Abstract
Comparisons of natural mortality rates can be used to identify essential habitat and nursery areas for fishery species. We estimated and compared natural mortality rates of juvenile white shrimp Litopenaeus setiferus using length-frequency and mark-recapture data and attempted to identify factors that may affect these mortality rates. Daily instantaneous natural mortality rates (95 % confidence interval (CI)) obtained from length-frequency data by following individual cohorts were 0.043 (0.031–0.054) and 0.014 (0.0–0.039). Combining all length-frequency data, converting to age-frequency data, and using two types of catch-curve analyses yielded estimates of 0.069 (0.042–0.095) and 0.060 (0.046–0.073). Mark-recapture estimates obtained in a separate study from two ponds were 0.129 (0.054–0.203) and 0.014 (−0.048–0.076). These estimates are comparable to previously reported values for this species, but we are the first to report a measure of precision with our estimates. In the mark-recapture study, mortality rates appeared to be related to predator abundance in ponds and flooding patterns of the surrounding marsh. The only mortality rate significantly different from any of the other estimates was the lower of the two length-frequency estimates, but this result should be interpreted with caution because of the uncertainty in that estimate, relative imprecision of our estimates, and confounding factors between the methods we used to estimate mortality. Despite this caveat, the results from our study can be used to improve population models for L. setiferus and our understanding of the role of marsh habitats as nursery areas.





Similar content being viewed by others
References
Anderson, D.R. 2008. Model based inference in the life sciences: A primer on evidence. New York, NY: Springer.
Baker, R., and T.J. Minello. 2010. Growth and mortality of juvenile white shrimp Litopenaeus setiferus in a marsh pond. Marine Ecology Progress Series 413: 95–104.
Baker, R., B. Fry, L.P. Rozas, and T.J. Minello. 2013. Hydrodynamic regulation of salt marsh contributions to aquatic food webs. Marine Ecology Progress Series 490: 37–52.
Baker, R., M. Fujiwara, and T.J. Minello. 2014. Juvenile growth and mortality effects on white shrimp Litopenaeus setiferus population dynamics in the northern Gulf of Mexico. Fisheries Research 155: 74–82.
Baxter, K.N., and W.C. Renfro. 1967. Seasonal occurrence and size distribution of postlarval brown and white shrimp near Galveston, Texas, with notes on species identification. Fishery Bulletin 66: 149–158.
Beck, M.W., K.L. Heck Jr., K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.P. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51: 633–641.
Brownie, C., D. R. Anderson, K. P. Burnham, and D. S. Robson. 1985. Statistical inference from band recovery data—a handbook. 2nd ed. U.S. Fish and Wildlife Service Resource Publication No. 156, 305 pp.
Chapman, D.G., and D.S. Robson. 1960. The analysis of a catch curve. Biometrics 16: 354–368.
Childers, D.L., J.W. Day Jr., and R.A. Muller. 1990. Relating climatological forcing to coastal water levels in Louisiana estuaries and the potential importance of El Niño-Southern Oscillation events. Climate Research 1: 31–42.
Dall, W., B.J. Hill, P.C. Rothlisberg, and D.J. Sharples. 1990. The biology of the Penaeidae. Advances in Marine Biology 27: 1–484.
Diop, H., W.R. Keithly Jr., R.F. Kazmierczak Jr., and R.F. Shaw. 2007. Predicting the abundance of white shrimp (Litopenaeus setiferus) from environmental parameters and previous life stages. Fisheries Research 86: 31–41.
Ditty, J.G. 2011. Young of Litopenaeus setiferus, Farfantepenaeus aztecus and F. duorarum (Decapoda: Penaeidae): A re-assessment of characters for species discrimination and their variability. Journal of Crustacean Biology 31: 458–467.
Edwards, R.R.C. 1977. Field experiments on growth and mortality of Penaeus vannamei in a Mexican coastal lagoon complex. Estuarine and Coastal Marine Science 5: 107–121.
