado) (Fig. 2d). Within the ISC, O2 distribution was homogenous throughout the water column, with values around 250 μM. Table 1 shows the average values, standard deviation, and ranges of temperature and salinity along with N2O, O2, and nutrient concentrations in the surface and subsurface estuarine area (RE) and throughout the entire water column in the marine area (ISC).
Spatial Distribution of Nitrous Oxide and Nutrients
The spatial distributions of N2O and nutrient concentrations are shown in Fig. 3. Regarding nutrient distributions, surface NO3 − varied between 1.70 and 24.9 μM, with the lowest concentrations in the surface layer of the RE and the highest concentrations toward the Gulf of Corcovado (Fig. 3a). In the ISC, NO3 − levels remained constant at around 20 μM, but increased with depth at around 26.6 μM, typical levels of subantarctic water (SAAW) (Fig. 3a). Low concentrations of NO2 − were registered from 0.03 to 0.45 μM in the surface water. In the stations with greatest exposure to marine influence, a slight increase in NO2 − was observed (Fig. 3b), particularly between Sts. 16 and 21 up to 25 m and between Sts. 32 and 38 from 50 to 150 m depth. Surface Si(OH)4 varied between 13.2 and 112 μM (Fig. 3c), with the lowest concentrations in the surface and subsurface layer of the ISC (Table 1). The highest values were found in the surface layer of the Reloncaví estuary averaging 61.8 ± 38.8 μM. Remarkably, a strong accumulation of Si(OH)4 was observed in the bottom water (<80 m depth) of Reloncaví estuary and the water column at the border of estuary con marine area (Sts. 14 and 16).
In addition, N2O concentration varied between 8.34 and 21.1 nM (corresponding to 80 % to 170 % saturation) with a mean ± SD value of 13.8 ± 2.01 nM for the estuarine and marine system (Fig. 3d; Table 1). Lower values were recorded in the surface layer (mean ± SD value 11.5 ± 1.30 nM) compared to the subsurface layer (mean ± SD value of 15 ± 1.4 nM). Undersaturated N2O levels, as low as 80 %, were only found in the surface layer between Sts. 7c and 14; these stations are located in the RE, the sound, and in the area immediately adjacent. However, within the subsurface layer of the RE and sound, an accumulation of N2O with levels of 14.5 nM (110 % saturation) was registered, which coincided with lower O2 concentrations (<150 μM). These were the lowest O2 concentrations registered in the study area (Table 1). In contrast, N2O in the Chiloe sea reached the highest concentration ~21.1 nM at St. 38, with a mean ± SD value of 12.6 ± 2.36 nM (Table 1). Within the ISC (from St. 14 to St. 33), vertical distribution of N2O was homogeneous throughout the water column; however, in the Gulf of Corcovado (Sts. 36 to 38), N2O concentrations increased with depth (below 100 m depth), indicating the influence of oceanic waters entering into the ISC through the Guafo mouth (Fig. 3d).
Scatter diagrams showed the relationship of N2O and nutrient contents vs. salinity in the surface layer in the RE (Fig. 4a, c) and throughout the entire water column (Fig. 4b, d). It was clearly discernible that most of the N2O content comes from more saline water, whereas the brackish waters were more depleted of this gas. A similar trend was observed for NO3 − and PO4 3− (not shown), but with Si(OH)4 vs. salinity, an inverse behavior was found (not shown), indicating an intense input coming from continental runoff.
N2O Exchange Across the Air–Sea Interface and Relationships Among Environmental Variables
The estimation of N2O air–sea fluxes based on surface N2O concentration and wind velocities ranged from −1.4 to 6.28 μmol m−2 day−1. N2O exchange across the air–sea interface along the RE estuary N2O varied between −1.57 and 55 μmol m−2 day−1 (mean ± SD = 0.86 ± 2.28). Negative fluxes were observed in the estuarine area near the river discharge zone (St. 7b) and in the Reloncaví sound (Sts. 3 and 8), whereas the highest N2O flux reaching 6.28 μmol m−2 day−1 was measured at St. 9, located on the border between the two areas under estuarine and marine influence. When this value was removed, the average flux in the RE was −0.03 ± 0.77 μmol m−2 day−1. N2O fluxes in the ISC (between Sts. 14 and 38) was on average 1.08 ± 1.41 with a range from −0.18 to 3.19 μmol m−2 day−1 being slightly higher than the RE. Sts. 16, 20, and 21 were the estimated N2O influxes and they probably were associated with thin surface layer of brackish water (Fig. 2a).
Table 2 shows the Spearman correlation analysis for environmental variables (temperature, salinity, O2, NO2 −, NO3 −, Si(OH)4, and N2O) separated into the two areas, those influenced by fresh water discharge or RE (Sts. from 7c to 9) and those with strong marine water influence or ISC (Sts. 14 to 38); the latter includes the entire water column, whereas the RE was separated in surface (<5 m depth) and subsurface layers. N2O correlated significantly and positively with salinity and also with NO3 − except in brackish water of the RE. Besides, N2O was negatively correlated with O2 and NO2 − except in surface water of the RE.
Discussion
Environmental Conditions in the Reloncaví Estuary
Our results were representative of an austral winter period in northern Chilean Patagonia that coincided with maximum precipitation in the area. Precipitation in the area fluctuates between 1800 and 3400 mm year−1, reaching maximum levels from June to August (Garreaud et al. 2013). The RE receives an annual water discharge of approximately 1100 m3 s−1 from various rivers, including Puelo (650 m3 s−1), Petrohué (255 m3 s−1), and Cochamo (20 m3 s−1) as well as the Canutillar hydroelectric plant (75.5 m3 s−1) (Castillo et al. 2016). The freshwater supply from the river discharges in the RE reached a maximum during June (León-Muñoz et al. 2013); thus, our sampling was carried out during periods of maximum freshwater discharge.
