Abstract
The magnoliid clade encompasses 18 extant families arranged in four orders, plus several extinct taxa, including some of the most ancient angiosperm fossils. The clade is characterized by paracytic stomata with a distinct pair of lateral subsidiary cells that flank the guard cells, though other stomatal types are also reported, including anomocytic and anisocytic. In contrast with monocots, the paracytic stomata of magnoliids develop from linear triads, and the lateral subsidiary cells are stomatal-lineage ground cells (SLGCs). Anisocytic stomata typically possess three SLGCs. Amplifying divisions are rare in magnoliids, but occur in some Piperales, in association with anisocytic stomata. Differences in mature stomatal types result from differences in cell shape and polarity at critical developmental stages. Stomatal clusters have been reported in Cinnamomum (Lauraceae) and Galbulimima (Himantandraceae), but neither are apparently formed by amplifying divisions, in contrast with eudicots. In Galbulimima, each peltate scale hair is surrounded by a ring of 3–8 non-contiguous stomata, each derived from different initial meristemoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two aspects of stomatal patterning have particular phylogenetic significance: the development of the stomatal apparatus and the spatial arrangement of stomata across the leaf surface. Focusing on magnoliid angiosperms, this paper fills some phylogenetically critical gaps in our understanding of stomatal development, and uses comparative and developmental data to examine their evolutionary and ecophysiological significance. More specifically, it aims to clarify the homologies of paracytic stomata in angiosperms and to determine whether amplifying divisions and stomatal clusters are present in the magnoliid clade (Magnoliidae or Magnolianae: Massoni et al., 2014).
A synthetic review of stomatal development is timely for the magnoliids. Although the mature stomata of extant magnoliids are relatively well known, few broad-scale studies have focused on their development, despite seminal work on Peperomia (Piperaceae) by Sachs and Novoplansky (1993) and reports of amplifying divisions and stomatal clusters in Cinnamomum (Lauraceae) by Zhao et al. (2006a, b). Indeed, most studies of stomatal development in magnoliids date from the pre-molecular era (Paliwal & Bhandari, 1962; Pant & Banerji, 1965; Pant & Gupta, 1966; Patel, 1971), when magnoliids were widely believed to represent the earliest relictual lineages among living angiosperms.
Characteristic aspects of stomatal development have been studied in detail in many plant groups, including both flowering and non-flowering plants (Rudall et al., 2013). Typically, stomatal development follows a “one-cell spacing” rule, which ensures the presence of at least one intervening ground cell between stomata – a pattern that is governed by key regulators such as TOO MANY MOUTHS (TMM) (Sachs, 1991; Nadeau & Sack, 2002; Hara et al., 2007). In the majority of vascular plants, stomata develop from meristemoids, which are pluripotent cells that form centers of differentiation (Bünning, 1952). A meristemoid typically undergoes a highly polarized (asymmetric) mitosis, resulting in a small cell and a larger sister cell that forms a stomatal-lineage ground cell (SLGC). The smaller daughter cell either forms a guard-cell mother cell (GMC), which divides symmetrically into two guard cells (GCs), or it forms another meristemoid that undergoes one or more asymmetric divisions (Fig. 2; see Table 1 for glossary of terms). In Arabidopsis and other eudicots, the bHLH protein SPEECHLESS (SPCH) initiates and promotes asymmetric mitoses; its paralogue MUTE regulates meristemoid differentiation and triggers the switch from a meristemoid to a GMC, which undergoes a symmetric mitosis. Finally, factors including FAMA inhibit further division and promote GC differentiation (Ohashi-Ito & Bergmann, 2006; Bergmann & Sack, 2007; Pillitteri et al., 2007; Spiegelhalder & Raissig, 2021).
The series of bHLH genes that control stomatal differentiation are well-conserved throughout the land-plant phylogeny (MacAlister & Bergmann, 2011; Ran et al., 2013; Chater et al., 2017; Bowles et al., 2022). Asymmetric mitoses characterize stomatal development in the majority of vascular plants (Payne, 1979; Rudall et al., 2013). Rare exceptions among angiosperms include the aquatic waterlily clade, in which protodermal cells give rise directly to GMCs without undergoing asymmetric division (Rudall & Knowles, 2013). Amplifying divisions, which occur in Arabidopsis and many other eudicots (Fig. 2E), are a rapid series of asymmetric mitoses confined to a single cell lineage, giving rise to a monoclonal complex of several relatively densely spaced stomata, often organized in a spiral arrangement (Zhao & Sack, 1999; Nadeau & Sack, 2002; Lau & Bergmann, 2012).
