Abstract
Potato (Solanum tuberosum L.) has a relatively high requirement for potassium (K+). In the face of the declining of potato yield and quality as a result of reduction of soil K+ content, it is necessary to determine how to improve the tolerance of potatoes to low-K+ stresses. The protein kinase AtCIPK23 is essential for K+ uptake, and its overexpression significantly enhances K+ uptake and tolerance to low-K+ in Arabidopsis. In this research, AtCIPK23 was transformed into an E3 potato cultivar successfully. In low-K+ conditions, the transgenic potato lines had lower levels of leaf chlorosis, higher K+ uptake rate, increased dry weight and K+ content. The non-transgenic lines displayed reduced stature while the transgenic lines exhibited sustained growth. These results indicate that ectopic expression of AtCIPK23 increases low-K+ tolerance in potato. This study demonstrated the potential use of the transgenic approach to increase potato production in low-K+ fields.
Resumen
La papa (Solanum tuberosum L.), tiene un requerimiento relativamente alto de potasio (K+). Encarando la reducción en rendimientos y de la calidad de la papa como resultado de una reducción en el contenido de K+ en el suelo, es necesario determinar cómo mejorar la tolerancia de la papa al agobio por bajo K+. La proteína kinasa AtCIPK23 es esencial para la absorción de K+, y su sobreexpresión aumenta significativamente la absorción de K+ y tolerancia a bajo K+ en Arabidopsis. En esta investigación, se incorporó AtCIPK23 a una variedad E3 con éxito. Bajo condiciones de bajo K+, las líneas trangénicas de papa tuvieron niveles más bajos de clorosis foliar, un nivel más alto de absorción de K+, aumento en peso seco y del contenido de K+. Las líneas no trangénicas expresaron altura reducida mientras que las trangénicas mostraron crecimiento sostenido. Estos resultados indican que la expresión ectópica de AtCIPK23 aumenta la tolerancia al bajo K+ en papa. Este estudio demostró el uso potencial del enfoque trangénico para aumentar la producción de papa en campos de bajo K+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.) is a major crop world wide, and its high yield, quality and disease resistance requires relative high potassium (K+) (Panique et al. 1997). 13.6 kg crop of potatoes needs 113 g K+ in average for vines and tubers (Dahnke and Nelson 1993). In previous research, we found that the amount of K+ affects the contents of water and starch in potato tuber significantly (Han et al. 2007). As K+ concentration in soil is depleted by farming and erosion, and the potato producers are reluctant to buy K+ fertilizer generally because of its higher price, K+ becomes increasingly depleted in potato soils. A wide range of measures must be taken to improve soil K+ fertility, at the same time, to isolate potato lines which can grow under a low-K+ nutrient status is another important way. In previous research, we found that potato cultivars have genotypic differences in responses to K+ deficiency and selected some low-K+ tolerance lines (Liu et al. 2005). However, the production of these low-K+ tolerant lines is still limited and some major cultivars have no low-K+ tolerance. It is necessary to find other methods to improve the tolerance of potato to low-K+ stress, such as finding low-K+ tolerance genes and using molecular biological and transgenic techniques for breeding new potato cultivars.
Recently, it was reported that a protein kinase, AtCIPK23, encoded by the LKS1 gene, regulates K+ uptake and confers low K+ tolerance in Arabidopsis (Xu et al. 2006). This signal pathway involves two calcium sensors (CBL1 and CBL9) physically interacting with their target protein kinase CIPK23, which, in turn, regulates the activity of voltage-gated K+ channel, AKT1, by phosphorylation (Li et al. 2006; Xu et al. 2006). Plasma membrane-localized CBL1– and CBL9–CIPK23 complexes simultaneously regulate K+ transportation in roots and stomatal guard cells, and play essential roles in the regulation of leaf transpiration and root K+ uptake in Arabidopsis (Cheong et al. 2007).