Freshwater discharge provokes a strong and permanent stratification as shown in Fig. 2. Indeed, the fjord was well stratified throughout the period and presented a narrow and permanent layer of brackish water. The depth of the upper layer slightly varied between seasons, remaining at about 4.5 m depth (Castillo et al. 2016). This suggests that stratification of the upper layer of the fjord is maintained seasonally, despite the occurrence of significant changes in river discharges and winds (Castillo et al. 2016). The presence of a sharp halocline clearly separates the surface layer with a relatively low temperature (9.1–11 °C) and salinity (2.5–32) from the subsurface layer, which has a relatively high temperature and salinity (Fig. 2a, b).
Stratification in the estuary impedes entrainment and vertical mixing between both layers. In fact, the estimated Richardson number (Ri < 0.25) in the area indicated a limited favorable condition for vertical mixing in the RE throughout the year (Castillo et al. 2012). It is important to note that the presence of a halocline also favors the accumulation of particles in the estuarine area, as observed by Farías (unpublished data). This may be due to a deceleration of particles as they sink caused by a strong salinity gradient. Thus, particle accumulation could generate microsites, where intense N remineralization could occur (Capone et al. 2008). Enoksson (1986) and Walter et al. (2006) recorded that the highest rates of nitrification in the Baltic Sea occurred below the halocline. This appears to also be the case in the RE, where nitrifying bacteria below the halocline may generate N2O (Fig. 3d).
Stratification and the estuarine circulation in the study area also control the nutrient and gas distribution. Nitrate-poor and silicate-rich brackish water is observed in the surface layer, while high salinity and nutrient-rich water (mainly NO3 − and PO4 3−) dominated the subsurface layer (Fig. 3a–c). A similar distribution of these variables has been reported by Silva et al. (1998) during the winter period in the RE. Our findings revealed that riverine and estuarine waters occupying the surface layer also have very low N2O concentrations, reaching undersaturated levels as low as 80 %. This coincided with the lowest levels of nutrients (NO3 − and PO4 3−), but highest concentrations of Si(OH)4, supporting the idea that Si(OH)4, may originate from the weathering of soils (Fig. 3c). The positive and negative correlations found between NO3 − and salinity and between Si(OH)4 and salinity in the estuarine area, respectively, suggest that water coming from the continental runoff input high levels of Si(OH)4 into the RE; however, this is not the case for N nutrient (Fig. 3a–c). Indeed, northern Chilean Patagonia is characterized by andosol-type soil affecting dissolved Si(OH)4 concentrations in the river system and explaining the particularly high regional rates of biogenic silica production (Vandekerkhove et al. 2016). Nitrogen fertilizers are not used on Patagonian soils (non-agricultural activities), and therefore, the N and P nutrient load should be very low in comparison with rivers and estuaries in the Northern Hemisphere, many of which are subjected to increasing levels of organic matter input and nutrient enrichment (Bange 2006a).
Rivers and estuaries account for approximately 20 % of the current global anthropogenic N2O emissions (Seitzinger and Kroeze 1998). For rivers and estuaries, approximately 90 % of N2O emissions are in the Northern Hemisphere, and China and India account for about 50 % of N2O emissions from rivers and estuaries (Beaulieu et al. 2011; Turner 2015). This pattern is consistent with the regional distribution of dissolved inorganic nitrogen export from rivers (Beaulieu et al. 2011). Indeed, most of these Northern Hemisphere ecosystems are affected by intense anthropogenic impacts, such as agriculture, that leads to eutrophic conditions with high nutrient (mainly NO3 −) and/or organic matter loading, which increases N2O content (Soetaert et al. 2006).
However, our findings showed undersaturated levels of N2O in brackish waters associated with low NO3− levels. It appears that the observed N2O undersaturation in the study area is not the result of overall denitrification in brackish water, since denitrification should not take place in well-oxygenated waters (Fig. 2d). Low N/P ratio, of around 5–6, observed in the surface layer of the RE indicates a nitrogen deficiency. As previously mentioned, denitrification is unlikely to occur; therefore, the low N/P ratio may be more suitably explained by NO3 − deficiency in freshwater inputs (Fig. 3b). The stratification present in the estuary also limits mixing and entrainment of nutrients from subsurface waters. This situation has been reported to create N limitation for photosynthesis (González et al. 2010), a situation persisting in most of Chilean Patagonian fjords, due to the presence of a low N/P ratio (<10) related to the theoretical (16:1) Redfield ratio (Iriarte et al. 2007; Labbé-Ibáñez et al. 2015). Thus, NO3 − and N2O deficiency associated with brackish water may occur in the numerous fjords within the Patagonian region. This pattern has been also observed in the Tubul–Raqui estuary, located in central Chile, where conditions of N2O undersaturation, or close to saturation was reported, acting as a N2O sink (Daniel et al. 2013).
Furthermore, low N2O levels (in the case that in situ N2O consuming process are occurring), indicated that the N2O producing process, such as nitrification or partial denitrification, is not significant in riverine/estuarine water due to low levels of dissolved organic matter and chlorophyll found in the RE. N2O production by nitrification or partial denitrification is primarily dependent on NH4 + and organic matter as electron donors and O2 and NO3 − as electron acceptors, respectively. Considering this, very low levels of fluorescent, dissolved organic carbon, chlorophyll-a, and also primary production were registered in the winter (González et al. 2010). Thus, these variables may also limit the processes involved on N2O generation in the water column.