Prevalence of Paracytic Stomata
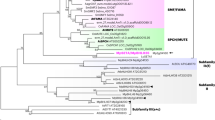
The magnoliid clade consists of 18 extant families arranged in four orders: Canellales, Laurales, Magnoliales, and Piperales (Massoni et al., 2014). It also encompasses several extinct taxa and some of the most ancient angiosperm fossils, including many in which the stomata are anatomically preserved (Friis et al. 2006; Doyle & Endress, 2010). To date, although relationships within the magnoliid clade are relatively well resolved, their broader relationships remain poorly understood, even where genomic data are available (Soltis & Soltis, 2019). Some analyses place the order Chloranthales (family Chloranthaceae) as sister to the magnoliid clade, and these two orders together as sister to all other angiosperms except the early-divergent ANA-grade lineages (Gitzendanner et al., 2018) (Fig. 1). Other analyses have tentatively indicated a sister-group relationship between magnoliids (plus Chloranthaceae and Ceratophyllaceae) and eudicots (e.g. Baker et al., 2022).
With regard to mature stomatal types, the majority of magnoliids possess paracytic stomata, which have a distinct pair of lateral subsidiary cells (LSCs) that flank the GCs (Tables 1 and 2). Other types are also reported, especially anomocytic stomata, the predominant type in Aristolochiaceae, and stomata surrounded by three or more cells – termed anisocytic or cyclocytic – in Piperaceae and Saururaceae (Tables 1 and 2; Figs. 2, 3, 4, 5 and 6). In Chloranthaceae, the putative sister family to Magnoliidae, various stomatal types have been documented, including paracytic and anomocytic (Metcalfe & Chalk, 1950; Baranova, 1983; Kong, 2001). The occurrence of mostly paracytic stomata in Canellales (Fig. 3), which is the sister order to Piperales, tentatively favors paracytic as the likely plesiomorphic condition for magnoliids. Taxa assigned to Magnoliidae also include many species known only from fossils, most of which possess paracytic stomata (Carpenter et al., 2007; Doyle & Upchurch, 2014).
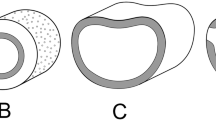
Diagrams of stomatal development. A–D Contrasting developmental pathways of paracytic stomata, showing A both lateral subsidiary cells (LSCs) mesogenous (e.g., in Magnolia), B both LSCs mesogenous, sometimes subdivided (e.g., in Annonaceae), C one LSC mesogenous, the other perigenous, both types sometimes subdivided (e.g., in Liriodendron), D both LSCs perigenous. E Amplifying divisions (e.g., Arabidopsis). F, G Development of stomata with F three stomatal lineage ground cells (SLGCs) (anisocytic stomata) and G more than three SLGCs (helicocytic stomata). H Development of non-contiguous stomatal clusters in Begonia. Yellow color indicates guard cells, red indicates meristemoid or guard-mother cell (GMC), pink indicates SLGCs, green indicates perigenous cells (not derived from meristemoid). Scales = 10 μm
Abaxial leaf surfaces in Canellales and Magnoliales, photographed from prepared microscope slides (A, B, F) or cleared leaf surfaces (C–E, G, H). A Canella alba (Canellaceae), mature paracytic stomata, each with a pair of lateral subsidiary cells (LSCs) flanking guard cells. B, C Drimys winteri (Winteraceae), mature paracytic stomata. D, E D. winteri, developing stages of stomatal development (GMC: guard-mother cell). F Magnolia grandiflora (Magnoliaceae), mature paracytic stomata (other epidermal cells with wany anticlinal walls). G M. cordata, mature paracytic stomata. H Liriodendron tulipifera (Magnoliaceae), mature paracytic stomata
Abaxial leaf surfaces in Piperales and Aristolochiales, photographed from prepared microscope slides (A, F) or cleared leaf surfaces (B–E, G–I). A Piper betle (Piperaceae), mature stomata, each surrounded by a ring or spiral of cells (gc: guard cells). B P. excelsum, mature stoma with surrounding cell spiral. C–E P. excelsum, different stages in stomatal development, showing meristemoids (m) with surrounding stomatal-lineage ground cells (SLGCs). F Asarum canadense (Aristolochiaceae), mature stomata. G A. europaeum, mature stoma with nearby trichome base (tb). H–I A. europaeum, developing stages with meristemoids, GMCs and guard cells
Stomata in Houttuynia cordata (Saururaceae). A–C, E TEMs of ultrathin sections showing cell contents (and cuticular ridge in A). (D, F, G) LMs of paradermal sections of prepared microscope slides. H, I Cleared young leaf surfaces imaged using differential interference contrast. A–D Mature stomata (lateral view in A). E, F Recently formed guard cells (gc). G–I Guard-mother cells (gmc). Scales = 10 μm
