Abstract
The New York Botanical Garden occupies 100 hectares (250 acres) in the north central portion of Bronx County, New York. The property is a public garden with the majority of the grounds under cultivation. The Thain Family Forest, margins of the Bronx River, rock outcrops and areas of undeveloped landscape are important refugia for spontaneous plants, both native and non-native. We compiled data from historic lists of spontaneously occurring species, conducted original field work and searched the William and Lynda Steere Herbarium to determine the historic and extant spontaneous vascular plant flora of the Garden from 1899 to 2015. This is the first published inventory of the wild flora since 1899. The historic and extant flora comprises 695 species and infraspecies in 363 genera and 121 families. The extant flora comprises 429 species and infraspecies in 263 genera and 108 families. A total of 264 (62%) of the extant species and infraspecies are native and 165 (38%) are not native. All species are vouchered by herbarium specimens collected between 1881 and 2015. All herbarium vouchers are databased, imaged and available online. Forty-six species and infraspecies are new Bronx County records. Among the rare extant species and infraspecies on the New York State Department of Environmental Conservation Active Inventory List are three critically imperiled (S1) and two imperiled (S2) taxa, and Carex aggregata that was thought to be historical (SH, no existing sites known) in New York State, but was found in 2009 on the grounds. The number of extant rare species and infraspecies, the total number of extant taxa and the percentage of native verus non-native species strongly supports the conclusion that The New York Botanical Garden is an important and significant refugium or hotspot for local biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The New York Botanical Garden was established in 1891 by an act of the State Legislature of New York to pursue a mission of botanical research, education, and horticulture. In 1895, founding director Nathaniel Lord Britton selected a 100-hectare site in the Bronx, New York’s northernmost borough, as the Garden’s location. Britton chose the site because it included a 20-hectare (50-acre) old-growth forest surrounded by fields with soils suitable for growing a diversity of plants to serve the Garden’s research and educational activities. Even as they were assembling living collections of plants from around the world, the Garden’s founders were documenting the spontaneous flora of the landscape. Early inventories of the Garden’s plant collections listed wild plants as well as cultivated specimens. Botanists deposited vouchers of wild plants collected in the Garden and adjacent Bronx Park in the Garden’s Herbarium. Native trees were identified and labeled for the public’s benefit.
Elizabeth Gertrude Knight Britton, noted bryologist and wife of Nathaniel Britton, took a particular interest in the native plants of the Garden. In the early 1900s she noted that populations of certain native species in the Garden were declining precipitously due to a variety of factors. Her concern about the fate of native plants at the Garden and beyond inspired her to become a founding member of the Wildflower Preservation Society.
The spontaneous flora of The New York Botanical Garden has changed significantly since the Britton’s time. As the Bronx went from a rural or suburban environment to a completely urban one, and as areas of the Garden were devoted to ornamental plantings, the proportion of native and non-native species changed in largely predictable patterns that have been observed throughout the region and indeed throughout the world. Introduced pathogens decimated once robust populations of American chestnut (Castanea dentata) and American elm (Ulmus americana) in the early 1900s, eastern hemlock (Tsuga canadensis) in the 1980s, and currently threaten our native ashes (Fraxinus species).
Determining the spontaneous flora of the grounds of a botanical garden presents a number of unique challenges. It is not always clear if a given plant is cultivated or spontaneous, particularly if it occurs in areas that were once actively cultivated but may be fallow. Some cultivated plants, particularly annuals, reoccur from year to year but only in or around locations where they are regularly planted. Some plants persist and spread vegetatively but do not appear to form spontaneous new populations. Some plants appear to be spontaneous but are present in such low numbers that their long-term viability as naturalized populations is uncertain.
In spite of these challenges, documenting the spontaneous flora of the Garden’s dynamic landscape serves many purposes. It provides invaluable information for horticulturists at the Garden and throughout the region, who can use these data to identify potential new invasive species. This information will improve regional efforts to prevent future invasions. These data also document biodiversity for ecological studies, regional floras and conservation efforts.
In 1916, Nathaniel Lord Britton and Elizabeth Gertrude Knight Britton argued that The New York Botanical Garden and other green spaces should serve as refuges for native plants in the City (E. K. Britton, 1916; N. L. Britton, 1916). In spite of significant stresses inherent in its urban location and substantial anthropogenic change to its spontaneous flora, the Garden remains a refugium for native species and perhaps because of these stresses, is a compelling location for botanical and ecological research.
The percentage of native (62%) versus non-native spontaneous species and infraspecies (38%) extant in the Garden today compares favorably with other natural areas in the region, including New York's Central Park with sixty percent non-native (DeCandido et al., 2007), Pelham Bay Park with sixty-one percent native (DeCandido, 2004) and Concord, Massachusetts with sixty-one percent native (Primack et al., 2009). Of course there are many factors complicating a comparison such as the total area covered by the flora, site history, location, and study methods. However, the data presented here are a first approximation and guide for research and conservation efforts.
Site description
The New York Botanical Garden (40.862N, 73.877W) is set on 100 hectares (250 acres) in Bronx County, New York. Designated a National Historic Landmark in 1967, the Garden features 50 curated gardens, displays, and plant collections and extensive natural areas including the 20-ha (50-acre) old-growth Thain Family Forest, the Bronx River and its floodplain, Twin Lakes, and the Mitsubishi Wild Wetland (Rachlin et al., 2007). Approximately 70 hectares (175 acres) of the landscape is intensively cultivated, paved, or otherwise developed. In addition to the spontaneous flora documented here, the Garden’s living collections include more than one million plants representing more than 14,000 taxa growing out of doors or under glass.
The Garden is located in the southwestern corner of the New England Physiographic Province, near the confluence of New England, Atlantic Coastal Plain, and Piedmont Provinces, and is characterized by warm humid summers and cold winters with periodic drought and regular severe storms (N. L. Britton, 1913; Anonymous, 1938, 1944). The mean average annual temperature measured at Central Park 12 km (10 mi) southwest of the Garden is 12.72° C (54.90°F) with a January average of 0.33° C (32.59°F) and a July average of 24.72° C (76.50°F) (NWS, 2014). The annual precipitation is 126.85 cm (49.94in), evenly distributed throughout the year (NWS, 2014).
The geology of the Garden includes all of the primary bedrock formations of New York City: Fordham Gneiss, Inwood Marble and Walloomsac Schist west of the Bronx River; Manhattan Schist in the center of the Garden; and the Hartland Formation in the eastern portion of the landscape (Merguerian, 2013). Bedrock throughout the Garden exhibits evidence of glaciation, including glacial striations, glacial erratics, and glacial potholes. The highest point is 43 m (141 ft) at the top of a rock outcrop in the center of the Forest and the lowest point is 10 m (32 ft) where the Bronx River passes beneath the Linnaean Bridge at Fordham Road (LaFave et al., 2003). The soils of upland areas consist largely of Charlton, Chatfield, and Hollis acidic sandy loams with varying depths to bedrock (Shaw et al., 2007). In wetlands and along the Bronx River floodplain, soils consist of Canandaigua (fine-silty), Tonawanda (coarse-silty), and Natchaug (loamy) silt loams or organic soils (Shaw, 2010). Recent studies have revealed that the Garden soils have a pH range of 3.9–8.0; have high concentrations of heavy metals including zinc, lead, and copper; are compacted due to excessive trampling; and exhibit hydrophobic properties due to long-term deposition of hydrocarbons from the burning of fossil fuels (White & McDonnell, 1988; Schuler, 2006, 2011; Gabel, 2014;).
Nathaniel Lord Britton and the Garden’s founding Board of Managers chose the Garden’s location in 1895 because the site included many features considered ideal for the establishment of an institution devoted to the study and display of plants: accessibility, rich and varied soil, beautiful scenery, and the largest remnant of natural forest in New York City (Britton et al., 1895). From the beginning, Britton and his scientific colleagues recognized that the wild plants growing in the Garden’s landscape could serve the institution’s mission. The 1896 “General Plan of The New York Botanical Garden” developed by Britton and landscape architect John Brinley with input from Calvert Vaux and Samuel Parsons, preserved the Forest, known then as the “Hemlock Grove,” in its entirety (Britton, 1904). Britton also decreed that the many mature native trees that shaded the old fields around the Hemlock Grove should be protected during the construction of roads and buildings. The Forest today is a 20 hectare (50 acre) uneven aged, mixed hardwood, remnant urban old-growth forest that has never been significantly cleared and effectively remains a natural ecosystem (McDonnell, 1988; Shaw et al., 2007; Loeb, 2011).
There have been many changes to the spontaneous flora of the Garden since the late 1800s. As early as 1902, Garden scientists documented that the over-collection of native wildflowers (e.g., Arisaema triphyllum and Epigaea repens) was leading to their decline (Britton, 1902; Copp, 1904; Britton, 1912a, 1912b; E. K. Britton, 1913). Chestnut blight (Cryphonectria parasitica [Murrill] Barr) led to the demise of 1500 Garden individuals of American chestnut in the early 20th century (Anonymous, 1911). Eastern hemlock, which represented 36% of the canopy trees in the Forest in 1937, has been nearly eliminated by two invasive insects: elongate hemlock scale (Fiorinia externa Ferris) and hemlock wooly adelgid (Adelges tsugae Annand) (Rudnicky & McDonnell, 1989; Schuler & Forrest, 2016). The trampling of soil by crowds of visitors early in the Garden's history was observed and mitigated by fencing the paths in the Hemlock Grove (Britton, 1904).
Although extensive research indicates that the Forest has never been cleared and remains an effectively natural ecosystem in the middle of one of the world’s most developed urban areas, its spontaneous flora has changed significantly since 1895 (McDonnell, 1988; Shaw et al., 2007; Loeb, 2011). In 1937 the most abundant canopy species based on density were Tsuga canadensis, Quercus spp., and Betula lenta (Rudnicky & McDonnell, 1989). In 2011 the most abundant canopy species based on density were Quercus spp., Prunus serotina, and Acer rubrum (Schuler & Forrest, 2016).