One explanation for the very low observed N2O levels is that water depleted of N2O drains into the fjord due to an intense N2O consumption occurring in pore water soils adjacent to the estuary. Subsequently, this water leaches and runs off into the riverine/estuarine waters. The lack of any correlation of N2O with environmental variables in the surface layer of RE suggests that N2O has a continental origin (Table 2). Soils can be a source and sink for atmospheric N2O (Freney et al. 1979); although most of the time the soil acts as a net emitter, N2O consumption has been reported for several terrestrial ecosystems, mostly for wetlands and peatlands (Schlesinger 2013). N2O consumption has been attributed exclusively to denitrifiers possessing NosZ, the nitrous oxide reductase enzyme system catalyzing N2O to N2 reduction (Zumft 1997). Sanford et al. (2012) suggested that non-denitrifying populations with a broad range of metabolisms and habitats are potentially significant contributors to N2O consumption, therefore controlling N2O emissions. However, the mechanisms and controls of N2O consumption in soil are not clear, and various biochemical routes have been identified in addition to the classical reduction of N2O during denitrification (van Groenigen et al. 2015); these routes include dissimilative reduction, a direct assimilatory N2O fixation via nitrogenase (Vieten et al. 2008; Farías et al. 2013). Recently, Wu et al. (2013) reported a significant N2O consumption occurring under aerobic conditions, with a rising N2O consumption with increased soil moisture content, and the complete blockage of N2O consumption with the sterilization of oxic soil. This suggests that consumption has a biological origin.
In contrast to the surface water layer, the majority of high nutrients levels and N2O accumulation occur in the subsurface waters of the RE, which are isolated from the surface due to strong stratification. These nutrients are unable to establish contact with the atmosphere as similar to gases; they cannot diffuse into the surface water, not even through turbulent mixing. In addition, a marked accumulation of N2O was observed in the subsurface waters of the Reloncaví fjord (including the sound), associated with relatively low O2 levels (Fig. 3). Valle-Levinson et al. (2007) found a low (but non-critical) level of dissolved O2 concentration (>2 mL L−1) near the mouth of the fjord, however the impact of seasonal variation on biological resources remains unknown. This area is close to the city of Puerto Montt, where urban waste discharge and intense salmon aquiculture activities occur (Iriarte et al. 2010). Within the area, aquaculture activities routinely input fertilizers (mainly ammonium and urea) and generate feed additions (particles) below coastal salmon farms. This could potentially modulate microbial activities, the seasonal phytoplankton blooms, and stimulate the growth of harmful algal blooms (Arzul et al. 1999; Iriarte et al. 2005). Particle accumulation favors the existence of microniches and intensifies and stimulates organic matter mineralization, which are processes involved in N2O and NO3 − generation through nitrification. However, if O2 levels are sufficiently low, particle accumulation may generate anoxic/suboxic microniches that could produce N2O through denitrification (Ganesh et al. 2014). In addition, N/P ratios between 9 and 11 were observed in the subsurface water, similar to the adjacent ISC, indicating that these ratio signals originate from the Interior sea of Chiloé.
Environmental Condition in the Interior Sea of Chiloé
Marine waters associated with the ISC contain high levels of NO3 − as well as N2O (see Fig. 3). N2O and NO3 − accumulation may be related to preformed N2O and NO3 − from marine sources and/or in situ production in the water column via nitrification, given the observed oxygenation condition. It is important to note that marine waters enter into the ISC through the Guafo mouth and transition into the deeper layer reaching the mouth of the estuary, in agreement with the pathway proposed by Silva and Palma (2008). In the Pacific Ocean adjacent to Chiloe island (42.5° S, 74° W), the water mass distribution indicated the presence of subantarctic water (SAAW) in the first 100 m (salinity >33) extending from the coast and further offshore (2000 km). This water mass originated from the subantarctic and transports cold and high salinity water with relatively high O2 and NO3− content (Silva 1983). As the SAAW is advected into the ISC, it mixes with freshwater creating a water mass with salinities between 31 and 33, called modified subantarctic water (MSAAW) (Sievers and Silva 2006; Silva and Palma 2008). The MSAAW occupied most of the Chilean fjord region of the ISC (surface to 200 m depth) (Peréz-Santos et al. 2014). In fact, salinity distribution (Fig. 2a) revealed that the 31 isohaline, which designates the upper limit of the MSAAW, occupied most of the water column in the ISC. Below the MSAAW the presence of subsurface equatorial waters (ESSWs) was detected, which also enters from the south through the Guafo mouth. The ESSW from equatorial origin is clearly discernible by low O2 levels, which may reach anoxia close to its formation area near the Equator (Canfield et al. 2010). It carries a high N2O content, since this water is subjected to intense nitrification and denitrification processes due to O2 depletion that occurs during the movement of the water mass toward the south, through the Peru–Chile undercurrent (Farías et al. 2009; Cornejo and Farías 2012). Indeed, our results also showed an incipient signal of ESSW present in deeper waters at St. 38 (Fig. 3d). Thus, N2O distribution in the ISC should show a clear association between water mass distribution and hydrographic/oceanographic processes, since estuarine circulation dominates (Valle-Levinson et al. 2007). Scatter diagrams of N2O vs. salinity and NO3 − vs. salinity (Fig. 4a, b), and the estimated correlations between these variables (Table 2) confirm the marine origin of these compounds as the most plausible explanation.