However, whether CIPK23 can confer low-K+ tolerance and plays essential roles in enhancement of potato yield is still unknown. E3 is one of the main potato cultivars suitable for planting in southwest China. It was bred from the Potato Research Center in Southern China and has good taste and tuber-shaped, high yield and resistance of late blight disease. We selected this potato cultivar to study the tolerance of transgenic potato lines ectopic expressing AtCIPK23 against low-K+ stress.
Materials and Methods
Plant Material, Growth Conditions and Plasmid
The E3 potato cultivar plants were propagated using single-node segments by tissue culture in MS medium containing 3% (w/v) sucrose and 0.6% (w/v) agar. The pH was adjusted to pH 5.8 before autoclaving and the cultures were incubated under a 16 h light (100 μmolm−2 s−1)/8 h dark cycle at 24°C in a growth chamber. Microtubers for plant transformation were induced on the plantlets grown in vitro as described by Liu et al. (1994).
The binary plasmid vector pBI121 (Jefferson et al. 1987) used in this study contained a 1.9 kb GUS expression cassette driven by the CaMV 35S promoter. To make a binary gene construct, a Xba I-Sma I DNA fragment carrying the full-length cDNA of AtCIPK23 (accession # NM102766) was inserted into the pBI121 binary vector at the Xba I-Sma I sites, located downstream of CaMV 35S promoter using routine molecular techniques. The pBI121 + AtCIPK23 vector was introduced into the disarmed Agrobacterium tumefaciens strain GV3101, and the positive clone was used for plant transformation. Agrobacterium tumefaciens strain GV3101 was cultivated in YEB medium (Vervliet et al. 1975).
Plant Transformation
The resulting Agrobacterium tumefaciens strains containing plasmid pBI121 with the AtCIPK23 gene were introduced into potato according to the microtuber transformation method described by Si et al. (2003). Agrobacterium tumefaciens GV3101 was cultured overnight at 28°C in YEB liquid medium supplemented with 50 mg/L kanamycin and 50 mg/L rifampicin with shaking at 250 rpm until A600 = 0.5 ~ 1.0. The bacteria were collected by centrifugation at 5000 × g for 6 min at 4°C and resuspended in 20 ml MS liquid medium (containing 3% w/v sucrose, pH 5.8)
Microtubers of 12–20 weeks old with a diameter of about 5 mm were cut into slices with a thickness of 1 to 2 mm. Following this, the microtuber slices were immersed in the aforementioned bacterial suspension for 8–10 min. The tuber slices were then dried on sterile filter papers before cultivation in the incubation medium [MS salts, indole-3-acetic acid (IAA) 0.3 mg/L, gibberellic acid (GA3) 0.2 mg/L, 6-benzyladenine (6-BA) 0.5 mg/L, sucrose 30 g/L, agar 8 g/L, pH 5.8]. After 36 h of co-cultivation in dark at 24°C, tuber slices were moved onto the shoot regenerating medium (MS salts, IAA 0.3 mg/L, GA3 0.2 mg/L, 6-BA 0.5 mg/L, zeatin 1 mg/L, sucrose 30 g/L, agar 8 g/L, pH 5.8) containing 200 mg/L cefapirin and 50 mg/L kanamycin to inhibit further growth of Agrobacterium, and incubated until small shoots were regenerated. When they were approximately 1 cm tall, regenerated shoots were excised and selected by rooting in MS medium containing 50 mg/L kanamycin and 200 mg/L cefapirin. Surviving plantlets with well-developed roots were propagated for further analysis.
PCR Analysis
Total genomic DNA was isolated from young leaves of the kanamycin resistant plants by the cetyltrimethyl ammonium bromide (CTAB) method and subjected to PCR analysis for the presence of transgene. Primers specific for the amplification of a 676 bp fragment used for detecting the npII gene were (FnptII) 5'- GCTATGACTGGGCACAACAG-3' and (RnptII) 5'-ATACCG TAAAGCACGAGGAA-3'. PCR was carried out using Taqr DNA polymerase (Takara, Shanghai) with 200 ng potato genomic DNA as a template. Plasmid DNA from the Agrobacterium carrying pBI121+ AtCIPK23 was used as positive control. PCR reactions for 29 cycles commenced with denaturing at 94°C for 30 s, followed by annealing at 57°C for 30 s and extension at 72°C for 1 min.