Fabrizio, M.C., M.E. Holey, P.C. McKee, and M.L. Toneys. 1997. Survival rates of adult lake trout in northwestern Lake Michigan, 1983–1993. North American Journal of Fisheries Management 17: 413–428.
Gosselink, J. G., C. L. Cordes, and J. W. Parsons. 1979. An ecological characterization study of the Chenier Plain coastal ecosystem of Louisiana and Texas. 3 vols. U.S. Fish and Wildlife Service, Office of Biological Services. FWS/OBS-78/9 through 78/11.
Gracia, A., and L.A. Soto. 1986. Estimación del tamaño de la población, crecimiento y mortalidad de los juveniles de Penaeus setiferus (Linnaeaus, 1767) mediante marcado recaptura en la Laguna Chacahito, Campeche, México. Anales del Centro de Ciencias del Mar y Limnología 13: 217–230.
Grant, A., P.J. Morgan, and P.J.W. Olive. 1987. Use made in marine ecology of methods for estimating demographic parameters from size/frequency data. Marine Biology 95: 201–208.
Haas, H.L., E.C. Lamon III, K.A. Rose, and R.F. Shaw. 2001. Environmental and biological factors associated with the stage-specific abundance of brown shrimp (Penaeus aztecus) in Louisiana: Applying a new combination of statistical techniques to long-term monitoring data. Canadian Journal of Fisheries and Aquatic Sciences 58: 2258–2270.
Haas, H.L., K.A. Rose, B. Fry, T.J. Minello, and L.P. Rozas. 2004. Brown shrimp on the edge: Linking habitat to survival using an individual-based simulation model. Ecological Applications 14: 1232–1247.
Haywood, M.D.E., and D.J. Staples. 1993. Field estimates of growth and mortality of juvenile banana prawns (Penaeus merguiensis). Marine Biology 116: 407–416.
Hoenig, J.M., N.J. Barrowman, W.S. Hearn, and K.H. Pollock. 1998. Multiyear tagging studies incorporating fishing effort data. Canadian Journal of Fisheries and Aquatic Sciences 55: 1466–1476.
Huston, M.A., and D.L. DeAngelis. 1987. Size bimodality in monospecific populations: A critical review of potential mechanisms. American Naturalist 129: 678–707.
Isley, J.J., and T.B. Grabowski. 2007. Age and growth. In Analysis and interpretation of freshwater fisheries data, ed. C.S. Guy and M.L. Brown, 187–228. Bethesda, Maryland: American Fisheries Society.
Kneib, R.T. 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanography and Marine Biology: An Annual Review 35: 163–220.
Kneib, R.T., and M.C. Huggler. 2001. Tag placement, mark retention, survival and growth of juvenile white shrimp (Litopenaeus setiferus Pérez Farfante, 1969) injected with coded wire tags. Journal of Experimental Marine Biology and Ecology 266: 109–120.
Knudsen, E.E., R.F. Paille, B.D. Rogers, and W.H. Herke. 1989. Effects of a fixed-crest weir on brown shrimp Penaeus aztecus growth, mortality, and emigration in a Louisiana coastal marsh. North American Journal of Fisheries Management 9: 411–419.
Knudsen, E.E., B.D. Rogers, R.F. Paille, and W.H. Herke. 1996. Juvenile white shrimp growth, mortality, and emigration in weired and unweired Louisiana marsh ponds. North American Journal of Fisheries Management 16: 640–652.
Laney, R.W. and B.J. Copeland. 1981. Population dynamics of penaeid shrimp in two North Carolina tidal creeks. Raleigh, NC: Report 81-1 Carolina Power & Light Co.
Latour, R.J., J.M. Hoenig, J.E. Olney, and K.H. Pollock. 2001. Diagnostics for multiyear tagging models with application to Atlantic striped bass (Morone saxatilis). Canadian Journal of Fisheries and Aquatic Sciences 58: 1716–1726.