Origin of N2O in an Area Subjected to Fluvial and Marine Influence
The excess of N2O (∆N2O) is one of the most relevant biogeochemical parameters in estimating N2O microbiological production. When ∆N2O is associated with AOU and NO3 −, it could indicate the biogeochemical N2O origin (Yoshinari 1976). Based on the correlation between ΔN2O and AOU and ΔN2O and NO3 − (Fig. 5), it is possible to estimate apparent N2O production as O2 is consumed (AOU), due to the remineralization of particulate organic matter associated to nitrification (Yoshinari 1976; Nevison et al. 2003). Moreover, the correlation between ΔN2O and NO2 −, which is a good index of suboxia/anoxia in OMZs (Codispoti and Christensen 1985; Cornejo and Farías 2012), can indicate the occurrence of denitrification.
Figure 5 shows linear relationships between ∆N2O vs. AOU and ∆N2O vs. NO3 − in the RE (Fig. 5a, b), in the ISC (Fig. 5c, d), and over the entire area (Fig. 5e, f). These relationships indicate that during the occurrence of oxidation of organic material (O2 consumption), NO3− is produced during nitrification along with N2O as a byproduct. Table 3 shows the slope obtained by linear regression analysis for ∆N2O vs. AOU, ∆N2O vs. NO3 − and its statistical significance. Differences in the slope were found when data was separated between the RE and the ISC, suggesting that physical processes (such as advection and mixing, which in turn affects gas solubility through temperature and salinity changes) and biogeochemical (nitrification) processes are differentially taking place, affecting N2O and NO3 − levels. The ratio of ΔN2O/AOU, which reflect N2O yield per mol of O2 consumed, fluctuated from 0.012 to 0.041 and was higher in the ISC than in the RE (Table 3). The ΔN2O/AOU ratios estimated over the entire area are relatively low compared to coastal areas (Bange 2006b; Nevison et al. 2003) but agree with the expected range compared to previously observed ratios in oceanic regions within cold temperate latitudes (Walter et al. 2006). The ∆N2O/NO3 − ratio ranged from 0.27 to 0.74, which was higher in the ISC (Table 3).
Since the slope between ∆N2O/AOU and ∆N2O/NO3 − superimposed physical and biogeochemical processes, it is important to separate the effect of in situ production from the production as a result of advection. Thus, it is important to estimate N2O supply by advection vs. the in situ production in the ISC.
We assume that the total N2O in the ISC is the sum of N2O production plus the offshore N2O supply from advection (Capelle et al. 2015), i.e.,
Since, we assume that N2O is transported along isopycnal surfaces from offshore water into the ISC, we would expect the N2O concentrations in the ISC, at a given isopycnal, to be equal to the offshore N2O concentrations at the same isopycnal in the absence of in situ production in the ISC, such that
We can rearrange Eq. (1) to solve for the amount of N2O produced over the ISC and substitute in N2O offshore yielding:
Figure 6 shows a meridional vertical distribution of N2O and O2 along Chilean coasts (Carrasco 2013). In front of Chiloé Island, the observed N2O and O2 levels along isopycnals belong to SAAW are lower in N2O and higher in O2 levels than those levels observed into the ISC’s water; therefore, the accumulation of N2O in the ISC is not associated with advection and/or isopycnal mixing of estuarine waters with SAAW. But ESSW, which is already in its southernmost boundary, has higher content of N2O as was seen by Cornejo and Farías (2012); if ESSW enters into ISC, it may provoke a diapycnal mixing which may be responsible for part of accumulated N2O.
N2O content in the ISC can be attributed to in situ nitrification during the transit of water masses into the ISC. Applying Eq. 2, most of N2O on the ISC was produced by nitrification in the water column and/or supplied by shelf sediments.
Nitrous Oxide Fluxes across the Air–Water Interface
Although rivers and estuaries do not make up a large area on a global scale, they are active sites for aquatic productivity and biogeochemical cycling. Indeed, air–sea N2O fluxes estimated in estuaries are relatively high in comparison to those estimated from the open ocean (EPA 2000). It has been estimated that estuaries and coastal areas contribute 35–60 % of all ocean N2O emissions (Bange 2006a). Although shallow water emissions are relatively high as a result of anthropogenic impacts on the N cycle, only a modest fraction of this N2O arises from natural N sources (Seitzinger et al. 2000). This is the reason by which they were classified as anthropogenic, rather than natural sources (Denman et al. 2007). There exist examples of European estuaries, such as Colde Estuary in England with a mean flux reaching 685.5 μmol N2O m−2 day−1 (Robinson et al. 1998) and the Siene Estuary in France with mean flux of 40 μmol N2O m−2 day−1 (Garnier et al. 2006), as well as Asian estuaries such as Chanjiang Estuary in China with mean flux of 87 μmol N2O m−2 day−1 (Zhang et al. 2008).