As the Garden’s native plant populations have changed, the number of naturalized species in the flora has increased. Some, such as Ficaria verna and Reynoutria spp., have become invasive and pose a serious threat to remaining populations of native plants. Recent efforts to control the spread of invasive species and re-establish populations of extirpated species in the Thain Family Forest indicate that some of the changes to the Garden’s spontaneous flora may be reversible (Schuler & Forrest, 2016).
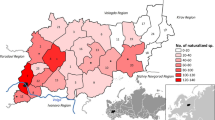
The boundaries of the Garden have shifted since 1895 (Fig. 1). In 1915, land east of the Bronx River including the former Lorillard Mansion and 56 hectares (140 acres) south of the Stone Mill were acquired (Britton et al., 1915). In 1937, approximately 48 hectares (120 acres) of land in the northeastern part of the Garden, including the historic shrub and willow collections and a portion of the Bronx River and its floodplain, were relinquished for the expansion of the Bronx River Parkway (Robbins, 1939; Robbins, 1941).
Materials and methods
Names of flowering plants, ferns, and mosses growing wild at the time the Garden was incorporated were published in a series of appendices to the Annual Reports in the Bulletin of The New York Botanical Garden in 1898, 1899, and 1900. The publications are unsigned and there are no introductions or explanation as to how the surveys were conducted, nor, more importantly, whether specimens were collected and preserved. Only in the case of the mosses is there any explicit mention of collections (Nash, 1900).
The first list (Britton, 1898) is an inventory of all plants "in the Grounds" of the Garden, including cultivated plants in nurseries, greenhouses, herbaceous grounds, borders, bog gardens, etc. The wild plants are marked with a "W", nursery plants with "N", greenhouse plants with "G" and so on. The list consists of binomials and trinomials alphabetical by genus and species with no families, authors, nor specimens cited. In the second list (Britton, 1899), those marked as "W" from the 1898 list were extracted and published under the heading "Wild Flora" apart from sections detailing the plants under cultivation. The list is nearly the same as the first list of binomials and trinomials but with the names grouped by family and arranged systematically by kingdom, subkingdom and family. Again, the names do not have authors and no specimens are cited. Some species were added to the list (e.g., Acer saccharinum, Cuscuta gronovii, Geranium maculatum, Ilex verticillata, Linum virginianum, Vaccinium corymbosum). In 1900, George V. Nash, the head gardener, published a list of additions to the 1899 list. This third list comprises mosses and 22 ferns and flowering plants (Nash, 1900).
After publication of the early Garden flora, only occasional accounts of various groups of wild plants (ferns, shrubs, and trees) were produced, some published and some not. From 1917 to 1920, Nash produced a series of works on the "Hardy Woody Plants in The New York Botanical Garden (Nash, 1917–1920). John Kunkel Small and Edward Johnston Alexander later published separate lists of native ferns (Small & Alexander, 1933a; Small, 1934), shrubs (Small & Alexander, 1933b), and trees (Small & Alexander, 1933c). The list of shrubs includes fifty species of native shrubs they report as "self-maintained" as well as thirty-five "Old World shrubs naturalized in the Garden". For the most part, however, their lists do not indicate which are naturalized and which are planted and they do not cite specimens.
Between 1950 and 1986, collecting on the grounds of the Garden was very sporadic. Not until after Michael Nee arrived at the Garden in 1986, was there an effort to systematically document the spontaneous flora. Nee collected numerous specimens on the grounds always indicating whether a species was likely spontaneous or cultivated. Nee kept a working manuscript of the Garden flora, adding species and observations as they were encountered.
From 1986 to 2015 and throughout all seasons, we explored the Garden grounds identifying and collecting those species not yet documented or exhibiting unusual morphological or phenological traits (such as unusual flowering times, invasiveness, etc.). We combined all names from the historic lists (Britton, 1898, 1899; Nash, 1900) and updated the nomenclature to modern equivalents. We then searched the herbarium for all these names as well as all names reported for Bronx County by the New York Flora Association (Weldy et al., 2015) and Taylor (1915). Specimens labeled "Bronx Park", "New York Botanical Garden", or variations of "Hemlock Grove" in the Bronx were identified, databased, imaged and cited here. Specimens marked as "cultivated" are not cited here. All specimens cited are housed at NY, unless otherwise noted. Species that persist from cultivation and spread vegetatively, but do not form new populations, such as Hosta lancifolia Engl., Hyacinthoides hispanica (Mill.) Rothm., Narcissus pseudonarcissus L., Pachysandra terminalis Siebold & Zucc., and Toona sinensis (A. Juss.) M. Roem. are not treated here. Rare escapes from cultivation found in and immediately around greenhouses or propogation areas are not reported here.
The historic and extant spontaneous flora (Appendix I) is arranged by major groups (ferns, gymnosperms, basal angiosperms [magnoliids], monocots and dicots) with families, genera and species arranged alphabetically. Fern taxonomy follows Smith et al. (2006). Flowering plant family taxonomy follows the classification of the Angiosperm Phylogeny Group (APG III, 2009). Author abbreviations follow Brummitt and Powell (1992). Misapplied names and synonyms from Britton (1898, 1899) and Nash (1900) are cited in braces with the species where the material applies.
New taxa named from spontaneous Garden plants (Appendix II) were found by searching the Garden's type database as well as literature searches. New Records (Appendix III) were found by searching the New York Flora Atlas for taxa not reported for Bronx County and comparing the list to the historic and extant flora (Appendix I). Rare plants (Appendix IV) were found by searching the New York State Department of Environmental Conservation's Active Inventory List (NYS DEC, 2016). Only extant species are reported as rare. Waifs as defined here are plants of anthropogenic origin that appear to be spontaneous but occur only as solitary individuals or only very localized populations. Some species collected on the grounds of the Garden were added to the New York State flora (Weldy et al., 2015), but were collected only once and apparently never spread further (e.g., Cynosurus echinatus and Rorippa indica). These are treated here as waifs. Some may be persistent from cultivation and are spreading vegetatively or they may be spontaneous but there is no definitive evidence indicating their status (e.g., Convallaria majalis and Hemerocallis fulva). Our approach is conservative. Some species treated here as waifs are local escapes from plants cultivated on the grounds (e.g., Cephalotaxus harringtonia and Ilex opaca). Others are escaped from plants cultivated elsewhere in the region (e.g., Cleome hassleriana Chod.). Sight records (Appendix V) are those species for which we found no voucher specimen. These taxa are not used in counts or analyses.
Vernacular names are from Gleason and Cronquist (1991), The New York Flora Atlas (Weldy et al., 2015) and USDA NRCS Plants Database (USDA NRCS, 2016). They are provided for convenience. As is the case with the majority of Cyperaceae and Poaceae, these names are contrived and may not be used by any living person familiar with the plants. Frequency values are site-specific for the Garden generally for the period between 1986 and 2015, but principally for the latter years. Frequency values are not meant as regional assessments. The categories used here are as follows: Historic–once present, but not found between 1986 and 2015; Rare–scarce, less than 2 colonies; Infrequent–uncommon, occasional, 2 to 5 colonies; Frequent–common, more than 5 colonies. A species may be reported as "Historic" even though the species is cultivated today. The species Campsis radicans (L.) Seem. ex Bureau. was reported as wild (without voucher) in the early flora (Britton, 1899), but today is known only from cultivation.
All analyses (counts of taxa, new records, lost and gained taxa, etc.) are based on current classification.
Results
The largest extant families are Asteraceae (40 species or infraspecies), Cyperaceae (35), Poaceae (32), Rosaceae (19) and Polygonacaee (18). The largest extant genus is Carex with 27 species or infraspecies, followed by Persicaria with ten, Viburnum and Cyperus each with seven, and Quercus with six (Table I).
Among the rare extant species and infraspecies (Appendix IV) on the New York State Department of Environmental Conservation Active Inventory List (NYS DEC, 2016), three are critically imperiled (S1) and two are imperiled (S2). The extant species Carex aggregata is currently listed as historic (not seen in 20–30 years) in New York State (NYS DEC, 2016), though we discovered it at the Garden in 2009. Some species, not rare in New York State, are nonetheless uncommon in New York City such as Maianthemum canadense (Canada Mayflower). This species still forms dense patches in the Thain Family Forest. Of the extant native species and infraspecies, 124 are currently rare on the grounds (47%). The families with the greatest number of locally rare extant taxa are Cyperaceae (15 rare species and infaraspecies), Poaceae (12) and Ericaceae (8). Many groups have experienced significant losses (e.g., native Orchidaceae, native Scrophulariaceae, and native Fabaceae). All tree species remain extant with the exceptions of Castanea dentata and Quercus coccinea. The Yellow Birch, Betula alleghaniensis Britton was not reported on any historic list, but apparently appeared spontaneously.
Seventeen families were gained since 1900 (Athyriaceae, Cystopteridaceae, Salviniaceae, Woodsiaceae, Ceratophyllaceae, Berberidaceae, Bignoniaceae, Boraginaceae, Caprifoliaceae, Elaeagnaceae, Grossulariaceae, Lythraceae, Malvaceae, Mazaceae, Menispermaceae, Nyctaginaceae, Paulowniaceae). Among those species and infraspecies gained since 1900 (283), native and non-native, the largest families are Asteraceae (38), Poaceae (22), Rosaceae (21), Brassicaceae (16) and Cyperaceae (14).
Among the 412 species and infraspecies reported in 1900, the largest families are Poaceae (64) species and infraspecies, Asteraceae (41), Cyperaceae (36), Rosaceae (13), and Fabaceae (11). The largest genus in 1900 was Carex with 28 species and infraspecies, followed by Dichanthelium with 13 and Ranunculus and Solidago with eight each. Eleven families reported in 1900 are no longer extant (Acoraceae, Aquifoliaceae, Aristolochiaceae, Cistaceae, Melanthiaceae, Myricaceae, Ophioglossaceae, Phrymaceae, Polygalaceae, Santalaceae, and Saxifragaceae). The largest groups of native species and infraspecies lost since 1900 (181) are Poaceae (29), Asteracae (19), Cyperaceae (15), Ranunculaceae (10), and Rosaceae (7). Some common weeds present in 1900 are no longer extant (e.g., Achillea millefolium, Elymus repens, Phleum pratense, and Saponaria officinalis). In 1900 there were 12 pteridophyte species reported for the Garden (Britton, 1899; Nash, 1900). Today there are 12 extant pteridophytes. Five were lost (Dryopteris cristata, Dryopteris intermedia, Equisetum fluviatile, Equisetum hyemale, and Sceptridium dissectum) and five were gained (Athyrium angustum, Azolla caroliniana, Cystopteris tenuis, Dryopteris carthusiana, and Woodsia obtusa). Some species were spontaneous in 1900, but are now only known from cultivation (e.g., Geranium maculatum, Juglans nigra, and Lobelia cardinalis) and are reported as Historic. In some cases we found no historical material collected from the grounds, but often found specimens from nearby areas. In the case of Osmunda regalis which is reported on the 1898 and 1899 lists, we found material from McLeans Woods (Holtzoff s.n.), Riverdale (Bicknell 11426a) and Pelham Ave (Burnham 701), but nothing from "The New York Botanical Garden" or "Bronx Park".