Abaxial leaf surfaces in Laurales (except Cassytha glabella, which has highly reduced scale leaves). All mature except developing leaf in (B). A, B Cinnomomum camphora (Lauraceae), A areole with stomata of paracytic, anisocytic, and unspecified types and B stomata at different stages of development, with guard cells (gc), guard-mother cells (gmc), meristemoids (m), and mesogenous lateral subsidiary cells (mlsc). C, D Cassytha glabella (Lauraceae), stem surface showing paracytic stomata with lateral subsidiary cells (lsc), arranged in a linear sequence perpendicular to the stem axis and axial cell files. E Ocotea guianensis (Lauraceae). F Nectandra salicifolia (Lauraceae). G Lindera praecox (Lauraceae). H Laurelia novae-zeylandiae (Atherospermataceae). I Hernandia cordigera (Hernandiaceae), gh: glandular hair. J Peumus boldus (Monimiaceae), single stoma. Scales = 10 μm
The predominance of paracytic stomata in magnoliids formerly indicated that this character is plesiomorphic in angiosperms (Baranova, 1972), though some authors have argued that it represents a derived condition (Carpenter, 2005). However, molecular phylogenetic placement of the ANA-grade lineages as successive sisters to all other extant angiosperms (Fig. 1) has necessitated reassessments of morphological evolution in early angiosperms and their relatives (Doyle, 2001, 2012; Doyle & Endress, 2010; Rudall & Bateman, 2019). Thus, the phylogenetic potential of paracytic stomata as a feature of some early-divergent angiosperms has remained equivocal, especially as this character state has taxonomically broad distribution throughout the angiosperms (Rudall et al., 2013).
Development from a Linear Triad
Stomatal development is notoriously difficult to track in magnoliids, for several reasons. Cell sizes are generally very small and ephemeral, perhaps partly due to relatively low genome sizes in this clade (Soltis et al., 2003; Pellicer et al., 2018). Furthermore, early developmental stages are often concealed among copious transitory trichomes that are shed as the leaf expands. The developmental origin of each cell can remain ambiguous even using the in vivo technique employed by Sachs and Novoplansky (1993) on some Piperaceae, which have relatively glabrous leaves compared with many other magnoliids.
Based on limited data, the paracytic stomatal apparatus of Drimys (Canellales) is typically formed by a triad of cells (Table 2; Fig. 2A, B). To establish a triad, a linear pair of mitoses derived from the same initial rectangular meristemoid produces a GMC flanked by two mesogene LSCs (i.e., two SLGCs). Specifically, the initial rectangular meristemoid divides in the orientation of its longer wall to form two unequal cells, of which the narrower cell divides again so that the resulting three cells are arranged with their longest walls adjoining each other, and the central cell becomes a GMC (Fig. 2A–C).
The paracytic stomata of magnoliids contrast strongly with those of grasses (Fig. 2D). In grasses and some other monocots with paracytic stomata (Fig. 2D), the LSCs are not SLGCs as they develop from adjacent cell lineages; they are recruited by the GCs during the functional maturation of the stomatal complex (Payne, 1979; Rudall et al., 2013, 2017). In grasses such as Brachypodium, this targeted lateral polarization of cells in adjacent files is controlled by the bHLH transcription factor MUTE, which has apparently adopted a novel role in commelinid monocots, compared with its primary role in eudicots to promote GC formation (Raissig et al., 2017; Spiegelhalder & Raissig, 2021). Conversely, in magnoliids and many other taxa with paracytic stomata, the LSCs are mesogenous (i.e., SLGCs), developing from the same meristemoid as the GCs (Fig. 2A–C). This distinction is important in evolutionary terms because it means that different types of LSC are controlled by different genes.
A broadly similar developmental pathway to that of Canellales occurs in Annonaceae and Magnoliaceae (Magnoliales) and also in some Lauraceae (Laurales) (Table 2). On the other hand, species in the order Piperales appear to vary in this respect. In Asarum europaeum (Aristolochiaceae), stomata are mostly mesoperigenous, each possessing only a single SLGC (Fig. 4F–I). Conversely, in some Piperaceae, from one to four of the surrounding cells can be SLGCs (Table 2; Fig. 4A–E).
The parasitic genus Cassytha (Lauraceae) has highly reduced scale leaves and bears stomata on its long twining stems (Fig. 6C, D). Although stomatal development is currently undocumented in this genus, it is interesting to compare the mature stomata with those of grasses. In both cases, the stomata are paracytic and borne in linear cell files, but GC orientation is radically different. In grasses, the long GC axis is oriented parallel to the stomatal cell file and the LSCs are recruited from adjacent cells files (see above). These two features – axial stomatal orientation and perigene LSCs – are highly constrained in grasses; for example, they remained unchanged even in severely affected stomatal mutants of barley (Zeiger & Stebbins, 1972). In contrast, in Cassytha, the long GC axis is invariably oriented perpendicular to the stomatal cell files and the LSCs are located in the same files, strongly indicating that they are SLGCs, as in other magnoliids.