Southern Blot Analysis
Genomic DNA samples (40 μg per sample) of six transgenic potato lines T5, T11, T15, T21, T22 and T23 were digested overnight at 37°C with EcoRI, which did not cut within the npt II coding sequence. A non-transgenic line was used as a negative control. After separation on 1% agarose gel, the DNA fragments were blotted onto a positively charged Hybond-N nylon membrane (Osmonics Magnacharge nylon transfer membrane, Amersham Pharmacia Biotec, Little Chalfont, UK) according to the manufacturer’s recommendations. The sequence generated by PCR using primers (F nptII and R nptII) was used as the probe in Southern blot analysis. Probe labeling, hybridization and signal detection were performed following the DIG Probe synthesis kit (Roche Diagnostics GmbH, Germany) manufacturer’s instructions. The membrane was washed under high stringency conditions (65°C) and the hybridized signals were visualized by exposure of the membrane to Fuji X-ray film at 25°C for 2 h.
RT-PCR Analysis
Total RNA was isolated from leaves by using TRIzol reagent (Invitrogen, Carlsbad, kCA, USA) and was used for RT-PCR as templates. Total RNA samples were treated extensively with RNase-free DNase1 to remove any contaminating genomic DNA. First-strand cDNA was then synthesized using MMLV reverse transcriptase (Stratagene, USA, http://www.stratagene.com) from 1 μg of total RNA with oligo d (T) 18 primers in a 20 μL reaction volume, and 1 μL of the reaction mixture was subjected to subsequent PCR in a 50 μL reaction volume. AtCIPK23 (248 bp) 5′-TTACTTCCAAATCTCGGCTAATGAG-3′ and 5′-GTCCCGTGGTAAGGTTCTTGTAAAAC-3′ was amplified using special primers for 28 cycles according to the following conditions for each cycle: 94°C for 40 s, 55°C for 40 s, and 72°C for 90 s. This was followed by a final cycle at 72°C for 5 min to allow completion of the polymerization. As a control for equal cDNA amounts in each reaction, PCR was performed with primers for EF-1α (293 bp) 5′-CCAGATTGGAAAACGGATATGCT-3′and 5′-CACCAGTTGGGTCCTTCTTGT-3′.
Test the Phenotypes, Plant Dry Weight and K+ Content of Seedlings
For low-K+ tolerance assays, the single-node segments of the plantlets were transferred to the low-K+ MS medium (LK) and MS medium (control) and treated for 20 days. For making the LK medium, first of all, a K+-free medium was prepared (Cheong et al. 2007), then we supplemented with KCl to get the K+ concentration of 0.5 mM, which was just 2.5% of normal K+ concentration in MS (20 mM KCl). The phenotypes were observed and the plant dry weight was measured after drying in the oven at 80°C for 48 h. For measurement of plant K+ content, the seedlings were rinsed four to five times with distilled water, treated in a muffle furnace at 575°C for 5 h and then dissolved in 0.1 M HNO3. K+ concentrations of the samples were determined using an atomic absorption spectrophotometer.
Kinetic K+ Uptake Experiment
Fifteen-day-old test-tube plantlets were pretreated in starvation solution (0.2 mM CaSO4, 5 mM Mes, pH 5.8) at 24°C for 3 days; the starvation solution was refreshed each day. Characteristics of kinetic K+ uptake were studied with the help of the K+ depletion method (Xu et al. 2006). The solution samples were collected every 2 h (solution contained 0.2 mM KCl, 0.2 mM CaSO4 and 5 mM Mes, pH 5.8), and depletion period was for 48 h. In order to determine evapotranspiration losses, the weight of each container, together with its labeled solution and plants, was measured using a top-loading balance. At intervals of 2 h, the measured loss of water in transpiration was replaced by the addition of deionized water. Subsequently, K+ concentration was determined, and K+ uptake parameters were calculated according to Yang et al. (2007).