The nomen nudum "Lobelia Canadensis" was reported by Nash (1900). In the Nash document, the name is listed with Lobelia cardinalis. These two were added to two other Lobelia from the grounds of the Garden (Lobelia inflata and Lobelia siphilitica), previously reported by Britton (1899). We searched the NY herbarium thoroughly and unsuccessfully for any specimen bearing the name "Lobelia Canadensis."
Discussion
All floras are inherently dynamic, subject to biotic and abiotic influences. In natural systems, floristic change usually occurs slowly (over thousands or millions of years) or episodically as a result of cataclysmic events like floods, landslides, droughts, etc. In contrast, recent studies show that modern, urban floras have undergone rapid and dramatic floristic change since the industrial revolution, particularly during the 20th century (Robinson et al., 1994; Pysek et al., 1995; Bertin, 2002; Standley, 2003; DeCandido, 2004; McKinney, 2006, 2008; DeCandido et al., 2007; Primack et al., 2009). Urban floras face unprecedented pressures including climate change, the heat-island effect, acid rain, air and water pollution, deposition of heavy metals, habitat destruction, construction, competition from introduced species, vandalism, soil compaction, etc.
Studies show a marked increase in non-native species and a decline in natives for urban ecosystems of our region. Staten Island as a whole lost almost 41% of its native species from the period 1879–1991 (Robinson et al., 1994). Concord, Massachusetts, an area with a long tradition of conservation has lost 27% of its native species since 1837 (Primack et al., 2009). Central Park has lost 70% of its native species since 1857 (DeCandido et al., 2007). Small populations like those that occur on islands or in isolated patches in urban areas have been shown to be more susceptible to extirpation than larger ones (Robinson et al., 1994). Williams et al. (2009) identified three sources of species in urban environments (native species extant in the environment; native species occurring regionally; non-native species introduced naturally or through human agency). Restoration efforts using native plants add a fourth category of species that may introduce or re-introduce viable species to an urban flora.
Factors contributing to changes in species composition within the Garden include the above regional factors as well as the following factors. Loss of territory of course meant a smaller area for plants. The Garden lost the northern floodplain forest in 1938, eliminating a large area suitable for silver maple (Acer saccharinum) and other species. Change in land use from farmland, pasture, homesites and industrial operations eliminates disturbance regimes particular to these activities, such as mowing for hay versus mowing for lawn. Some were probably brought in from more rural areas with livestock and machinery. The alteration of habitat such as grading and construction eliminated areas where plants could grow. Predation from rabbits, rats and other animals impact the vegetation. Gathering of plants by the public; trampling and soil compaction and ground fires impacted the vegetation in the past.
In 1900, much of the Bronx was rural with farms and scattered homesites. There was a Bedford Park bog with Dichanthelium, Pogonia ophioglossioides, Rhexia, and other bog plants. In her book, Bog Trotting for Orhids, Grace Greylock Niles (1904) described the area with abundant wildflowers: "Along the higher ridges, the brilliant Rock Pinks (Phlox subulata L.) bloom abundantly. Their mossy-mats creep over the hills from Bronx Park to Yonkers." The abundance of Poaceae and Asteraceae in the historic flora suggests that open meadows were a prominent feature of the landscape. Indeed, hay was being cut and sold for revenue within the grounds until at least 1897 (Britton, 1899). Land west of the Bronx River and adjacent to the Metro North railroad tracks (formerly the Harlem division of the New York Central and Hudson River Railroad) at the time of incorporation was described as "mainly open fields which had been under cultivation up to the time that the Bronx Park was established…" (Eustis, 1902: 105). Into the 1920s there was a meadow behind the Museum building with typical meadow species such as Achillea millefolium, Coreopsis lanceolata, Silphium perfoliatum, and Tragopogon pretensis. Other meadow species collected on the grounds early in the Garden's history include Asclepias incarnata and Gentiana andrewsii.
Some species were not recorded from the grounds during the 1898–1900 surveys, but were almost certainly present (e.g., non-natives: Morus alba, Verbascum thapsus; natives: Populus deltoides, Persicaria punctata, Persicaria pensylvanica, Persicaria lapathifolia, Rubus allegheniensis, and Podophyllum peltatum). On the other hand Allium tricoccum Aiton was collected from the "vicinity" (e.g., McLeans Woods, Williams Bridge, Bedford Park and Riverdale) and was cultivated, but apparently never wild collected on the grounds of the Garden. Similarly, it is surprising that Lysimachia borealis (Raf.) U. Manns & A. Anderb. (Primulaceae) has never been found on the grounds when it has been collected on Long Island, Staten Island, and Westchester County. The orchid flora of The New York Botanical Garden originally consisted of just five vouchered species: Calopogon tuberosus, Corallorhiza maculata, Platanthera hookeri, Spiranthes cernua, and Spiranthes lacera. This is surprising because the greater New York City metro area historically contained 45 species. A couple of possible hypotheses may explain the relative paucity of orchids. First, most native orchids are edge or open growing plants that do not compete well in wooded or shrubby habitats. However, regionally common woodland species such as Cypripedium acaule Aiton or Goodyera pubescens (Willd.) R. Br. have never been collected from what eventually became the Thain Family Forest. Second, as noted above, much of the land surrounding and comprising the grounds of the Garden were meadows, where many orchids regionally common to such habitats, e.g., Platanthera lacera (Michx.) G. Don or Platanthera psycodes (L.) Lindl., were collected. Thus, this paucity of orchids may be due not to a deficiency of suitable habitat, but may instead be due to overcollection by the local population prior to the establishment of the Garden, although it is impossible to verify this hypothesis. Today, the only spontaneous orchid to occur within the Garden is Epipactis helleborine, a common and regionally expanding Eurasian orchid typically found in disturbed garden beds, sidewalk cracks, and wooded edges. Why this non-native species continues to maintain healthy populations, vs. the extirpation of all five native species, is an intriguing question with wider implications for the native flora of urban refuges.
Some species were collected before 1898 but not reported on the early lists: Actaea pachypoda was collected in "Woods, Bronx Park" in May 1891 (S. Clarke s.n.) and again by Charles Gilly (444) in 1940 from "New York Botanical Garden, Bronx Park"); Agrimonia rostellata was collected by W. Clute & P. Wilson (s.n.) on 1 September 1899; Amsonia tabernaemontana was collected in a "field near greenhouses" in 1893 (S. Clarke s.n.), but not reported on the early list, and collected again in 1940 from "Bronx Park" by Charles Gilly (311) and again "in waste grassy clearing, not cultivated" by Harold Moldenke (20593) in 1950; Cardamine parviflora was collected from "Fordham Hemlock woods" by Eugene Bicknell (4454) in 1881; Rosa palustris was collected by George Nash (s.n.) in Bronx Park in 1896; Lespedeza repens was collected by E. Bicknell (5170 and s.n.) in September 1891; Vicia sativa was collected in "Bronx Park" May 1891 by S. Clarke (s.n.).
Most curiously, perhaps, is the addition of Picea mariana to the wild flora of the Garden by Nash in the supplement to the larger list (Nash, 1900). This is a boreal species at the southern end of its range, occurring in bogs and frigid swamps. We found several specimens gathered from cultivated trees dating from the period, but none from wild or spontaneous individuals. The species is not formally excluded here from the Garden flora. If accepted generally, the native occurrence of this species in the Bronx is a county record (albeit based on historic occurrence).
The changed garden boundaries complicate comparison between the historic flora and the extant flora, especially when attempting to compare species per unit area for the historic and modern periods. Because species were not collected continuously throughout the Garden's history and the early collectors often only reported the locality as "Bronx Park" it is impossible to know what species occurred in exactly what area at what time. However, for the modern period (1986 to 2015), we did attempt to document every species with precise localities and day of collection. So for the modern period and for the current boundaries, it is possible to calculate species per unit area.
The Garden was established during a dynamic period in botanical science, especially in the area of nomenclature. The "American Code of Botanical Nomenclature" was being debated and Nathaniel Britton was a leading proponent. Eventually, the American Code became the basis for the code used today, but the process was dynamic and contentious. The early Garden flora was published during this period of flux in plant nomenclature and employed novel nomenclatural concepts such as tautonyms (e.g., Benzon benzoin, Malus malus, Opuntia opuntia, Sassafras sassafras) that were dropped from the adopted code. Britton and colleagues were meticulous classifiers of taxonomic groups, often recognizing segregate genera long lumped under the Linnean system. Later workers such as Gleason and Cronquist (1991) again lumped some of these species into larger genera (e.g., Corydalis sempervirens for Capnoides sempervirens, and Prenanthes trifoliolata for Nabalus trifoliolatus), which are once again being recognized in a narrower sense, largely on the basis of molecular evidence in addition to the morphological data available in 1900.