Amplifying Divisions are Rare in Magnoliids
Amplifying divisions represent a series of asymmetric mitoses derived from the same initial meristemoid, of which the resulting SLGCs can divide asymmetrically two or more times, thereby forming a loose inward spiral of cells incorporating one or more stomata (Fig. 2E). This non-linear pattern was termed helicocytic by Payne (1979); it relies on cell shapes that are isodiametric rather than rectangular or elongated, so that amplifying divisions are typically angled relative to other cells (Zhao & Sack, 1999; Serna, 2009). Amplifying divisions are absent from monocots, even from species with relatively broad, non-linear leaves (Tomlinson, 1974; Rudall et al., 2017). They are also rare in early-divergent (ANA- or ANITA-grade) angiosperm lineages (Rudall & Knowles, 2013). Furthermore, taken in their strictest sense, amplifying divisions are apparently absent from non-angiosperms, even among species with reticulate leaf venation, such as Gnetum and fossil Bennettitales (Rudall & Bateman, 2019; Rudall & Rice, 2019). Thus, amplifying divisions are primarily a eudicot feature. Anisocytic stomata (which have three subsidiary cells: Table 1) frequently occur in eudicots, though they are relatively rare in other taxa. They are common in the asterid and rosid eudicot clades, (Wilkinson, 1979; Rudall et al., 2018), including Arabidopsis and other Brassicaceae, in which they are surrounded by three SLGCs (Zhao & Sack, 1999). They are formed by formed by two divisions of the initial meristemoid, of which the second is an amplifying division.
Amplifying divisions are rare in magnoliids, but their occurrence in some species remains equivocal, because neighboring epidermal cells can undergo secondary spacing divisions (i.e., expansion divisions, rather than amplifying divisions). However, in some magnoliids (e.g., Houttuynia; Saururaceae), mature and developing stomata are surrounded by a ring-like or spiral arrangement of cells that are likely to be SLGCs, here clearly visible at a range of developmental stages (Fig. 5). Peterson et al. (2010) plausibly hypothesized that stomatal complexes in Houttuynia could result from an extended series of divisions caused by delayed MUTE-like activity. Similarly, stomata are surrounded by a ring-like or spiral cell arrangement in some other magnoliids, notably in some Piperaceae (Piper, Fig. 4A–E), resembling the helicocytic stomata of many eudicots (Payne, 1970).
During formation of a typical anisocytic stoma in Peperomia pellucida (Piperaceae), the meristemoid cuts off three unequal neighboring cells on its three sides, forming three successive intersecting walls in a spiral sequence, with a triangular GMC in the center (Pant & Banerji, 1965). Thus, it is arguably the case that some magnoliids undergo amplifying divisions during stomatal development.
Stomatal Clusters in Magnoliids
Stomatal clusters can be formed in various ways in different species. Occasional examples of contiguous stomatal clusters that have been reported spasmodically throughout land plants are essentially teratological: the GCs of adjacent stomata are in contact with each other, thus breaking the “one-cell spacing” rule. In contrast, non-contiguous stomatal clusters characterize many xerophytic eudicots. They are exemplified by the stomatal clusters in some Begonia species (Fig. 2H), which represent monoclonal complexes of two or more stomata each separated by at least one ground cell (termed “compound helicocytic stomata” by Payne, 1970). The Begonia-type clusters are derived by an extended series of amplifying divisions from the same initial meristemoid, often arranged in a loose spiral (Rudall et al., 2018). Gan et al. (2010) observed a positive correlation between stomatal clustering and drought tolerance, and suggested that this feature enhances water efficiency by reducing the surface area for water loss by transpiration (see also Tsai et al., 2022).
Non-contiguous stomatal clusters have been reported in about 20 angiosperm families (Payne, 1970; Wilkinson, 1979; Gan et al., 2010), most of which are eudicots. In some cases, their developmental pathway remains undetermined. For example, in many xerophytes, such as Nerium oleander, the stomata are protected by depressions, crypts or grooves in interveinal regions (areoles) on the leaf surface (Dickison, 2000). These depressions trap a chamber of relatively humid air, reducing the diffusion gradient of water vapour. Thus, stomatal clustering is positively correlated with drought tolerance (Gan et al., 2010; Tsai et al., 2022).