Result
Potato Transformation and Identification of Transgenic Lines
The visible shoots emerged in the co-cultivated explants (Fig. 1A) after 10–12 days of culture in selecting medium containing 50 mg/L kanamycin (Fig. 1b, c and d). Those that were rooted in the medium containing 50 mg/L kanamycin and 200 mg/L cefapirin were regarded as transgenic candidates compared the controls of E3 which had no root initiation (Fig. 1e). All of these transgenic lines were transplanted into pots containing compost. The transgenic lines exhibited typical cultivar morphology in the greenhouse (data not shown).
Molecular Analysis of Transgenic Lines
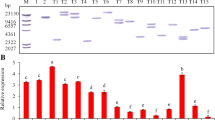
The presence of the transgene in kanamycin-resistant plants was determined by amplification of a 676 bp DNA fragment with nptII-specific primers in a number of putative transgenic lines (Fig. 2a).
Molecular characterization of transgenic potato lines. T5, T11, T15, T21, T22 and T23 are transgenic lines. NT is a non-transgenic line. V is a positive pBI121-AtCIPK23 vector. M is the DNA ladder. a Polymerase chain reaction (PCR) analysis using primers specific to the nptII gene. b Southern analysis of EcoR I digested genomic DNA for checking transgene copy number using the nptII gene as a probe. c Reverse transcription (RT)-PCR analysis for the RNA expression using primers specific to AtCIPK23 gene and ef-1α primer
The PCR positive plants were analyzed by Southern hybridization to identify the integration and copy number of the transgene. Some transgenic lines showed one or two copy insertions of the transgene in plants tested and different banding pattern denoting that they are independent transgenic lines. One copy of T-DNA was detected in the transgenic plant lines T11, T15, T21 and T23, while two copies were detected in the transgenic lines T5 and T22. (Fig. 2b)
The transgenic lines T5, T11, T15, T21, T22 and T23 showed distinct transgene expression at the transcript level by semi-quantitative reverse transcription (RT-PCR) using AtCIPK23 gene-specific primers, while this was not shown in non-transgenic line (Fig. 2c).
AtCIPK23 Transgenic Lines Showed the Phenotype of Enhanced Tolerance to Low-K+ Stress
After growing for 20 days in MS media, non-transgenic line were indistinguishable from the transgenic lines (T15, T21, T23) (Fig. 3AMS). When grown in LK, the non-transgenic lines displayed reduced stature and high levels of leaf chlorosis, but the transgenic lines (T15, T21, T23) retained normal stature and exhibited significantly lower levels of leaf chlorosis (Fig. 3ALK). These growth characteristics are indicative of the low-K+ tolerant phenotypes of the transgenic lines.
Plant phenotypes and dry weights, K+ content, K+ uptake test. a The low-K+ tolerance phenotypes of transgenic lines. Transgenic lines (T15, T21, and T23) and the non-transgenic line (E3) had grown in MS (up) or LK (down) for 20 days. b Comparison of plant dry weights in MS or LK for 20 days. c Comparison of plant K+ content in MS for 20 days. d Comparison of plant K+ content in LK for 20 days. eComparison of K+ uptake by K+-depletion methods. T15, T21 and T23 were transgenic lines. E3 was the non-transgenic line. All the data are presented as means ± SE (n = 3)
Plant Dry Weight and K+ Content Analysis
We further investigated the plantlet dry weight and K+ content in non-transgenic and transgenic lines T15, T21 and T23 after growing for 20 days in MS and LK. The plant dry weight of the non-transgenic line was indistinguishable from that of the transgenic lines (T15, T21 and T23) in MS (Fig. 3BMS). But in LK, the plant dry weight of the non-transgenic line was significantly lower than that of every transgenic line (T15, T21 or T23) (Fig. 3BLK). For example, the T23 in LK displayed 72% of the plant dry weight of T23 in MS and non-transgenic line in LK showed only 42% of the plant dry weight compared to that in MS.