Historic names not in use today were traced through the successive editions of the "Britton and Brown" floras and correlated with contemporaneous specimens from the area whenever possible. In most cases the synonomy was straightforward and unambiguous (e.g., Ailanthus glandulosa became Ailanthus altissima). In some cases, however the disposition of a name has not been straightforward. Contemporaneous specimens from "Bronx Park" (G. Nash 319) and "Bronx Meadow" (E. Bicknell 10312) labeled as Panicularia fluitans and Glyceria fluitans, respectively, are in fact specimens of Glyceria septentrionalis. We put "Panicularia fluitans" in braces with Panicularia septentrionalis, although the name is actually a synonym of Glyceria fluitans (Soreng, 2003), which is also reported here with the synonym Panicularia brachyphylla Nash in braces. Another example is Malva rotundifolia which was reported as wild at by Britton in 1898. In the 1913 edition of the Britton and Brown flora the species is reported as "common nearly throughout our territory" and depicted with smooth mericarps (Britton & Brown, 1913). In the 1952 edition (Gleason, 1952), the name as we know it today, Malva neglecta, is said to be "included in Malva rotundifolia of Gray, B & B., Small, Rydb." The name is actually misapplied to both Malva neglecta and Malva pusilla, but more commonly to the former. The latter is more common westward and is not currently known from New York (Weldy et al., 2015). The species Malva rotundifolia does occur in the region, but is uncommon and has rugose mericarps. We found no historic specimens of wild material from the grounds of the Garden annotated with the name Malva rotundifolia, however, there are specimens of Malva neglecta collected by Britton and others from the New York region annotated as Malva rotundifolia (e.g., Bicknell 5769 from Van Cortlandt Park). We applied the name to Malva neglecta.
Some species were listed twice under two different names in the Britton (1899) list. For example, both Poa flava and Tricuspis seslerioides are listed under Poaceae. Both names are synonyms of Tridens flavus and are listed as such in the Britton and Brown flora of 1913. Botrychium dissectum was reported by Britton (1899) and later Nash (1900) reported Botrychium obliquum. Today these are considered synonyms of Sceptridium dissectum and are so treated here. Carex lurida and Carex tentaculata are both listed in the 1899 list. The latter is a synonym of the former and is listed as such in the Britton and Brown flora of 1913. The same is true for Carex costellata and Carex virescens, both of which are reported on the 1899 list and were later listed under Carex virescens in the 1913 Britton and Brown flora. Sisyrinchium angustifolium and Sisyrinchium graminoides E. P. Bicknell are both recognized by Britton (1899). Today we view the species as a single, if somewhat variable entity (Sisyrinchium angustifolium). However, these examples are few and the vast majority of names on the historic list correspond to valid species today (though sometimes with different names).
Some of the names from the historic list are based on misidentifications or alternate application of a name. Hylodesmum nudiflorum (L.) H. Ohashi & R. R. Mill., reported as Meibomia nudiflora (Britton, 1898), is based on a misidentified specimen. The Bicknell specimen (s.n.) from "Bronx Park", collected on 20 September 1896, is Desmodium perplexum. The name Eragrostis purshii was applied to a specimen collected on 1 September 1899 by W. Clute and P. Wilson (s.n.). The specimen is Eragrostis pectinacea, a species persisting in one locality on the grounds today. Lespedeza frutescens reported by Britton (1898) is based on misidentified specimens of Lespedeza violacea.
Nineteenth Century specimens labeled "Bronx Park" could include plants actually collected from what is now the Wildlife Conservation Society (Bronx Zoo) or areas of Bronx Park now north of the Garden (see Fig. 1), although most were collected after 1896 when the Garden was established. No taxa were added to the spontaneous flora (Appendix I) solely on the basis of a specimen labeled "Bronx Park". All historic taxa are either reported by Britton (1898, 1899), Nash (1900) or explicitly cited as "New York Botanical Garden" on preserved specimens.
We cite all voucher specimens seen for a particular taxon from the flora area, rather than just one "representative specimen" as is sometimes done. If only one specimen per taxon is cited and that specimen is later determined to be misidentified, it is not possible to exclude the name because there is always the possibility other, correctly identified specimens could exist. If we have similarly erred in the application of a name, future workers will be able to confidently exclude that taxon from the flora because we have cited all specimens seen by us that are the basis for that report. The same holds true for application of synonyms and misidentifications.
Species reported by Britton (1899) and Nash (1900) that are not known to form spontaneous new populations but may spread vegetatively in a very localized area are treated here as waifs: Hemerocallis fulva, Nepeta cataria, and Ornithogalum umbellatum. These names are not counted in the historic flora. The report by Britton (1898) of Syringa vulgaris L. as wild is certainly an error. The species is only known to be persistent from cultivation. We are not aware of any credible reports of spontaneous lilacs anywhere in the region and indeed, none was collected. The species is excluded here. Populus alba, also long-persistent from cultivation, but never spontaneous was listed by Britton (1899). No spontaneous voucher was found and it is excluded here. Hibiscus syriacus is also a long-lived shrub or small tree and may appear spontaneous, but we have seen no truly spontaneous populations. Like the preceeding, no spontaneous vouchers were found, and the species is excluded here.
The relative abundance of a species often fluctuates from year to year, depending on many factors including climactic variation, disturbance, etc. For example, in some years, in some areas, Persicaria extremiorientalis may be abundant in a location and scarce or absent in others (Atha et al., 2010). In a very few cases (e.g., Achillea millefolium or Poa pratensis), a species is reported from the historic list and has undoubtedly been here ever since, but we have seen no modern voucher specimen from the grounds of the Garden. These species are cited here as historic, though they are likely extant today. Rorippa indica persisted as a weed on the grounds of the Garden at least from 1947 to 1949, but has since vanished from New York State (Weldy et al., 2015). The species is treated here as a waif.
Plants from the Garden’s living collection have sometimes escaped cultivation and become established in the spontaneous flora of the landscape. Viburnum plicatum forma tomentosum was documented as naturalizing in the Forest in 1935 (McLean, 1935). Today, Ficaria verna, Phellodendron amurense, and Reynoutria spp. have become invasive and pose a serious threat to remaining populations of native plants. Recent efforts to control the spread of invasive species and re-establish populations of extirpated species in the Thain Family Forest indicate that some of the changes to the Garden’s spontaneous flora may be reversible (Schuler & Forrest, 2016).
Since 2008, the Garden has conducted ecological restoration—or the process of assisting the recovery of an ecosystem that has been degraded, damaged or destroyed—in the 50 acre, remnant old-growth forest (SER, 2004). The main goal has been to improve forest health through active management informed by research. In this context, a healthy forest is defined as an ecosystem that is dominated by naturally regenerating populations of native plants that respond to and recover from disturbances of all scales with minimal human intervention, provides suitable habitat to a diversity of organisms in a structurally diverse, mixed-aged woodland, and sustains natural processes that support biodiversity (Schuler & Forrest, 2016). Forest inventories conducted in 1985, 2002, 2006, and 2011 have quantitatively revealed the expansion of non-native plant species in the forest (Rudnicky & McDonnell, 1989; Schuler & Forrest, 2016). Out of the 75 invasive plant species that are listed on the New York State Prohibited and Regulated List (NYS DEC, 2014), there are 34 extant species: Acer platanoides, Acer pseudoplatanus, Alliaria petiolata, Ampelopsis brevipedunculata, Anthriscus sylvestris, Aralia elata, Artemisia vulgaris, Celastrus orbiculatus, Cirsium arvense, Clematis terniflora, Euonymus alatus, Euonymus fortunei, Ficaria verna, Frangula alnus, Humulus japonicus, Lonicera japonica, Lonicera maackii, Lonicera morrowii, Lythrum salicaria, Microstegium vimineum, Miscanthus sinensis, Persicaria perfoliata, Phellodendron amurense, Potamogeton crispus, Reynoutria × bohemica, Reynoutria japonica, Reynoutria sachalinensis, Rhamnus cathartica, Robinia pseudoacacia, Rosa multiflora, Rubus phoenicolasius, Silphium perfoliatum, Vincetoxicum nigrum, and Vincetoxicum rossicum. It should be clarified that some of these plants are native to North America, just not to this region, e.g., Robinia pseudoacacia and Silphium perfoliatum. In addition, Berberis thunbergii and Phragmites australis are known to grow on the grounds of the Garden but, have not been vouchered, and Arthraxon hispidus, Euphorbia cyparissias, and Lespedeza cuneata are reported as historic but have not been collected recently. Interestingly, species of concern statewide and regionally, like Berberis thunbergii, Euonymus alatus, and Rhamnus cathartica are present only in limited numbers. Only half of the statewide list are currently extant or known to have been part of the Garden’s flora, leaving speculation for the potential of additional invasion in the future or possibly the habitat requirements for the remaining listed species do not exist in the Garden’s current 250 acres.
Invasive species of concern actively managed in 2008–2015 were Acer platanoides, Ailanthus altissima, Alliaria petiolata, Ampelopsis brevipedunculata, Aralia elata, Celastrus orbiculatus, Corydalis incisa, Ficaria verna, Hedera helix, Lonicera japonica, Lonicera maackii, Microstegium vimineum, Phellodendron amurense, Persicaria perfoliata, Reynoutria × bohemica, Reynoutria japonica, Reynoutria sachalinensis, Rosa multiflora, and Viburnum dilatatum. Extant non-native species that are present in inventory data but are not actively managed include Acer pseudoplatanus, Humulus japonicus, Paulownia tomentosa, Prunus avium, Prunus incisa, Prunus sargentii, Prunus subhirtella, Prunus × yedoensis, Rhamnus frangula, and Rubus phoenicolasius. Forest inventory data identify priority species and target locations for management. Literature is consulted for the best management practices for individual species with special focus on techniques that are proven effective at controlling a given species without causing unintended negative impacts on forest ecosystem processes (Schuler & Forrest, 2016). Published best management practices do not always result in the best control. Frequent monitoring and repeating the forest inventory sampling every five years reveals efficacy of control treatments (Schuler & Forrest, 2016). In addition, there is the constant threat of introduction of new invasive species as in the case in recent years of Corydalis incisa and Persicaria perfoliata. In both cases, the infestations were discovered early and mechanical management has been effective. Microstegium vimineum is abundant regionally, is observed periodically (despite treatment) along the Forest trails, and requires constant monitoring and management to prevent its spread. The monitoring for new invasive species and new infestation of extant species is constant.