Stomatal clusters have been reported in only two families of magnoliids: Lauraceae (Cinnamomum: Zhao et al., 2006a, b) and Himantandraceae (Baranova, 1972). However, neither of these cases represent clusters that are derived from amplifying divisions. In Cinnomomum camphora, successive development of stomata occurs within each areole, resulting in groups of non-contiguous stomata (Zhao et al., 2006a, b). However, as shown here (Fig. 6B), at least some of the stomata in C. camphora are paracytic and were formed by developmental triads.
The case of the tropical Australasian shrub Galbulimima (Himantandraceae) is remarkable in that each peltate scale hair on the abaxial leaf surface is surrounded by a regular ring of 3–8 non-contiguous stomata (Fig. 7), with the long axes of their GCs oriented circumferentially around the hair bases (Bailey et al., 1943; Baranova, 1972). This arrangement is often referred to as a stomatal cluster. However, although their development has not been studied, the stomata are clearly derived from different initial meristemoids. The scales each form an umbrella over the stomata, potentially trapping water vapor.
Stomata around trichome bases (hb). A Stomatal ring in Galbulimima belgraveana (Himantandraceae), reproduced from Baranova (1972, listed as Himantandra parvifolia). B Trichome base in Trimenia weinmanniifolia (Trimeniaceae), with radiating cells including stomata. scale = 10 μm
Morphogenetic factors responsible for the non-random arrangement of stomata in Galbulimima remain unknown. Several authors have observed interactions between different epidermal cell types during ontogeny, especially between developing stomata and hairs (Bünning & Sagromsky, 1948). Bünning (1952) also noted a mutual inhibition between loci of active protoplasmic growth, suggesting that in Begonia, this localized inhibition results in the positioning of stomatal clusters over large intracellular spaces in the mesophyll. It is not unique for cells (including stomata) to radiate around trichome bases, but Galbulimima displays an unusual degree of regularity, and unusual stomatal orientation. By contrast, in the early-divergent angiosperm Trimenia (Trimeniaceae: Fig. 7A, B), stomata are located close to the trichome base but do not form a clear circumferentlal ring.
Among other magnoliids, Sachs & Novoplansky (1993) examined the developmental cellular interactions of stomata and glandular hairs in Peperomia (Piperaceae, Piperales) and noted oriented divisions of the cells surrounding both the stomata and the glands. Descriptions by Bongers (1973) included images of stomata radiating round glandular hairs in Zygogynum (as Bubbia; Winteraceae, Canellales). Koster and Baas (1981) illustrated a ring of (paracytic) stomata surrounding a glandular trichome in Horsfieldia crassifolia (Myristaceae, Magnoliales), with various orientations. They compared the stomatal ring in some Horsfieldia species with a similar phenomenon in some eudicots, such as Kostermansia (Malvaceae), in which the bases of the epidermal scales are surrounded by a ring of anisocytic stomata (Baas, 1972).
Conclusions and Perspectives
The predominance of paracytic stomata in magnoliids is well-documented. However, the possession of paracytic stomata represents a morphological condition that can mask different underlying developmental pathways. Comparative developmental studies of stomata have demonstrated strong contrasts between monocots and other angiosperms. In monocots (which include the grasses), amplifying divisions are absent; mature stomata are either anomocytic or paracytic, and the paracytic stomata invariably have perigenous LSCs (Rudall et al., 2017). In grasses, new technologies that allow affordable and efficient high-throughput sequencing and gene editing have enabled the discovery of a novel role for the MUTE transcription factor in promoting the four-celled paracytic stomatal complex (Raissig et al., 2017; Spiegelhalder & Raissig, 2021). By inference, it seems likely that this role was co-opted early in the monocot phylogeny, though the relatively compact stomatal complexes of grasses are functionally highly dynamic; the LSCs are physiologically active and can fuel the GCs, which themselves regulate the stomatal aperture (Franks & Farquhar, 2007; Gray et al., 2020).
In contrast, the paracytic stomata of magnoliids mostly develop from linear triads that represent a GMC flanked by two SLGCs. This mesogenous developmental pathway mirrors that observed in some living Gnetales and fossil Bennettitales; both are seed-plant groups with paracytic stomata, and both have had putative associations with the angiosperm stem groups (Rudall & Bateman, 2019; Rudall & Rice, 2019). Among extant early-divergent angiosperms, mesogene neighbor cells (SLGCs) can occur in Amborella and Austrobaileyales but are absent from Nymphaeales (Rudall & Knowles, 2013). Indeed, the absence of amplifying divisions during stomatal development has been tentatively correlated with aquatic environments, leading Doll et al. (2021) to hypothesize that this type of development might be advantageous in these conditions, perhaps allowing more rapid stomatal differentiation directly from protodermal cells.