These fluxes are up to one or two orders of magnitude higher than the measurements in this study. The average flux in the RE measured within this study area is −0.034 ± 0.77 μmol m−2 day−1; this value is only comparable with rivers and estuarine waters without any anthropogenic influences (EPA 2000). Our findings showed that N2O exchange across the air–sea interface in estuarine and sound waters varied widely from −1.57 to 5.65 μmol m−2 day−1. In the Reloncaví estuary and some stations within the ISC (St. 16, 20, 21), conditions of N2O undersaturation were observed, and therefore, N2O influx was registered (up to 1.57 μmol m−2 day−1). N2O influx in the RE is likely to be associated with low NO3 − values coming from freshwater into the surface layer. Thus, the area under riverine and estuarine influence acted as a N2O sink or close to equilibrium with the atmosphere, Coincidently, Tubul–Raqui estuary located in the central area of Chile presented an averaged flux of −2.2 ± 1.3 μmol m−2 day−1 (Daniel et al. 2013). In contrast, the area under marine influences as the ISC behaved either as a light sink or as a light source; however, the magnitude of O2 exchange reflects the tropic conditions in surface waters. This estimate is very low in relation to estuaries with high organic matter discharge or surrounded by fertilized fields. In addition, the observed stratification that suppresses turbulence is predominantly associated to a positive buoyancy flux caused by the influence of freshwater discharge (MacIntyre et al. 2010). This buoyancy force competes with mixing via wind, hindering gas fluxes and isolating the surface from the subsurface water.
Conclusion
Low levels of N2O, and even N2O influx closer to estuarine waters, were registered in the RE. In this area low levels of N2O were associated with very low NO3 − levels but high Si(OH)4 along with oligotrophic and oxygenated conditions. Low N2O level does not cause partial denitrification in water.
Conversely, relatively high N2O concentrations were found in the subsurface layer (>5 m) of the RE; accumulation in the subsurface water layer of the estuary was associated with lower O2 concentrations, which seems to be the result of a relatively incipient eutrophication along with a relatively longer residence time of the fjord water.
In the ISC, N2O fluxes are variable (from sink to source), and N2O content reflects up to 60 % of those advected by SAAW from the open ocean to the ISC. Nitrification seems to be the most viable process in the production of N2O, acting over the entire study area, mainly in the subsurface layer of the RE and the ISC. In addition, the SAAW advection into the ISC has little influence in the N2O and NO3 − content in the ISC. However, the income of N2O and NO3 − rich waters from ESSW that enters into the ISC throughout Guafo mouth could provoke a diapycnal mixing being responsible for part of accumulated N2O.
References
Arzul, G., M. Seguel, I. Guzman, and E. Erard-LeDenn. 1999. Comparison of allelopathic properties in three toxic Alexandrium species. Journal of Experimental Marine Biology and Ecology 232: 285–295. doi:10.1016/S0022-0981(98)00120-8.
Bange, H.W., S. Rapsomanikis, and M.O. Andreae. 1996. Nitrous oxide emissions from the Arabian Sea. Geophysical Research Letters 23: 3175–3178.
Bange, H.W. 2006a. Nitrous oxide and methane in Europa coastal waters. Estuaries, Coastal and Shelf Science 70: 361–374.
Bange, H.W. 2006b. The importance of the oceanic nitrous oxide emissions. Atmospheric Environment 40: 198–199.
Battle, M., M. Bender, T. Sowers, P.P. Tans, J.H. Butler, J.W. Elkins, J.T. Ellis, T. Conway, N. Zhang, P. Lang, and A.D. Clarket. 1996. Atmospheric gas concentrations over the past century measured in air from firn at the South Pole. Nature 383: 231–235.
Beaulieu, J.J., W.D. Shuster, and J.A. Rebholz. 2011. Nitrous oxide emission from denitrification in stream and river networks. Proceedings of the National Academy of Sciences of the United States of America 108: 214–219.
Bouwman, A.F., K.W. Van der Hoek, and J.G.J. Olivier. 1995. Uncertainties in the global distribution of nitrous oxide. Journal of Geophysical Research 100: 2785–2800.
Buschmann, A.H., F. Cabello, K. Young, J. Carvajal, D.A. Varela, and L. Henriquez. 2009. Salmon aquaculture and coastal ecosystem health in Chile: analysis of regulations, environmental impacts and bioremediation systems. Ocean & Coastal and Management 52: 243–249.
Canfield, D.E., F.J. Stewart, B.L. Thamdrup, T. De Brabandere, E.F. Dalsgaard, N. Delong, P. Revsbech, and O. Ulloa. 2010. A Cryptic Sulfur Cycle in Oxygen-Minimum-Zone Waters off the Chilean Coast. Science 330: 1375–1378.
Capelle D.W, Dacey J.W. and P.D., Tortell. 2015. An automated, high through-put method for accurate and precise measurements of dissolved nitrous-oxide and methane concentrations in natural waters Limnology and Oceanography. Methods 13: 345–355. doi:10.1002/lom3.10029.
Capone, D.G., D. Bronk, M. Mulholland, and E.J. Carpenter. 2008. Nitrogen in the Marine Environment, 2nd edn. San Diego: Academic/Elsevier 1729 pp.
Carrasco, C. 2013. El rol del Agua Intermedia Antártica (AAIW) en la distribución de gases (O2y N2O) en el Pacífico Sur Oriental y su influencia en la ventilación de la zona de mínima de oxígeno. Tesis pata optar Programa de Magíster en Ciencias mención en Oceanografía. Universidad de Concepción, Concepción.
Castillo, M.I., O. Pizarro, U. Cifuentes, N. Ramírez, and L. Djurfeldt. 2012. Subtidal dynamics in a deep fjord of southern Chile. Continental Shelf Research 49: 73–89.
Castillo, M.I., U. Cifuentes, O. Pizarro, L. Djurfeldt, and M. Cáceres. 2016. Seasonal hydrography and surface outflow in a fjord with a deep sill: the Reloncaví fjord. Chile. Ocean Science 12: 533–544.