When cultured in MS for 20 days, the K+ content of plants increased 5% in T23 but decreased 10% and 6% in T15 and T21 compared with the non-transgenic line (Fig. 3c). These differences were not significant. But when cultured in LK for 20 days, the K+ content of plants of T15, T21 and T23 was 35%, 71% and 50% higher than that of the non-transgenic line respectively (Fig. 3d). These differences were significant. These K+ content analysis results indicate that AtCIPK23 conferred the transgenic lines increases of K+ accumulation. It may be because AtCIPK23 enhances the K+ uptake ability of transgenic lines when subjected to low-K+ stress.
Kinetic K+ Uptake Analysis
To confirm that AtCIPK23 enhances the K+ uptake ability of transgenic lines when subjected to low-K+ stress, we measured the K+ uptake rates of the non-transgenic and transgenic lines. Three independent transgenic lines showed higher K+ uptake rates than the non-transgenic line (Fig. 3e). As shown in Table 1, the T23 had the highest Vmax, the lowest Km and Cmin for K+ uptake, while the non-transgenic line had lowest Vmax, increased Km and Cmin. Compared with the non-transgenic line, the Vmax of the transgenic lines showed 53.8%, 19.2% and 15.4% increases in T23, T21 and T15 respectively, while the Km of these transgenic lines decreased to 78 μM, 85 μM and 94 μM, respectively, compared to 108 μM for the non-transgenic line. These results demonstrate that ectopic expression of AtCIPK23 resulted in a significant increase in the maximum K+ uptake rate and K+ affinity. The Cmin for K+ uptake of the transgenic lines T15, T21 and T23 was decreased to 38 μM, 49 μM and 36 μM, respectively, compared to 61 μM for the non-transgenic line (Table 1), indicating that the transgenic lines may initiate K+ uptake at much lower K+ concentration compared to the non-transgenic line.
Discussion
Here we report that the AtCIPK23 cDNA was fused to the promoter CaMV35S and transformed into the E3 potato cultivar by the Agrobacterium tumeficians-mediated transformation approach. Molecular analyses identified several transgenic lines with high expression of AtCIPK23. A trial of the single-node segments in vitro indicated that these transgenic lines exhibited sustained growth, lower levels of leaf chlorosis and accumulated higher levels of K+ in LK medium compared to the non-transgenic line. In K+ depletion experiment, transgenic lines showed higher K+ uptake rates and K+ affinity than non-transgenic line. These results indicate that the ectopic expression of AtCIPK23 in potato improves the K+ uptake ability under low-K+ conditions and confers enhanced low-K+ tolerance. This study demonstrated the potential use of our transgenic approach in developing new potato cultivars with increased low-K+ tolerance, minimizing growth defects in low-K+ fields, which would provide a potential agricultural application of the transgenic potato lines.
The acquisition of low-K+ tolerance indicates that the AtCIPK23 can function in potato during low-K+ conditions and that AtCIPK23 is able to regulate downstream genes associated with K+ uptake. However, in normal K+ conditions, the transgenic lines did not shown advantages in K+ accumulation compared with the non-transgenic line. This could be due to the fact that both non-transgenic and transgenic lines can absorb enough K+ for normal and healthy growth, and the fact that absorption of K+ in the roots is regulated by the demands of plants themselves (Yan et al. 1997). Therefore, the contribution of AtCIPK23 gene to the K+ accumulation and K+ -use efficiency was decreased under the normal external K+ level. Our researches about the transgenic potato’s yield, quality, disease resistance and other morphological, physiological, biochemical parameters are in progress under the strict control conditions of transgenic security.
Southern blot analysis allowed determination of copy numbers and the intact integration of the transgene in the examined plants. Several independent transgenic lines were obtained with one to two copies of the transgene integrated into the genome. A low copy of AtCIPK23 in the transgenic plants was considered as a good result, because several studies showed the positive correlation between a high copy number of transgenes and cytosine methylation-mediated silencing (Matzke and Matzke 1995; Lechtenberg et al. 2003). We selected three independent lines with single copy transgenes and observed variations in plant dry weight, plant K+ contents, K+ uptake rate and growth among them. These differences could be due to slight variations in the expressions level of AtCIPK23, which might be caused by different positional or other unknown effects of transgenes.