This review highlights the critical importance of cell shape and polarity in understanding stomatal diversity. Differences between paracytic and anisocytic stomata result primarily from differences in protodermal cell shapes and division orientation at critical stages in leaf expansion. Specifically, in paracytic stomatal complexes, which develop from linear triads, the initial meristemoid is not triangular. In contrast, anisocytic stomatal complexes develop from triangular meristemoids by helicocytic patterning. The presence in some magnoliids (Piperaceae and Saururaceae) of anisocytic stomata with three SLGCs, or sometimes with a ring-like or spiral (helicocytic) arrangement of SLGCs, indicates a clear developmental similarity with the eudicot clade. More targeted future studies can apply new technologies to help determine the evolution of stomatal patterning in angiosperms.
References
Baas, P. 1972. The vegetative anatomy of Kostermannia malayana Soegeng. Reinwardtia 8: 335–344.
Bailey, I. W., C. G. Nast & A. C. Smith. 1943. The family Himantandraceae. Journal of the Arnold Arboretum 24: 190–206.
Bailey, I. W. & A. C. Smith. 1942. Degeneriaceae, a new family of flowering plants from Fiji. Journal of the Arnold Arboretum 23: 356–365.
Baker, W.J., P. Bailey, V. Barber, A. Barker, S. Bellot, D. Bishop, L. R. Botigue, G. Brewer, T. Carruthers, J. J. Clarkson, J. Cook, R. S. Cowan, S. Dodsworth, N. Epitawalage, E. Francoso, B. Gallego, M. G. Johnson, J. T. Kim, K. Leempoel, O. Maurin, C. Mcginnie, L. Pokorny, S. Roy, M. Stone, E. Toledo, N. J. Wickett, A. R. Zuntini, W. l. Eiserhardt, P. J. Kersey, I. J. Leitch & F. Forest. 2022. A Comprehensive Phylogenomic Platform for Exploring the Angiosperm Tree of Life. Systematic Biology 71: 301–319.
Baranova, M. 1972. Systematic anatomy of the leaf epidermis in the Magnoliaceae and some related families. Taxon 21: 447–469.
Baranova, M. 1983. On the laterocytic stomatotype in angiosperms. Brittonia 35: 93–102.
Bergmann, D. C. & F. D. Sack. 2007. Stomatal development. Annual Review of Plant Biology 58: 163–181.
Bongers, J. M. 1973. Epidermal leaf characters of the Winteraceae. Blumea 2I: 381–411.
Bowles, A. M. C., J. Paps & U. Bechtold. 2022. Water-related innovations in land plants evolved by different patterns of gene co-option and novelty. New Phytologist 235: 732–742.
Bünning, E. 1952. Morphogenesis in plants. Pp. 105–140. In: Avery, G. S., (ed.), Survey of biological progress, vol II. Academic Press, New York, USA.
Bünning, E. & H. Sagromsky. 1948. Die Bildung des Spaltöffnungsmusters in der Blattepidermis. Zeitschrift für Naturforschung 3b: 203–216.
Carlquist, S. 1964. Morphology and Relationships of Lactoridaceae. Aliso 5: 421–435.
Carlquist, S. 1992. Vegetative Anatomy and Relationships of Eupomatiaceae. Bulletin of the Torrey Botanical Club 119: 167–180.
Carpenter, K. J. 2005. Stomatal architecture and evolution in basal angiosperms. American Journal of Botany 92: 1595–1615.
Carpenter, K. J., G. J. Jordan & R. S. Hill. 2007. A toothed Lauraceae leaf from the early Eocene of Tasmania, Australia. International Journal of Plant Science 168: 1191–1198.
Chater, C. C., R. S. Caine, A. J. Fleming, J. E. Gray. 2017. Origins and evolution of stomatal development. Plant Physiology 174: 624–638.
Dickison, W. C. 1996. Stem and leaf anatomy of Saruma henryi Oliv., including observations on raylessness in the Aristolochiaceae. Bulletin of the Torrey Botanical Club 123: 261–267.
Dickison, W. C. 2000. Integrative plant anatomy. Academic Press, San Diego, California, USA.
Doll, Y., H. Koga & H. Tsukaya. 2021. The diversity of stomatal development regulation in Callitriche is related to the intrageneric diversity in lifestyles. Proceedings of the National Academy of Sciences, USA 118: e2026351118.
Doyle, J. A. 2001. Significance of molecular phylogenetic analyses for paleobotanical investigations on the origin of angiosperms. Palaeobotanist 50: 167–188.
Doyle, J. A. 2012. Molecular and fossil evidence on the origin of angiosperms. Annual Review of Earth and Planetary Sciences 40: 301–326.
Doyle, J. A. & P. K. Endress. 2010. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: Magnoliidae and eudicots. Journal of Systematics and Evolution 48: 1–35.