Cicerone, R.J., and R.S. Orelamland. 1998. Biogeochemical aspects of atmospheric methane. Global Biogeochemical Cycles 2: 299–327.
Codispoti, L.A., and J. Christensen. 1985. Nitrification, denitrification and nitrous oxide cycling in the eastern tropical South Pacific Ocean. Marine Chemistry 16: 277–300.
Cohen, Y., and L. Gordon. 1978. Nitrous oxide in the oxygen minimum of the eastern tropical North Pacific: evidence for its consumption during denitrification and possible mechanisms for its production. Deep Sea Research 25: 509–524.
Cole, J. A. 1988. Assimilatory and dissimilatory reduction of nitrate to ammonia. In The nitrogen and suphur cycles, eds. Cole & Fergurson, 161–218. Cambrige University Press.
Cornejo, M., and L. Farías. 2012. Following the N2O consumption in the oxygen minimum zone of the eastern South Pacific. Biogeosciences 9: 3205–3212.
Daniel, I., M. DeGrandpre, and L. Farías. 2013. Greenhouse gas emissions from Tubul-Raqui estuary (central Chile 36°S). Estuarine and Costal Shelf Sciences 134: 31–44. doi:10.1016/j.ecss.2013.09.019.
Denman, K.L., Brasseur, G., Chidthaisong, A., Ciais, P., Cox, P.M., Dickinson, R.E., Hauglustaine, D., Heinze, C., Holland, E., Jacob, D., Lohmann, U., Ramachandran, S., da Silva Dias, P.L., Wofsy, S.C. and X., Zhang. 2007. Couplings between changes in the climate system and biogeochemistry. In: Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M. & Miller, H.L. (eds.), Climate change. 2007. The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 499-587. http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter7.pdf. Accessed 18 Apr 2016.
De Bie, M.J.M., J.J. Middelburg, M. Starink, and H.J. Ladbroke. 2002. Factors controlling nitrous oxide at the microbial community and estuarine scale. Marine Ecology Progress Series 24: 1–9. doi:10.3354/meps240001.
Enoksson, V. 1986. Nitrification rates in the Baltic Sea: Comparison of three isotope techniques. Applied and Environmental Microbiology 51: 244–250.
Farías, L., M. Castro-González, M. Cornejo, J. Charpentier, J. Faúndez, N. Boontanon, and N. Yoshida. 2009. Denitrification and nitrous oxide cycling within the upper oxycline of the oxygen minimum zone off the eastern tropical South Pacific. Limnology and Oceanography 54: 132–144.
Farías, L., J. Faundez, C. Fernádez, M. Cornejo, S. Sanhueza, and C. Carrasco. 2013. Biological N2O fixation in the eastern South Pacific Ocean. PloS One 8(5): e63956.
Freney, J.R., O.T. Denmead, and J.R. Simpson. 1979. Nitrous oxide emission from soils at low moisture contents. Nature 273: 530–532.
Ganesh, S., D.J. Parris, E.F. DeLong, and F.J. Stewart. 2014. Metagenomic analysis of size-fractionated picoplankton in a marine oxygen minimum zone. The ISME Journal 1: 1–25.
Garnier, J., A. Cebron, G. Tallec, G. Billen, M. Sebilo, and A. Martinez. 2006. Nitrogen behaviour and nitrous oxide emission in the tidal Seine River estuary (France) as influenced by human activities in the upstream watershed. Biogeochemistry 77: 305–326.
Garreaud, R., P. Lopez, M. Minvielle, and M. Rojas. 2013. Large-scale control on the Patagonian climate. Journal of Climate 26: 215–230.
González, H.E., Calderón, M.J., Castro, L., Clément, A., Cuevas, L.A., Daneri, G., Iriarte, J.L., Lizárraga, L., Martínez, R., Menschel, E., N. N. Silva, Carrasco, C., Valenzuela, C., Vargas, C.A., and C. Molinet. 2010. Primary production and plankton dynamics in the Reloncaví Fjord and the interior Sea of Chiloé, Northern Patagonia. Marine Ecology Progress Series 402, 13–30.
Grasshoff, K., M. Ehrhardt, and K. Kremling. 1983. Methods of sea water analysis. Weinheim: Verlag Chem 63-97 pp.
Howarth, R.W., G. Billen, D. Swaney, A. Townsend, N. Jaworski, K. Lajtha, A. Downing, R. Elmgreen, N. Caraco, T. Jordan, F. Berendse, J. Freney, V. Kudeyarov, P. Murdoch, and Z. Zhao-liang. 1996. Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: Natural and human influences. Biogeochemistry 35: 181–226.
Howarth, R., and H.W. Paerl. 2008. Coastal marine eutrophication: control of both nitrogen and phosphorus is necessary. Proceedings of the National Academy of Sciences of the United States of America 105(49): E103.
IPCC. 2013. The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. T.F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P.M. Midgley. Cambridge University Press, Cambridge, United Kingdom 1535 pp.
Iriarte, J.L., R.A. Quiñones, and R.R. González. 2005. Relationship between biomass and enzymatic activity of a bloom-forming dinoflagellate (Dinophyceae) in the southern Chile (41° S): A field approach. Journal of Plankton Research. 27: 159–166.
Iriarte, J.L., H.E. González, K.K. Liu, C. Rivas, and C. Valenzuela. 2007. Spatial and temporal variability of chlorophyll and primary productivity in surface waters of southern Chile (41.5–43° S). Estuarine, Coastal and Shelf Science 74: 471–480.