When invasive plants are removed, it is necessary to plant desirable plants in management areas to prevent the reestablishment of the species removed or the establishment of another invasive species. From 2008 through 2015, Garden staff and volunteers spent a total of 21,500 hours in invasive plant management and restoration planting. Collectively, they have planted 25,220 individual plants plus seed mix including 11,700 herbaceous plants, 1720 shrubs, and 11,800 trees. Over 100 different species have been planted.
Restoring the historic flora and expanding the populations of rare extant species in the Forest has been an objective for ongoing restoration work. This flora has provided a list of historic or rare extant species of concern to be considered in future restoration projects. To date, the following historic or regionally rare species have been planted from regionally sourced seed: Anemone canadensis, Anemone nobilis, Anemone virginica, Anemone quinquefolia, Athryium filix-femina, Caltha palustris, Carex grayii, Carex plantaginea, Carex platyphylla, Corylus americana, Dryopteris marginalis, Elymus hystrix, Geranium maculatum, Geum canadensis, Iris versicolor, Lilium superbum, Lobelia siphilitica, Monarda fistulosa, Osmunda regalis, Osmundastrum cinnamomeum, Polystichum acrostichoides, Salix discolor, and Thelypteris palustris. Carex aggregata, Carex amphibola, Carex cephalaphora, and Carex debilis were vegetatively propagated from populations on the grounds of The New York Botanical Garden.
Literature Cited
Anonymous. 1911. Washington Post. Trees soon extinct. Washington Post, Washington D.C. 14 August 1911.
———. 1938. Journal of The New York Botanical Garden. 1938. Notes, news, and comment: storm damage. 10: 246.
———. 1944. Journal of The New York Botanical Garden. 1944. Storm damage. 10: 238.
APG III. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121.
Atha, D., M. H. Nee & R. F. C. Naczi. 2010. Persicaria extremiorientalis (Polygonaceae) is established in the flora of the eastern United States of America. Journal of the Torrey Botanical Society 137: 333–338.
———, R. Alvarez, D. Feeser, M. Feder, Z. Wang & R. Kelly. 2016. Gamochaeta pensylvanica (Asteraceae) is established in the New York flora. Phytoneuron 2016-22: 1–4.
Bertin, R. I. 2002. Losses of native plant species from Worcester, Massachusetts. Rhodora 104: 325–349.
Britton, E. K. 1912a. Thoughtless destruction of Jack-in-the-Pulpit. Journal of The New York Botanical Garden 13: 68–69.
———. 1912b. Wild plants needing protection. Journal of The New York Botanical Garden, published series 1912–1929.
———. 1913. Disappearing wild flowers. Torreya 20: 101.
———. 1916. Public parks as preserves of native plants. Torreya 16: 182–183.
Britton, N. L. 1898. Report of the Secretary and Director-in-Chief for 1897. Appendix 3. List of plants in the grounds of The New York Botanical Garden and in the temporary greenhouses, 1897. Bulletin of The New York Botanical Garden 1: 137–161.
———. 1899. Report of the Secretary and Director-in-Chief for 1898. Appendix 3. List of plants in the grounds, 1898, A. Wild flora. Bulletin of The New York Botanical Garden 1: 195–203.
———. 1902. The preservation of native plants. Journal of The New York Botanical Garden 3: 1–3.
———. 1904. The Hemlock Grove. Bulletin of The New York Botanical Garden. 6: 10–11.
———. 1913. A destructive storm. Journal of The New York Botanical Garden 9: 160–161.
———. 1916. Descriptive guide to the grounds, buildings, and collections. Bulletin of The New York Botanical Garden 9: 179–292.
——— & A. Brown. 1913. An illustrated flora of the northern United States, Canada and the British possessions, from Newfoundland to the parallel of the southern boundary of Virginia, and from the Atlantic Ocean westward to the 102d meridian. 2d ed. rev. and enl. C. Scribner, New York.
———, W. G. Thompson & J. Haag. 1915. Grant, by the City, of the use of additional land in Bronx Park. Journal of The New York Botanical Garden. 16: 85–95.
———, W. Gilman Thompson & J. F. Kemp. 1895. Unpublished letter in the Minutes of the Scientific Directors, LuEsther T. Mertz Library, New York Botanical Garden.
Brummitt, R. K. & C. E. Powell. 1992. Authors of plant names. Royal Botanic Gardens, Kew. 732 pp.
Copp, G. G. 1904. Protection of the wildflowers. Journal of The New York Botanical Garden 5: 112–118.
DeCandido, R. 2004. Recent changes in plant species diversity in urban Pelham Bay Park, 1947–1998. Biological Conservation. 120: 129–136.
———, N. Calvanese, R. Alvarez, M. Brown & T. M. Nelson. 2007. The naturally occurring historical and extant flora of Central Park, New York City, New York 1857–2007. Journal of the Torrey Botanical Society 134: 552–569.
Eustis, J. E. 1902. The Commissioner's report of the work of the department of parks for the borough of the Bronx for the year 1902. 72–131.
Gabel, D. 2014. Horticulture soil test results for the past 5 years. Unpublished data.
Gleason, H. A. 1952. The new Britton and Brown illustrated flora of the northeastern United States and adjacent Canada, 3 volumes. New York Botanical Garden, New York.
——— & A. Cronquist. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd ed. New York Botanical Garden, New York. 910 pp.
LaFave, White & McGivern. 2003. Contour Map of The New York Botanical Garden. Theresa, New York. Unpublished map.
Loeb, R. 2011. Old growth urban forests. Springer: New York. 78 pp.
McDonnell, M. J. 1988. A forest for New York. The Public Garden 3: 28–31.
McKinney, M. L. 2006. Urbanization as a major cause of biotic homogenization. Biological Conservation: 247–260.
———. 2008. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosystems 11: 161–176.
McLean, F. F. 1935. Japanese Viburnum runs wild in the Garden. Journal of The New York Botanical Garden. 36: 140.
Merguerian, C. 2013. Description of Stop 39: Outcrop immediately east of Old Snuff Mill. On the Rocks Field Trips. Duke Geological Laboratory, Westbury, New York.
Nash, G. V. 1900. Report of the General Assistant on the Plantations. Wild flora (additions to the list published in Bulletin No. 4). Bulletin of The New York Botanical Garden 1: 392–393.
———. 1917–1920. Hardy woody plants in The New York Botanical Garden. Journal of The New York Botanical Garden 18.
NWS. 2014. National Weather Service. Normals and extremes in Central Park, NY 1869 to 2014. Retrieved December 4, 2014 from National Weather Service, WFO-Upton, 175 Brookhaven Ave. Bldg. NWS-1Upton, NY 11973. http://www.erh.noaa.gov/okx/climate/records/nycnormals.htm
NYS DEC. 2014. New York State Department of Environmental Conservation. Prohibited and regulated invasive species. 6 NYCRR Part 575. http://www.dec.ny.gov/regulations/2359.html. Accessed 18 February 2016.
———. 2016. New York State Department of Environmental Conservation. 2016. Active Inventory List. http://www.dec.ny.gov/animals/66348.html#status. Accessed 18 February 2016.
Niles, G. G. 1904. Bog trotting for orchids. G. P. Putnam & Sons. 310 pp.
Primack, R. B., A. J. Miller-Rushing & K. Dharaneeswaran. 2009. Changes in the flora of Thoreau's Concord. Biological Conservation. 142: 500–508.
Pysek, P., K. Prach, M. Rejmánek & P. M. Wade (eds.). 1995. On the role of alien species in urban flora and vegetation. In: Plant Invasions – General Aspects and Special Problems, pp. 85–103. SPB Academic, Amsterdam (Netherlands).
Rachlin, J. W., B. E. Warkentine & A. Pappantoniou. 2007. An evaluation of the ichthyofauna of the Bronx River, a resilient urban waterway. Northeastern Naturalist 14(4): 531–544.
Robbins, W. J. 1939. Report of the Director for 1938. Journal of The New York Botanical Garden 40: 2.
———. 1941. Annual report for the Director 1940. Journal of The New York Botanical Garden 42: 1.
Robinson, G. R., M. E. Yurlina & S. N. Handel. 1994. A century of change in the Staten Island flora: ecological correlates of spcecies losses and invasions. Bulletin of the Torrey Botanical Club 121: 119–129.
Rudnicky, J. L. & M. J. McDonnell. 1989. Forty-eight years of canopy change in a hardwood-hemlock forest in New York City. Journal of the Torrey Botanical Club 116: 52–64.
Schuler, J. A. 2006. Soil test results from the Tanyosho Pines. Unpublished data.
———. 2011. Soil test results from the Permanent Forest Reference Plots in the Thain Family Forest. Unpublished data.
——— & T. A. Forrest. 2016. Thain Family Forest Management Plan. The New York Botanical Garden, Bronx, New York.
SER. 2004. Society for Ecological Restoration International Science & Policy Working Group. The SER International Primer on Ecological Restoration. www.ser.org & Tucson: Society for Ecological Restoration International.
Shaw, R. K. 2010. Soils of the Native Forest. The natural history of The New York Botanical Garden. unpublished data.
———, J. Isleib & L. Reinhardt. 2007. Soil survey of Bronx River watershed, Bronx, NY. U.S. Department of Agriculture, Natural Resources Conservation Service.
Small, J. K. 1934. Native ferns in The New York Botanical Garden. Journal of The New York Botanical Garden 35: 148–151.
——— & E. J. Alexander. 1933a. Native ferns in The New York Botanical Garden (Illustrated), A guide for the botanist and naturalist. Typescript, New York Botanical Garden library, 65 pp.
——— & ———. 1933b. Native Sshrubs in The New York Botanical Garden, A guide for the botanist and naturalist. Typescript, New York Botanical Garden library. Unpaginated,
——— & ———. 1933c. Native trees in The New York Botanical Garden, A guide for the botanist and naturalist. Typescript, New York Botanical Garden library. Unpaginated, and a few pages out of place or missing.
Smith, A. R., K. M. Pryer, E. Schuettpelz, P. Korall, H. Schneider & P. G. Wolf. 2006. A classification for extant ferns. Taxon 55: 705–731.
Soreng, R. J. 2003. Glyceria. In: Catalogue of New World Grasses (Poaceae): IV. Subfamily Pooideae. Contributions to the United States National Herbarium 48: 371–379.