Doyle, J. A. & G. R. Upchurch. 2014. Angiosperm clades in the Potomac Group: what have we learned since 1977? Bulletin of the Peabody Museum of Natural History 55: 111–134.
Franks, P. J. & G. D. Farquhar. 2007. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiology 143: 78–87.
Friis, E. M., K. R. Pedersen & P. R. Crane. 2006. Cretaceous angiosperm flowers: innovation and evolution in plant reproduction. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 251–293.
Gan Y., L. Zhou, Z. J. Shen, Y. Q. Zhang & G. X. Wang. 2010. Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Botanical Studies 51: 325–336.
Gitzendanner, M. A., P. S. Soltis, G. K. S. Wong, B. R. Ruhfel & D. E. Soltis. 2018. Plastid phylogenomic analysis of green plants: a billion years of evolutionary history. American Journal of Botany 105: 291–301.
Gray, A., L. Liu & M. Facette. 2020. Flanking support: how subsidiary cells contribute to stomatal form and function. Frontiers in Plant Science 11: 881.
Hara, K., R. Kajita, K. U. Torii, D. C. Bergmann & T. Kakimoto. 2007. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes and Development 21: 1720–1725.
Kong, H. Z. 2001. Comparative morphology of leaf epidermis in the Chloranthaceae. Botanical Journal of the Linnean Society 136: 279–294.
Koster, J. & P. Baas. 1981. Comparative leaf anatomy of the Asiatic Myristicaceae. Blumea 27: 115–173.
Lau, O. S. & D. C. Bergmann. 2012. Stomatal development: A plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development 139: 3683–3692.
MacAlister, C. A. & D. C. Bergmann. 2011. Sequence and function of basic helix-loop-helix proteins required for stomatal development in Arabidopsis are deeply conserved in land plants. Evolution and Development 13: 182–192.
Mandal, M., S. Mitra & D. Maity. 2012. Structure of polymorphic stomata in Canella winterena (L.) Geartn. (Canellaceae). Feddes Repertorium 123: 295–303.
Massoni, J., F. Forest & H. Sauquet. 2014. Increased sampling of both genes and taxa improves resolution of phylogenetic relationships within Magnoliidae, a large and early-diverging clade of angiosperms. Molecular Phylogenetics and Evolution 70: 84–93.
Metcalfe, C. R. & L. Chalk. 1950. Anatomy of the Dicotyledons. Clarendon Press, Oxford, UK.
Nadeau, J.A. & F. D. Sack. 2002. Stomatal development in Arabidopsis. The Arabidopsis Book 1: e0066.
Ohashi-Ito, K. & D. C. Bergmann. 2006. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. The Plant Cell 18: 2493–2505.
Paliwal, G. S. & N. N. Bhandari. 1962. Stomatal development in some Magnoliaceae. Phytomorphology 12: 409–412.
Pant, D. D. & R. Banerji. 1965. Structure and ontogeny of stomata in some Piperaceae. Journal of the Linnean Society (Botany) 378: 223–228
Pant, D. D. & K. L. Gupta. 1966. Development of stomata and foliar structure of some Magnoliaceae. Journal of the Linnean Society (Botany) 379: 265–277.
Patel, R. J. 1971. Epidermal structure and development of stomata in some Annonaceae. Annals of Botany 35: 1205–1212.
Payne, W. W. 1970. Helicocytic and allelocytic stomata: unrecognized patterns in the Dicotyledonae. American Journal of Botany 57: 140–147.
Payne, W. W. 1979. Stomatal patterns in embryophytes: their evolution, ontogeny and interpretation. Taxon 28: 117–132.
Pellicer, J., O. Hidalgo, S. Dodsworth & I. J. Leitch. 2018. Genome size diversity and its impact on the evolution of land plants. Genes 9: 88.
Peterson, K. M., A. L. Rychel & K. U. Torii. 2010. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. The Plant Cell 22: 296–306.
Pillitteri, L. J., D. B. Sloan, N. L. Bogenschutz & K. U. Torii. 2007. Termination of asymmetric cell division and differentiation of stomata. Nature 445: 501–505.
Raissig, M. T., J. L. Matos, M. X. Anleu Gil, A. Kornfeld, A. Bettadapur, E. Abrash, H. R. Allison, G. Badgley, J. P. Vogel, J. A. Berry & D. C. Bergmann. 2017. Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 355: 1215–1218.
Ran, J. H., T. T. Shen, W. J. Liu & X. Q. Wang. 2013. Evolution of the bHLH genes involved in stomatal development: implications for the expansion of developmental complexity of stomata in land plants. PLOS One 8: e78997.
Rudall, P. J. & R. M. Bateman. 2019. Leaf surface development and the plant fossil record: stomatal patterning in Bennettitales. Biological Reviews 94: 1179–1194.