Iriarte, J.L., H.E. González, and L. Nahuelhual. 2010. Patagonian Fjord Ecosystems in Southern Chile as a Highly Vulnerable Region: Problems and Needs. Ambio: A Journal of the Human Environment 39(7): 463–466.
Kock, A., and H.W. Bange. 2015. Counting the oceans greenhouse gas emissions. Eos 96: 10–13.
Labbé-Ibáñez, P., J.L. Iriarte, and S. Pantoja. 2015. Respuesta del microfitoplancton a la adición de nitrato y ácido silícico en fiordos de la Patagonia chilena. Latin American Journal of Aquatic Research 43(1): 80–93.
LaMontagne, M.G., R. Duran, and I. Valiela. 2003. Nitrous oxide sources and sinks in coastal aquifers and coupled estuarine receiving waters. The Science of the Total Environment 309: 139–149.
León-Muñoz, J., R. Marcé, and J.L. Iriarte. 2013. Influence of hydrological regime of an Andean river on salinity, temperature and oxygen in a Patagonia fjord. Chile. New Zealand Journal of Marine and Freshwater Research 47(30): 515–528.
Lukas, R., and E. Lindstrom. 1991. The mixed layer of the western equatorial Pacific Ocean. Journal of Geophysical Research 96: 3343–3358.
McAuliffe, C. 1971. Gas chromatographic determination of solutes by multiple phase equilibrium. Chemical Technology 1, 46–51pp.
MacIntyre, S., A. Jonsson, M. Jansson, J. Aberg, D.E. Turney, and S.D. Miller. 2010. Buoyancy flux, turbulence, and the gas transfer coefficient in a stratified lake. Geophysical Research Letters 37: L24604. doi:10.1029/2010GL044164.
Musenze, R.S., U. Werner, A. Grinham, J. Udy, and Z. Yuan. 2014. Methane and nitrous oxide emissions from a subtropical estuary (the Brisbane River estuary, Australia). Science of the Total Environment 472: 719–729.
Nevison, C., R. Weiss, and D.J. Erickson. 1995. Global oceanic emissions of nitrous oxide. Journal of Geophysical Research 100: 15809–15820.
Nevison, C.D., J.H. Butler, and J.W. Elkins. 2003. Global distribution of N2O and the ΔN2O-AOU yield in the subsurface ocean. Global Biogeochemical Cycles 17: 11–19.
Nightingale, P.D., G. Malin, C.S. Law, A.J. Watson, P.S. Liss, M.I. Liddicoat, J. Boutin, and R.C. Upstill-Goddard. 2000. In situ evaluation of air-sea gas exchange parameterizations using novel conservative and volatile tracers. Global Biogeochemical Cycles 14: 373–387.
NOAA. 2011. Halocarbons and other atmospheric trace gases. http://www.esrl.noaa.gov/gmd/hats/combined/N2O.html. Accesed 21 april 2016
Peréz-Santos, I., J. Garcés-Vargas, W. Schneider, L. Ross, S. Parra, and A. Valle-Levinson. 2014. Double-diffusive layering and mixing in Patagonian fjords. Progress in Oceanography 129: 35–49.
Peters, H., M.C. Gregg, and J.M. Toole. 1988. On the parameterization of equatorial turbulence. Journal of Geophysical Research 93: 1199–1218.
Poth, M., and D. Focht. 1985. 15N kinetic analysis of nitrous oxide production by Nitrosomonas europaea: an examination of nitrifier denitrification. Applied and Environmental Microbiology 49: 1134–1141.
Price, J.F., R.A. Weller, and R. Pinkel. 1986. Diurnal cycling: Observations and models of the upper ocean response to diurnal heating, cooling, and wind mixing. Journal of Geophysical Research 91: 8411–8427.
Ravishankara, A.R., J.S. Daniel, and R.W. Portmann. 2009. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 326: 123–125. doi:10.1126/science.1176985.
Robinson, A.D., D.B. Nedwell, R.M. Harrinson, and B.O. Ogilvie. 1998. Hypernutrified estuaries as source of N2O emission to the atmosphere: the estuary of the River Colne, Essex, UK. Marine Ecology. Progress Series 164: 59–71.
Sanford, R.A., D.D. Wagner, Q. Wu, J.C. Chee-Sanford, S.H. Thomas, C. Cruz-Garcia, G. Rodriguez, A. Massol-Deya, K.K. Krishnani, K.M. Ritalahti, S. Nissen, K.T. Konstantinidis, and F.E. Loffler. 2012. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proceedings of the National Academy of Sciences of the United States of America 109: 19709–19714. doi:10.1073/pnas.1211238109.
Schlesinger, W.H. 2013. An estimate of the global sink for nitrous oxide in soils. Global Change Biology 19: 2929–2931. doi:10.1111/gcb.12239.
Seitzinger, P., and C. Kroeze. 1998. Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Global Biogeochemical Cycles 12(1): 93–113.
Seitzinger, S.P., C. Kroeze, and R.V. Styles. 2000. Global distribution of N2O emission from system: natural emissions and anthropogenic effects. Chemosphere Global Change Science 2: 267–279.
Sievers, H.A., and N. Silva. 2006. Masas de agua y circulación en los canales y fiordos australes. En: avances en el conocimiento de las aguas interiores chilenas. Silva, N. and Palma, S. (Eds). CONA-PUCV, pp. 53–58.
Silva, N. 1983. Masas de agua y circulación en la región norte de Chile, latitudes 18–32° S (Operación oceanográfica Mar Chile XI–ERFEN II). Ciencia y Tecnología del Mar 7: 47–84.