Standley, L. A. 2003. Flora of Needham, Massachusetts. 100 years of floristic change. Rhodora 105: 354–378.
Taylor, N. 1915. Flora of the vicinity of New York, Memoirs of The New York Botanical Garden 5. 1–683.
USDA NRCS. 2016. The PLANTS Database. National Plant Data Team, Greensboro, NC 27401-4901 USA. http://plants.usda.gov. Accessed 15 January 2016.
Weldy, T., D. Werier & A. Nelson. 2015. New York flora atlas. New York Flora Association. http://www.newyork.plantatlas.usf.edu/. Accessed 18 February 2016.
White, C. S. & M. J. McDonnell. 1988. Nitrogen cycling processes and soil characteristics in an urban versus rural forest. Biogeochemistry 5: 243–262.
Williams, N. S. G., M. W. Schwartz, P. A. Vesk, M. A. McCarthy, A. K. Hahs, S. E. Clemants, R. T. Corlett, R. P. Duncan, B. A. Norton, K. Thompson & M. J. McDonnell. 2009. A conceptual framework for predicting the effects of urban environments on floras. Journal of Ecology 97: 4–9.
Acknowledgments
We are grateful to Jerry and Carol Bodian for their long-time service to The New York Botanical Garden and especially for their diligent and painstaking search of the William and Lynda Steere Herbarium for specimens of spontaneous plants collected on the grounds of the Garden and also for their assistance in the Digital Imaging Laboratory photographing specimens for the C. V. Starr Virtual Herbarium. We also thank the head curators of the Steere Herbarium, from Harold Rickett to Barbara Thiers, for their wise stewardship of this invaluable collection. Equally, we want to express our appreciation for the many librarians over the years who have been entrepreneurial collectors and custodians of vital botanical literature in the Garden’s LuEsther T. Mertz Library. Thank you to David Werier for helpful discussions and for pointing out the existence of Potentilla anglica specimens collected on the grounds of the Garden. Thank you Fordham University intern Marissa Vaccarelli for searching the BKL collection. We are grateful to our colleagues Ben Torke for pointing out wild plants of Gaultheria procumbens, Robbin Moran for identifying the ferns and John Mickel for sharing his extensive knowledge of the ferns of the Garden. Finally, we would like to thank Carmen and John Thain for their support of restoration and research in the Thain Family Forest and two anonymous members of the Garden’s Board of Managers for their generous support of The New York Botanical Garden’s Center for Conservation Strategy, in which the first author serves as Conservation Program Manager.
Author contributions
Conceived project: MN; conducted field work: DA, JS, MN, MP, RN; identified specimens: DA, MN for all except RN for Cyperaceae and Poaceae and Robbin Moran for ferns; wrote manuscript: DA, JS, MP, MR, TF; compiled data: DA, MR; analyzed data DA, MR.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix I. The Historic and Extant Spontaneous Vascular Plant Flora of The New York Botanical Garden
Ferns
Aspleniaceae — Asplenium platyneuron (L.) Britton, Sterns & Poggenb., Ebony Spleenwort. D. Atha 8557; T. Edmondson 1422; D. McClelland 353. Frequent.
Athyriaceae — Athyrium angustum (Willd.) C. Presl, {Asplenium felix-foemina}, Ladyfern. E. Roy 110. Rare.
Cystopteridaceae — Cystopteris tenuis (Michx.) Desv., Upland Brittle Bladderfern. D. Atha 14744. Rare.
Dennstaedtiaceae — Dennstaedtia punctilobula (Michx.) T. Moore, {Dicksonia punctilobula}, Hayscented Fern. D. McClelland 348, 373; C. Morenberg 93; G. Nash s.n.; E. Roy 98. Infrequent.
Dryopteridaceae — Dryopteris carthusiana (Vill.) H. P. Fuchs, Spinulose Woodfern. D. Atha 14745; D. McClelland 349; E. Roy 85. Rare.
Dryopteridaceae — Dryopteris cristata (L.) A. Gray, Crested Woodfern. W. Clute s.n. Historic.
Dryopteridaceae — Dryopteris intermedia (Muhl. ex Willd). A. Gray, {Dryopteris spinulosa intermedia}, Fancy Woodfern. E. Bicknell 11489. Historic.
Dryopteridaceae — Polystichum acrostichoides (Michx.) Schott, {Dryopteris acrostichoides}, Christmas Fern. G. Nash s.n.; E. Roy 84. Rare.
Equisetaceae — Equisetum arvense L., Field Horsetail. D. Atha & M. Nee 8027; J. Knowles 9; G. Nash s.n. Rare.
Equisetaceae — Equisetum fluviatile L., Water Horsetail. E. Bicknell 11584B; W. Clute s.n. Historic.
Equisetaceae — Equisetum hyemale L., Scouringrush Horsetail. W. Clute & P. Wilson s.n.; G. Nash s.n. Historic.
Onocleaceae — Onoclea sensibilis L., Sensitive Fern. D. Atha 7581; E. Britton s.n.; M. Nee & K. Cameron 54354. Infrequent.
Ophioglossaceae — Sceptridium dissectum (Spreng.) Lyon, {Botrychium obliquum}, Lacefrond Grape Fern. H. House s.n.; L. Underwood s.n. Historic.
Osmundaceae — Osmundastrum cinnamomeum (L.) C. Presl, {Osmunda cinnamomea}, Cinnamon Fern. D. McClelland 329; G. Nash s.n. Rare.
Salviniaceae — Azolla caroliniana Willd., Carolina Mosquito Fern. D. Atha 1912. Rare.
Thelypteridaceae — Thelypteris noveboracensis (L.) Nieuwl., {Dryopteris noveboracensis}, New York Fern. D. Atha 7580, 7659, 14715; E. Roy 91. Rare.
Woodsiaceae — Woodsia obtusa (Spreng.) Torr., Cliff Fern. D. McClelland 356. Infrequent.
Gymnosperms
Cupressaceae — Juniperus virginiana L. var. virginiana, {Juniperus virginiana}, Eastern Red Cedar. D. Atha 6911; G. Nash s.n.; P. Wilson 10809, s.n. Infrequent.
Ginkgoaceae — Ginkgo biloba L., Ginkgo. J. Schuler 17. Not Native. Waif. Rare.
Pinaceae — Pinus strobus L., Eastern White Pine. D. Atha 15045. Rare.
Pinaceae — Tsuga canadensis (L.) Carrière, Eastern Hemlock. H. Moldenke 4572; G. Nash s.n.; M. Nee 37822, 54277, 54283; E. Roy 39; J. Small s.n.; P. Wilson 10811, s.n. Infrequent.
Taxaceae — Cephalotaxus harringtonia (Knight ex J. Forbes) K. Koch, Harrington's Cephalotaxus. D. Atha 6914; M. Nee 59042. Not Native. Waif. Frequent.
Magnoliids
Aristolochiaceae — Asarum canadense L., {Asarum reflexum}, Canadian Wild Ginger. A. Vail s.n.; C. Gilly 292; G. Nash 170. Historic.
Ceratophyllaceae — Ceratophyllum demersum L., Hornwort. M. Nee & D. McClelland 54421. Rare.
Lauraceae — Lindera benzoin (L.) Blume, {Benzoi n benzoin}, Spicebush. D. Atha 6947, 9038; H. Beck 1333; A. Carvalho & W. Thomas 6889; J. Knowles s.n.; M. Nee & J. Beitel 35907; M. Nee & K. Cameron 54248; W. Nieder 8, 9; E. Roy 5, 24; P. Wilson 167, 10819. Frequent.
Lauraceae — Sassafras albidum (Nutt.) Nees, {Sassafras sassafras}, Sassafras. D. Atha 6973, 6990, 8593; W. Cahilly 27; C. Gilly 281; E. Humphreys s.n.; G. Nash 77, 78; M. Nee 43495; M. Nee & K. Cameron 54342; E. Roy 41; P. Wilson 10859. Frequent.
Magnoliaceae — Liriodendron tulipifera L., Tuliptree. R. Dragonetti 7; C. Gilly 2; D. McClelland 331; G. Nash 147; M. Nee 31284; E. Roy 37, 44; P. Wilson s.n. Frequent.
Magnoliaceae — Magnolia kobus DC., Kobushi Magnolia. D. Atha 13349; W. Nieder 2, 7; E. Roy 14. Not Native. Waif. Infrequent.
Magnoliaceae — Magnolia × soulangeana Soul.-Bod., Saucer Magnolia. M. Nee & K. Cameron 54247. Not Native. Waif. Rare.
Magnoliaceae — Magnolia tripetala (L.) L., Umbrella Magnolia. D. Atha 9034; D. McClelland 316. Not Native. Waif. Rare.
Saururaceae — Houttuynia cordata Thunb., Chameleon Plant. D. Atha 13718. Not Native. Waif. Rare.
Saururaceae — Saururus cernuus L., Lizard's Tail. D. Atha 8591; A. Gibson 1090; C. Gilly 81; M. Nee & K. Cameron 54494; P. Wilson 137. Infrequent.
Monocots
Acoraceae — Acorus calamus L., Sweet Flag. G. Nash s.n. Not Native. Historic.
Alismataceae — Alisma subcordatum Raf., {Alisma plantago-aquatica}, Southern Waterplantain. C. Gilly 153; J. Monachino 449, 450. Historic.
Alismataceae — Sagittaria latifolia Willd., Common Arrowhead. D. Atha & M. Nee 8020. Rare.
Alliaceae — Allium vineale L., Field Garlic. D. Atha 6926; J. Gowdy s.n.; D. McClelland 333. Not Native. Frequent.
Amaryllidaceae — Galanthus nivalis L., Snowdrop. D. Atha & L. Collins 14229; M. Nee & K. Cameron 54240; E. Roy 121. Not Native. Waif. Frequent.
Araceae — Arisaema triphyllum (L.) Schott subsp. triphyllum, Jack-in-the-Pulpit. C. Gilly s.n., 282; N. Holmgren 656 (UTC); D. McClelland 338; G. Nash 32; M. Nee & K. Cameron 54357; W. Nieder 26; A. Vail s.n.; E. Wolfson 7. Rare.