MacDonald, P., and J. Du. 2012. Mixdist: Finite mixture distribution models. R package version 0.5-4. http://CRAN.R-project.org/package=mixdist.
Minello, T.J. 1993. Chronographic tethering: A technique for measuring prey survival time and testing predation pressure in aquatic habitats. Marine Ecology Progress Series 101: 99–104.
Minello, T.J., and R.J. Zimmerman. 1983. Fish predation on juvenile brown shrimp, Penaeus aztecus Ives: The effect of simulated Spartina structure on predation rates. Journal of Experimental Marine Biology and Ecology 72: 211–231.
Minello, T.J., R.J. Zimmerman, and E.X. Martinez. 1989. Mortality of young brown shrimp Penaeus aztecus in estuarine nurseries. Transactions of the American Fisheries Society 118: 693–708.
Minello, T.J., G.A. Matthews, P.A. Caldwell, and L.P. Rozas. 2008. Population and production estimates for decapod crustaceans in wetlands of Galveston Bay, Texas. Transactions of the American Fisheries Society 137: 129–146.
Miranda, L.E., and P.W. Bettoli. 2007. Mortality. In Analysis and interpretation of freshwater fisheries data, ed. C.S. Guy and M.L. Brown, 229–277. Bethesda, Maryland: American Fisheries Society.
Moore, D.S., and G.P. McCabe. 2006. Introduction to the practice of statistics, 5th ed. New York: W. H. Freeman and Company.
NMFS. 2010. Marine fisheries habitat assessment improvement plan. Report of the National Marine Fisheries Service Habitat Assessment Improvement Plan Team. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-F/SPO-108, 115 pp.
NOAA Fisheries Toolbox. 2013. Instantaneous Rates (IRATE) Program, Version 2.0. http://nft.nefsc.noaa.gov.
O’Brien, C.J. 1994. Population dynamics of juvenile tiger prawns Penaeus esculentus in south Queensland, Australia. Marine Ecology Progress Series 104: 247–256.
Penland, S., and J.R. Suter. 1989. The geomorphology of the Mississippi River Chenier Plain. Marine Geology 90: 231–258.
Pérez Farfante, I. 1970. Diagnostic characters of juveniles of the shrimps Penaeus aztecus aztecus, P. duorarum duorarum, and P. brasiliensis (Crustacea, Decapoda, Penaeidae). U.S. Fish and Wildlife Service Special Scientific Report, Fisheries No. 599. 26 pp.
Pérez-Castañeda, R., and O. Defeo. 2003. A reciprocal model for mortality at length in juvenile pink shrimps (Farfantepenaeus duorarum) in a coastal lagoon of Mexico. Fisheries Research 63: 283–287.
Pérez-Castañeda, R., and O. Defeo. 2005. Growth and mortality of transient shrimp populations (Farfantepenaeus spp.) in a coastal lagoon of Mexico: role of the environment and density-dependence. ICES Journal of Marine Science 62: 14–24.
Pine, W.E., K.H. Pollock, J.E. Hightower, T.J. Kwak, and J.A. Rice. 2003. A review of tagging methods for estimating fish population size and components of mortality. Fisheries 28: 10–23.
Powell, L.A. 2007. Approximating variance of demographic parameters using the delta method: A reference for avian biologists. The Condor 109: 949–954.
R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Ricker, W.E. 1975. Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada 191: 1–382.
Rockwood, L.L. 2006. Introduction to population ecology. Malden, MA: Blackwell Publishing.
Roth, B.M., K.A. Rose, L.P. Rozas, and T.J. Minello. 2008. Relative influence of habitat fragmentation and inundation on brown shrimp Farfantepenaeus aztecus production in northern Gulf of Mexico salt marshes. Marine Ecology Progress Series 359: 185–202.
Rozas, L.P., T.J. Minello, and D.D. Dantin. 2012. Use of shallow lagoon habitats by nekton of the northeastern Gulf of Mexico. Estuaries and Coasts 35: 572–586.