Silva, N., C. Calvete, and H.A. Sievers. 1998. Masas de agua y circulación general para algunos canales australes chilenos entre Puerto Montt y laguna San Rafael (Crucero CIMAR-Fiordo 1). Ciencia y Tecnología del Mar 21: 17–48.
Silva, N., and S. Palma. 2008. Progress in the oceanographic knowledge of Chilean interior waters, from Puerto Montt to Cape Horn, 161 pp. Valparaíso: Comité Oceanográfico Nacional/Pontificia Universidad Católica de Valparaíso.
Soetaert, K., J. Middelburg, and C. Heip. 2006. Long-term change in dissolved inorganic nutrients in the heterotrophic Scheldt estuary (Belgium, The Netherlands). Limnology and Oceanography 51(1, part 2): 409–423.
Subpesca. 2016. Informe ambiental de acuicultura 2014 http://www.subpesca.cl . Accesed 10 april 2016.
Thompson, T.M, Butler, J. H., Daube, B. C., Dutton, G. S., Elkins, J.W., Hall, B. D., Hurst, D. F., King, E. S., Kline, D. B., Lafleur, B.G., and J. Lind. S. Lovitz, D. J. Mondeel, S. A. Montzka, F. L. Moore, J. D. Nance, J. L. Neu, P. A. Romashkin, A. Scheffer, and W. J. Snible. 2004. 5. Halocarbons and other Atmospheric Trace Species. In: Climate Monitoring and Diagnostics Laboratory, Summary Report No. 27, 2002–2003, eds. R. C. Schnell, A.-M. Buggle, and R. M. Rosson, 115–133. NOAA CMDL, Boulder, CO.
Tilbrook, B.D., and D.M. Karl. 1995. Methane sources, distributions and sinks from California coastal waters to the oligotrophic North Pacific gyre. Marine Chemistry 49: 51–64.
Turner, J.T. 2015. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Progress in Oceanography 130: 205–428.
US Environmental Protection Agency (US EPA). 2000. Methane and Nitrous oxide emission from natural sources. Washington, DC: U.S. EPA, Office of Atmospheric Programs.
Valle-Levinson, A., N. Sarkar, R. Sanay, D. Soto, and J. León. 2007. Spatial structure of hydrography and flow in a Chilean fjord, Estuario Reloncaví. Estuaries and Coasts 30: 113–126.
Vandekerkhove, E., S. Bertrand, B. Reid, A. Bartels, and B. Charlie. 2016. Sources of dissolved silica to the fjords of northern Patagonia (44–48°S): the importance of volcanic ash soil distribution and weathering. Earth Surf Process Landform 41: 499–512. doi:10.1002/esp.3840.
Van Groenigen, J.W., D. Huygens, P. Boeckx, Th.W. Kuyper, I.M. Lubbers, T. Rütting, and P.M. Groffman. 2015. The soil N cycle: new insights and key challenges. The Soil 1: 235–256.
Vieten, B., F. Conen, B. Seth, and C. Alewell. 2008. The fate of N2O consumed in soils. Biogeosciences 5: 129–132.
Wu, D., W. Dong, O. Oenema, Y. Wang, I. Trebs, and C. Hu. 2013. N2O consumption by low-nitrogen soil and its regulation by water and oxygen. Soil Biology and Biochemistry 60: 165–172.
Weiss, R.F., and B.A. Price. 1980. Nitrous oxide solubility in water and seawater. Marine Chemistry 8: 347–359.
Walter, S., H.W. Bange, U. Breitenbach, and D.W.R. Wallace. 2006. Nitrous oxide in the north Atlantic Ocean. Biogeosciences 3: 607–619. doi:10.5194/bg-3-607-2006.
Wanninkhof, R. 1992. Relationship between wind speed and gas exchange over the ocean. Journal of Geophysical Research 97(C5): 373–7382.
Yoshinari, T. 1976. Nitrous oxide in the sea. Marine Chemistry 2(4): 189–202. doi:10.1016/0304-4203(76)90007-4.
Zhang, G.L., J. Zhang, J. Ren, J. Li, and M. Liu. 2008. Distributions and sea to air fluxes of Methane and nitrous Oxide in the North East China Sea in summer. Marine Chemistry 110: 42–55.
Zhang, G.L., J. Zhang, S.M. Liu, J.L. Ren, and Y.C. Zhao. 2010. Nitrous oxide in the Changjiang (Yangtze River) estuary and its adjacent marine area: riverine input, sediment release and atmospheric fluxes. Biogeosciences 7: 3505–3516. doi:10.5194/bg-7-3505-2010.
Zumft, W.G. 1997. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61(4): 533–616.
Acknowledgments
This research was funded by National Oceanographic Committee (Comité Oceanográfico Nacional) (CONA) and the Chilean navy, as part of the multidisciplinary project CIMAR 19. Support from CONICYT/FONDAP program no. 1511009 (CR2), FONDECYT no. 1161138 (Laura Farías), and FONDECYT no. 3150162 (Mariela A. Yevenes) is also appreciated. Technical support is also appreciated from graduate students Karen Sanzana and Sebastián Loyola.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Bradley Eyre
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yevenes, M.A., Bello, E., Sanhueza-Guevara, S. et al. Spatial Distribution of Nitrous Oxide (N2O) in the Reloncaví Estuary–Sound and Adjacent Sea (41°–43° S), Chilean Patagonia. Estuaries and Coasts 40, 807–821 (2017). https://doi.org/10.1007/s12237-016-0184-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0184-z