Araceae — Lemna minor L., Common Duckweed. G. Nash 511; M. Nee & K. Cameron 54331; P. Wilson s.n. Infrequent.
Araceae — Peltandra virginica (L.) Schott, Arrow Arum. D. Atha 2222, 8991; C. Gilly 43; J. Gunderson 8; M. Nee 43605; M. Nee & K. Cameron 54453; E. Roy 101. Infrequent.
Araceae — Symplocarpus foetidus (L.) Nutt., {Spathyema foetida}, Skunk Cabbage. N. Holmgren 630; C. Morenberg 90; M. Nee & K. Cameron 54265; W. Nieder 6; R. Rennert s.n.; E. Roy 53. Infrequent.
Araceae — Wolffia brasiliensis Wedd., Brazilian Watermeal. H. Beck 1373; M. Nee 43607; M. Nee & K. Cameron 54332. Rare.
Asparagaceae — Asparagus officinalis L., Asparagus. G. Nash 166. Not Native. Historic.
Asparagaceae — Convallaria majalis L., Lily of the Valley. D. Atha 7049; N. Holmgren 699 (UTC); D. duMouchel s.n.; M. Nee & K. Cameron 54300. Not Native. Waif. Rare.
Asparagaceae — Maianthemum canadense Desf., {Unifolium canadense}, Canada Mayflower. C. Gilly 313; N. Holmgren 702 (UTC); D. McClelland 327; C. Morenberg 91; M. Nee & K. Cameron 54356; W. Nieder 47; E. Wolfson 2. Frequent.
Asparagaceae — Maianthemum racemosum (L.) Link, {Vagnera racemosa}, False Solomon Seal. D. Atha 7371; W. Cahilly 38, 97008; S. Clarke s.n.; C. Gilly 172; D. McClelland 325; M. Nee & K. Cameron 54344; W. Nieder 55; E. Wolfson 3. Frequent.
Asparagaceae — Ornithogalum umbellatum L., Star of Bethlehem. J. Fargione 2; M. Nee & K. Cameron 54350. Not Native. Waif. Infrequent. Reported by Britton (1898).
Asparagaceae — Othocallis siberica (Haw. ex Andr.) Speta, Siberian Squill. D. Atha 11554; M. Nee & K. Cameron 54254. Not Native. Waif. Rare.
Asparagaceae — Polygonatum biflorum (Walter) Elliott var. biflorum, {Polygonatum biflorum}, Smooth Solomon Seal. N. Holmgren 697 (UTC); E. Roy 63; M. Wolf & R. Dragonetti 11. Infrequent.
Asparagaceae — Polygonatum biflorum (Walter) Elliott var. commutatum (Schult. & Schult. f.) Morong, {Polygonatum commutatum}, Smooth Solomon Seal. D. Atha 7370, 7638; G. Nash 140. Rare.
Asparagaceae — Polygonatum pubescens (Willd.) Pursh, Hairy Solomon Seal. D. McClelland 328; G. Nash 86; M. Nee & K. Cameron 54320; W. Nieder 42; E. Wolfson 6. Infrequent.
Colchicaceae — Uvularia perfoliata L., Perfoliate Bellwort. C. Gilly 297. Historic.
Colchicaceae — Uvularia sessilifolia L., Sessileleaf Bellwort. D. Atha 8131; M. Eaton s.n.; T. Edmondson 1074; C. Gilly 265; J. Gunderson 7; R. Naczi 12496; G. Nash 47; W. Nieder 17; E. Wolfson 5. Rare.
Commelinaceae — Commelina communis L., Asiatic Dayflower. D. Atha 6795, 7760, 10601, 13728; R. Dragonetti 10; C. Gilly 53; W. Nieder 65 (3 sheets); D. McClelland 339; J. Monachino 11748; R. Naczi & D. Atha 12686; F. Pennell 6714; M. Petrino 4; C. Ruiz 9; P. Wilson 83. Not Native. Frequent.
Commelinaceae — Murdannia nudiflora (L.) Brenan., Nakedstem Dewflower. R. Naczi & D. Atha 12673. Not Native. Waif. Rare.
Commelinaceae — Tradescantia virginiana L., Virginia Spiderwort. D. Atha 8049; C. Gilly 12. Not Native. Rare.
Cyperaceae — Carex aggregata Mack., Glomerate Sedge. R. Naczi 12535. SH but discovered at the Garden in 2009. Rare.
Cyperaceae — Carex albicans Willd. ex Spreng. var. albicans, {Carex varia}, Whitetinge Sedge. R. Naczi 12384. Infrequent.
Cyperaceae — Carex albicans Willd. ex Spreng. var. emmonsii (Torr.) Rettig, Emmons Sedge. M. Nee & K. Cameron 54296; R. Naczi 13682; W. Nieder 4, 12. Infrequent.
Cyperaceae — Carex amphibola Steud., Eastern Narrowleaf Sedge. R. Naczi 12501, 12538; G. Nash 149. Rare.
Cyperaceae — Carex annectens (E. P. Bicknell) E. P. Bicknell, {Carex xanthocarpa}, Yellowfruit Sedge. R. Naczi 12497. Rare.
Cyperaceae — Carex blanda Dewey, {Carex laxiflora blanda}, Eastern Woodland Sedge. R. Naczi 12498. Infrequent.
Cyperaceae — Carex cephalophora Muhl. ex Willd., Ovalleaf Sedge. R. Naczi 12480. Frequent.
Cyperaceae — Carex communis Bailey var. communis, {Carex pedicellata}, Fibrousroot Sedge. G. Nash 157. Historic.
Cyperaceae — Carex crinita Lam. var. crinita {Carex crinita}, Fringed Sedge. R. Naczi 12488. Rare.
Cyperaceae — Carex debilis Michx. var. debilis, {Carex flexilis}, Whiteedge Sedge. R. Naczi 12534. Rare.
Cyperaceae — Carex digitalis Willd. var. digitalis, {Carex digitalis}, Slender Woodland Sedge. R. Naczi 12482. Rare.
Cyperaceae — Carex gracillima Schwein., Graceful Sedge. G. Nash 151. Historic.
Cyperaceae — Carex granularis Muhl., Limestone Meadow Sedge. R. Naczi 12537. Rare.
Cyperaceae — Carex grayi Carey, {Carex asa-grayi}, Gray’s sedge. J. Monachino 76; G. Nash 272, 311; F. Pennell s.n. Historic.
Cyperaceae — Carex grisea Wahlenb., Inflated Narrowleaf Sedge. R. Naczi 12539; M. Nee & K. Cameron 54339. Rare.
Cyperaceae — Carex hirtifolia Mack., {Carex pubescens}, Pubescent Sedge. G. Nash 201. Historic.
Cyperaceae — Carex hystericina Muhl. ex Willd., {Carex hystricina}, Bottlebrush Sedge. E. Bicknell 1751. Historic.
Cyperaceae — Carex laxiculmis Schwein. var. laxiculmis, {Carex laxiculmis}, Spreading Sedge. R. Naczi 12536. Rare.
Cyperaceae — Carex lupulina Muhl., Hop Sedge. E. Bicknell s.n., s.n.; G. Nash 261, 428. Historic.
Cyperaceae — Carex lurida Wahlenb., {Carex tentaculata}, Shallow Sedge. R. Naczi 12487; M. Nee 43635; M. Nee & D. McClelland 54444. Frequent.
Cyperaceae — Carex muhlenbergii Schkuhr ex Willd. var. enervis Bott, {Carex muhlenbergii}, Muhlenberg’s Sedge. R. Naczi 12481. Frequent.
Cyperaceae — Carex pallescens L., {Carex triceps}, Pale Sedge. G. Nash 196. Historic.
Cyperaceae — Carex pellita Muhl. ex Willd., {Carex lanuginosa}, Wooly Sedge. G. Nash 145. Historic.
Cyperaceae — Carex pensylvanica Lam., Pennsylvania Sedge. D. Atha 7433; R. Naczi 12390, 13683; W. Nieder 3. Frequent.
Cyperaceae — Carex radiata (Wahlenb.) Small, {Carex rosea}, Eastern Star Sedge. D. Atha 2612, 7379; J. Monachino 2; M. Nee 54338; M. Nee & D. McClelland 54447; R. Naczi 12476, 12478. Frequent.
Cyperaceae — Carex reznicekii Werier, Reznicek’s Sedge. R. Naczi 13687. Rare.
Cyperaceae — Carex rugosperma Mack., Parachute Sedge. R. Naczi 12388. Rare.
Cyperaceae — Carex scoparia Schkuhr ex Willd., Broom Sedge. G. Nash 250. Historic.
Cyperaceae — Carex sparganioides Muhl. ex Willd., Bur-reed Sedge. G. Nash 150; R. Naczi 12500. Rare.
Cyperaceae — Carex sprengelii Dewey ex Spreng., Sprengel’s Sedge. R. Naczi 12489. Rare.
Cyperaceae — Carex squarrosa L., Squarrose Sedge. G. Nash 424. Historic.
Cyperaceae — Carex stipata Muhl. ex Willd., Awlfruit Sedge. G. Nash 195. Historic.
Cyperaceae — Carex stricta Lam., Upright Sedge. G. Nash 198. Historic.
Cyperaceae — Carex swanii (Fernald) Mack., Swan’s Sedge. D. Atha 7436; R. Naczi 12477, 12483; G. Nash 324; J. Schuler & C. Zhou 3. Frequent.
Cyperaceae — Carex tenera Dewey, Quill Sedge. R. Naczi 12484. Infrequent.
Cyperaceae — Carex tonsa (Fernald) E. P. Bicknell, Shaved Sedge. R. Naczi 12389. Infrequent.
Cyperaceae — Carex umbellata Schkuhr ex Willd., Parasol Sedge. R. Naczi 12385, 12387, 13686. Infrequent.
Cyperaceae — Carex virescens Muhl., {Carex costellata}, Ribbed Sedge. J. Monachino s.n; R. Naczi 12499; G. Nash 159. Infrequent.
Cyperaceae — Carex vulpinoidea Michx., Fox Sedge. R. Naczi 12495. Rare.
Cyperaceae — Cyperus bipartitus Torr., {Cyperus diandrus}, Slender Flatsedge. D. Atha 8144; R. Naczi, M. Nee & D. Atha 13382. Infrequent.