References
Cheong, Y.H., G.K. Pandey, J.J. Grant, O. Batistic, L. Li, B.G. Kim, S.C. Lee, J. Kudla, and S. Luan. 2007. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. The Plant Journal 52: 223–239.
Dahnke, W.C., and D.C. Nelson. 1993. Soils and fertilizers. In Potato production and pest management in North Dakota and Minnesota, ed. H.L. Bissonnette et al., 29–34. St. Paul: Minnesota Ext Serv Bull 26. Univ of Minnesota.
Han, X.A., X.Q. Yang, S.M. Yang, and X.Y. Wang. 2007. Potassium nutrition characteristics of different potato strains. Journal of Sichuan Agricultural University 25: 392–396 (In Chinese with an English abstract).
Jefferson, R.A., T.A. Kavanagh, and M.W. Bevan. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6: 3901–3907.
Lechtenberg, B., D. Schuberty, A. Forsbachz, M. Gils, and R. Schmidt. 2003. Neither inverted repeat T-DNA configurations nor arrangements of tandemly repeated transgenes are sufficient to trigger transgene silencing. The Plant Journal 34: 507–551.
Li, L., B.G. Kim, Y.H. Cheong, G.K. Pandey, and S. Luan. 2006. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. PNAS 103: 12625–12630.
Liu, F., Y.Y. HU, S. NI, and X.Y. Wang. 2005. Genotypic difference in response of potato plant to deficient potassium. In Potato industry and the development in northeast of China, ed. D.Y. Qu et al., 148–158. Harbin: Harbin Engineering University (In Chinese with an English abstract).
Liu, J., C.H. Xie, and D.E. Huang. 1994. Formation mechanism of potato microtubers—influence of dark and day light on tuber initiation. Chinese Potato Journal 8: 138–141.
Matzke, M.A., and A.J.M. Matzke. 1995. How and why do plants inactivate homologous (trans) genes. Plant Physiology 107: 679–685.
Panique, E., K.A. Kelling, E.E. Schulte, D.E. Hero, W.R. Stevenson, and R.V. James. 1997. Potassium rate and source effects on potato yield, quality, and disease interaction. American Journal of Potato Research 74: 379–398.
Si, H.J., C.H. Xie, and J. Liu. 2003. An efficient protocol for Agrobacterium mediated transformation with microtuber and the introduction of an antisense class I patatin gene into potato. Acta Agronomica Sinica 29: 801–805.
Vervliet, G., M. Holsters, H. Teuchy, M. Van Montagu, and J. Schell. 1975. Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. The Journal of General Virology 26: 33–48.
Xu, J., H.D. Li, L.Q. Chen, Y. Wang, L.L. Liu, L. He, and W.H. Wu. 2006. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360.
Yan, W.D., W.M. Shi, H.Q. Liao, and Z.H. Cao. 1997. Genotypic difference of plants in K-enrichment capability and the distribution of K in plant rhizosphere. Pedosphere 7: 165–170.
Yang, T.Z., L.M. Lu, W. Xia, and J.H. Fan. 2007. Characteristics of potassium-enriched, flue-cured tobacco genotype in potassium absorption, accumulation, and in-ward potassium currents of root cortex. Agricultural Sciences in China 6: 1479–1486.
Acknowledgements
We thank Dr. Weihua Wu (State Key Laboratory of Plant Physiology and Biochemistry, China Agricultural University) for providing AtCIPK23 gene and pBI121 vector for the experiments. We also thank Dr. Conghua Xie (Key Laboratory of Horticulture Plant Biology, Huazhong Agricultural University) for technical assistance in potato transformation and molecular analysis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiyao Wang and Jia Li contributed equally to this work.
This project funded by the Application Foundation of Sichuan Province, China (O3JY029—036—2).
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wang, X., Li, J., Zou, X. et al. Ectopic Expression of AtCIPK23 Enhances Tolerance Against Low-K+ Stress in Transgenic Potato. Am. J. Pot Res 88, 153–159 (2011). https://doi.org/10.1007/s12230-010-9173-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-010-9173-0