Ruxton, G.D. 2006. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behavioral Ecology 17: 688–690.
Salini, J.P., S.J.M. Blaber, and D.T. Brewer. 1990. Diets of piscivorous fishes in a tropical Australian estuary, with special reference to predation on penaeid prawns. Marine Biology 105: 363–374.
Schenker, N., and J.F. Gentleman. 2001. On judging the significance of differences by examining the overlap between confidence intervals. The American Statistician 55: 182–186.
Smith, M.W., A.Y. Then, C. Wor, G. Ralph, K.H. Pollock, and J.M. Hoenig. 2012. Recommendations for catch-curve analysis. North American Journal of Fisheries Management 32: 956–967.
Turner, R.E. 1977. Intertidal vegetation and commercial yields of penaeid shrimp. Transactions of the American Fisheries Society 106: 411–416.
Turner, R.E. 1990. Landscape development and coastal wetland losses in the northern Gulf of Mexico. American Zoologist 30: 89–105.
Vetter, E.F. 1988. Estimation of natural mortality in fish stocks: A review. Fishery Bulletin 86: 25–43.
Wang, Y.-G., and M.D.E. Haywood. 1999. Size-dependent natural mortality of juvenile banana prawns Penaeus merguiensis in the Gulf of Carpentaria, Australia. Marine and Freshwater Research 50: 313–317.
Webb, S., and R.T. Kneib. 2004. Individual growth rates and movement of juvenile white shrimp (Litopenaeus setiferus) in a tidal marsh nursery. Fishery Bulletin 102: 376–388.
Williams, A.B. 1969. A ten-year study of meroplankton in North Carolina estuaries: Cycles of occurrence among Penaeidean shrimps. Chesapeake Science 10: 36–47.
Zimmerman, R.J., T.J. Minello, and G. Zamora Jr. 1984. Selection of vegetated habitat by brown shrimp, Penaeus aztecus, in a Galveston Bay salt marsh. Fishery Bulletin 82: 325–336.
Zimmerman, R.J., T.J. Minello, and L.P. Rozas. 2000. Salt marsh linkages to productivity of penaeid shrimps and blue crabs in the Northern Gulf of Mexico. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 293–314. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Acknowledgments
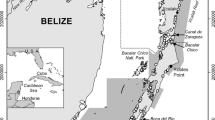
This research was conducted through the NOAA National Marine Fisheries Service (NMFS) Southeast Fisheries Science Center (SEFSC) by personnel from the Galveston Laboratory and the Estuarine Habitats and Coastal Fisheries Center in Lafayette, Louisiana. In particular, we thank Shawn Hillen, Juan Salas, Jennifer Doerr, Rick Hart, and Lainey Broussard for help in the field and Alex Cummings for help processing samples in the laboratory. We would also like to thank David Richard at Stream Wetland Services and the staff at Murphree Wildlife Management Area for providing lodging during the study and the staff of the Texas Point National Wildlife Refuge for access to the field site. We thank Rick Hart for information on white shrimp stock assessments and Phil Caldwell for conducting the GIS analysis needed to weight the length-frequency distributions and for creating Fig. 1. Tom Minello, Ronnie Baker, and two anonymous reviewers provided helpful suggestions that improved the original manuscript. A NOAA Habitat Assessment Improvement Program grant, a Teaching Assistantship from the University of Louisiana at Lafayette, a Rockefeller Wildlife Scholarship, a Coastal Conservation Association/Ted Beaulieu Sr. Scholarship, a research grant from the Wetland Foundation, and the NMFS SEFSC provided funding to support this project. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the NOAA NMFS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Wim J. Kimmerer
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 89 kb)
Rights and permissions
About this article
Cite this article
Mace, M.M., Rozas, L.P. Estimating Natural Mortality Rates of Juvenile White Shrimp Litopenaeus setiferus . Estuaries and Coasts 38, 1580–1592 (2015). https://doi.org/10.1007/s12237-014-9901-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-014-9901-7