Rudall, P. J., E. D. Chen & E. Cullen. 2017. Evolution and development of monocot stomata. American Journal of Botany 104: 1122–1141.
Rudall, P. J., J. Hilton & R. M. Bateman. 2013. Several developmental and morphogenetic factors govern the diversity of stomatal development in land plants. New Phytologist 200: 598–614.
Rudall, P. J., A. C. M. Julier & C. A. Kidner. 2018. Ultrastructure and development of non-contiguous stomatal clusters and helicocytic patterning in Begonia. Annals of Botany 122: 767–776.
Rudall, P. J. & E. V. W. Knowles. 2013. Ultrastructure of stomatal development in early-divergent angiosperms reveals contrasting patterning and pre-patterning. Annals of Botany 112: 1031–1043.
Rudall, P. J. & C. L. Rice. 2019. Epidermal patterning and stomatal development in Gnetales. Annals of Botany 124: 149–164.
Sachs, T. 1991. Pattern formation in plant tissues. Cambridge University Press, Cambridge, UK.
Sachs, T. & N. Novoplansky. 1993. The development and patterning of stomata and glands in the epidermis of Peperomia. New Phytologist 123: 567–574.
Serna, L. 2009. Cell fate transitions during stomatal development. BioEssays 31: 865–873.
Soltis, D. E. & P. S. Soltis. 2019. Nuclear genomes of two magnoliids. Nature Plants 5: 6–7.
Soltis, D. E., P. S. Soltis, M. D. Bennett & I. J. Leitch. 2003. Evolution of genome size in the angiosperms. American Journal of Botany 90: 1596–1603.
Spiegelhalder, R. P. & M. T. Raissig. 2021. Morphology made for movement: formation of diverse stomatal guard cells. Current Opinion in Plant Biology 63: 102090
Takemori, N. K., C. Bona & N. Alquini. 2003. Anatomia comparada das folhas de espécies de Peperomia (Piperaceae). I. Ontogênese do tecido aqüífero e dos estômatos. Acta Botanica Brasiliensis 17: 387–394.
Tomlinson, P. B. 1974. Development of the stomatal complex as a taxonomic character in the monocotyledons. Taxon 23: 109–128.
Tsai, M. Y., C. Kuan, Z. L. Guo, H. A. Yang, K. F. Chung & C. M. K. Ho. 2022. Stomatal clustering in Begonia improves water use efficiency by modulating stomatal movement and leaf structure. Plant-Environment Interactions. DOI: https://doi.org/10.1002/pei3.10086.
Wilkinson, H. P. 1979. The plant surface (mainly leaf). Part 1: stomata. Pp. 97–117. In: C. R. Metcalfe & L. Chalk [eds.], Anatomy of the dicotyledons, 2nd edn., vol. 1. Clarendon Press, Oxford, UK.
Zeiger, E. & G. L. Stebbins. 1972. Developmental genetics in barley: a mutant for stomatal development. American Journal of Botany 59: 143–148.
Zeng, G., B. Liu, H. van der Werff, D. K. Ferguson & Y. Yang. 2014. Origin and evolution of the unusual leaf epidermis of Caryodaphnopsis (Lauraceae). Perspectives in Plant Ecology, Evolution and Systematics 16: 296–309.
Zhao, L. & F. D. Sack. 1999. Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. American Journal of Botany 86: 929–939.
Zhao, X., Y. Yang, Z. Shen, H. Zhang, G. Wang & Y. Gan. 2006a. Stomatal clustering in Cinnamomum camphora. South African Journal of Botany 72: 565–569.
Zhao, X., X. Dai, G. Wang, Z. Shen, H. Zhang & M. Qiu. 2006b. Developmental mechanism and distribution pattern of stomatal clusters in Cinnamomum camphora Russian Journal of Plant Physiology 53: 310–315.
Acknowledgements
All images except Fig. 7D were made by the author from the collection of permanent microscope slides located at the Royal Botanic Gardens, Kew, or from cleared developing leaves sampled from Kew’s living collections. Figure 7D was reproduced from Baranova (1972), with permission from John Wiley and Sons (License Number 5293601116120, issued Apr 21, 2022). Elisabeth Chen sectioned the material of Houttuynia cordata illustrated in Fig. 5 during her placement year at Kew from Imperial College in 2012. Pieter Baas kindly supplied information on stomatal clusters. Richard Bateman and two anonymous reviewers critically reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rudall, P.J. Phylogenetic, Developmental and Functional Aspects of Stomatal Patterning: Lessons from Magnoliids. Bot. Rev. 89, 1–18 (2023). https://doi.org/10.1007/s12229-023-09287-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-023-09287-9