Cyperaceae — Cyperus esculentus L., Yellow Nutsedge. D. Atha 7927; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12572; R. Naczi, M. Falk, J. Schuler & D. Atha 12602, 12606; M. Nee 43579. Not Native. Frequent.
Cyperaceae — Cyperus iria L., Ricefield Flatsedge. D. Atha & M. Nee 8006; R. Naczi & D. Atha 12675; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12575; R. Naczi, M. Falk, J. Schuler & D. Atha 12603. Not Native. Infrequent.
Cyperaceae — Cyperus lupulinus (Spreng.) Marcks, {Cyperus filiculmis}, Great Plains Flatsedge. D. Atha 7916, 8047. Rare.
Cyperaceae — Cyperus microiria Steudel., Asian Flatsedge. R. Naczi & D. Atha 12681. Not Native. Rare.
Cyperaceae — Cyperus squarrosus L., Bearded Flatsedge. R. Naczi & D. Atha 12677; R. Naczi, M. Falk, J. Schuler & D. Atha 12601. Infrequent.
Cyperaceae — Cyperus strigosus L., False Nutsedge. D. Atha 7845; R. Naczi, M. Falk, J. Schuler & D. Atha 12604; M. Nee 54532. Infrequent.
Cyperaceae — Eleocharis obtusa (Willd.) Schult., {Eleocharis ovata}, Blunt Spikerush. W. Clute & P. Wilson s.n. Historic.
Cyperaceae — Schoenoplectus tabernaemontani (C. C. Gmel.) Palla, {Scirpus lacustris}, Softstem Bulrush. G. Nash 427. Historic.
Cyperaceae — Scirpus hattorianus Makino, {Scirpus atrovirens}, Mosquito Bulrush. R. Naczi 12502; M. Nee 54450. Infrequent.
Cyperaceae — Trichophorum planifolium (Spreng.) Palla, {Scirpus planifolius}, Bashful Bulrush. G. Nash 146. Historic.
Dioscoreaceae — Dioscorea villosa L., Colicroot. D. Atha 6841, 7583. Rare.
Hydrocharitaceae — Elodea canadensis Michx., Common Waterweed. D. Atha 8103. Rare.
Hypoxidaceae — Hypoxis hirsuta (L.) Coville, Common Goldstar. D. Atha 8130; R. Naczi 12532; G. Nash s.n. Rare.
Iridaceae — Iris pseudacorus L., Pale Yellow Iris. D. Atha 7378, 10597; C. Gilly 5; M. Kearns 27; D. McClelland 330; E. Roy 123. Not Native. Rare.
Iridaceae — Iris versicolor L., Blueflag. C. Gilly 4; G. Nash 142. Historic.
Iridaceae — Sisyrinchium angustifolium Mill., {Sisyrinchium graminoides}, Narrowleaved Blue-eyed Grass. D. Atha 10617; W. Cahilly 32; R. Dragonetti 5; M. Nee & K. Cameron 54454; P. Wilson s.n. Rare.
Juncaceae — Juncus tenuis Willd., Poverty Rush. D. Atha 7565, 7844, 7945; C. Gilly 83; W. Gonzalez 1; A. Hollick s.n.; R. Naczi 12531; M. Nee & K. Cameron 54472. Frequent.
Juncaceae — Juncus torreyi Coville, Torrey’s Rush. D. Atha & M. Nee 8001. Rare.
Juncaceae — Luzula multiflora (Ehrh.) Lej. subsp. multiflora, {Juncoides campestre}, Common Woodrush. D. Atha 7048, 7435; R. Naczi 12528; G. Nash s.n.; M. Nee & K. Cameron 54297. Rare.
Liliaceae — Erythronium americanum Ker Gawl., American Troutlily. D. Atha 6950: N. Holmgren 641 (UTC); S. Mori & C. Gracie 18816; M. Nee & K. Cameron 54251; W. Nieder 16. Frequent.
Liliaceae — Lilium superbum L., Turkscap Lily. G. Nash 413. Historic.
Melanthiaceae — Trillium cernuum L., Nodding Trillium. T. Edmondson s.n. Historic.
Melanthiaceae — Veratrum viride Aiton, Green False Hellebore. G. Nash 190. Historic.
Orchidaceae — Calopogon tuberosus (L.) Britton, Stearns & Poggenb. var. tuberosus, Tuberous Grasspink. G. Nash 267. Historic.
Orchidaceae — Corallorhiza maculata (Raf.) Raf. var. maculata, Spotted Coralroot. M. Eaton s.n. Historic.
Orchidaceae — Epipactis helleborine (L.) Crantz, Broadleaf Helleborine. D. Atha 13717; D. Atha & M. Nee 7992; W. Gonzalez 9; J. Lendemer 37816. Not Native. Frequent.
Orchidaceae — Platanthera hookeri (Torrey ex A. Gray) Lindl., Hooker’s Orchid. C. Gilly 333. Historic.
Poaceae — Agrostis capillaris L., {Agrostis alba}, Rhode Island Bent. G. Nash 317, 340, 391, s.n. Not Native. Historic.
Poaceae — Agrostis gigantea Roth, {Agrostis alba vulgaris}, Black Bent. P. Wilson 1261. Not Native. Historic.
Poaceae — Agrostis perennans (Walter) Tuck., {Agrostis intermedia}, Autumn Bent. D. Atha 8129, 14833; D. Atha & M. Nee 7990; W. Bastedo s.n.; G. Nash 466, 482, 543, s.n. Infrequent.
Poaceae — Alopecurus pratensis L., Meadow Foxtail. D. duMouchel s.n.; R. Stewart s.n.; P. Wilson s.n. Not Native. Historic.
Poaceae — Andropogon virginicus L., Broomsedge Bluestem. D. Atha 8086; K. Kimball s.n.; D. McClelland 354; G. Nash 580; E. Roy 118. Infrequent.
Poaceae — Anthoxanthum odoratum L., Sweet Vernalgrass. D. Atha 2610, 6992, 7434; W. Gonzalez 3; N. Holmgren 655 (UTC); M. Nee & D. McClelland 54286; P. Wilson s.n. Not Native. Frequent.
Poaceae — Aristida dichotoma Michx., Churchmouse Threeawn. D. Atha 8119; E. Bicknell s.n.; C. Gilly 209; G. Nash 587; M. Nee & D. Atha 56506. Rare.
Poaceae — Arrhenatherum elatius (L.) P. Beauv. ex J. Presl & C. Presl, Tall Oatgrass. F. Pennell s.n.; P. Wilson 114. Not Native. Historic.
Poaceae — Arthraxon hispidus (Thunb.) Makino, Small Carpetgrass. J. Monachino 397. Not Native. Waif. Historic.
Poaceae — Beckmannia syzigachne (Steud.) Fernald, American Sloughgrass. F. McCarthy s.n. Not Native. Waif. Historic.
Poaceae — Bromus commutatus Schrad., {Bromus racemosus}, Bald Brome. J. Monachino 23; G. Nash 203, 216, 263; R. Stewart s.n. Not Native. Historic.
Poaceae — Bromus hordeaceus L. subsp. hordeaceus, Soft Brome. D. Atha 7440. Not Native. Rare.
Poaceae — Calamagrostis canadensis (Michx.) P. Beauv., Bluejoint. G. Nash 396. Historic.
Poaceae — Cinna arundinacea L., Sweet Woodreed. D. Atha & M. Nee 8023; G. Nash 468, s.n.; J. Monachino 170, s.n; D. du Mouchel s.n. Rare.
Poaceae — Cynosurus echinatus L., Bristly Dogstail Grass. J. Monachino 559. Not Native. Waif. Historic.
Poaceae — Dactylis glomerata L., Orchardgrass. D. Atha 7437; G. Nash 257; M. Nee & K. Cameron 54340. Not Native. Frequent.
Poaceae — Danthonia compressa Austin, Flattened Oatgrass. D. Atha 7428; J. Gonzalez 2; R. Naczi 12475, 12485, 12529; G. Nash 262, 280. Infrequent.
Poaceae — Danthonia spicata (L.) P. Beauv. ex Roem. & Schult., Poverty Oatgrass. D. Atha 7429; R. Naczi 12486; G. Nash 228, 251, 325; J. Schuler & C. Zhou 2; R. Stewart & F. Pennell s.n. Infrequent.
Poaceae — Deschampsia flexuosa (L.) Trin., Wavy Hairgrass. R. Naczi 12527. Rare.
Poaceae — Dichanthelium boreale (Nash) Freckmann, {Panicum bicknellii}, Northern Panicgrass. Bicknell s.n.; G. Nash 346. Historic.
Poaceae — Dichanthelium boscii (Poir.) Gould & C. A. Clark, {Panicum porterianum}, Bosc's Panicgrass. G. Nash 279, 339. Historic.
Poaceae — Dichanthelium clandestinum (L.) Gould, {Panicum clandestinum}, Deertongue Puberulent Panicgrass. D. Atha 7637; R. Naczi 12494; G. Nash 274, 314. Infrequent.
Poaceae — Dichanthelium columbianum (Scribn.) Freckmann, {Panicum tsugetorum}, Variable Panicgrass. E. Bicknell 11040; G. Nash 287, 417, 483. Historic.
Poaceae — Dichanthelium commutatum (Schult.) Gould subsp. ashei (Ashe) Lelong, {Panicum ashei}, Ashe's Variable Panicgrass. G. Nash 490. Historic.
Poaceae — Dichanthelium commutatum (Schult.) Gould subsp. commutatum, {Panicum commutatum, Panicum commutatum minor}, Variable Panicgrass, E. Bicknell 10564, 10565, 10566; G. Nash 491. Historic.
Poaceae — Dichanthelium depauperatum (Muhl.) Gould, {Panicum depauperatum}, Starved Panicgrass. D. Atha 7430; E. Bicknell 10688, 10692; G. Nash 286, 330, 416. Rare.
Poaceae — Dichanthelium dichotomum (L.) Gould, {Panicum dichotomum}, Cypress Panicgrass. E. Bicknell 10630; G. Nash 229, 282, 494. Historic.
Poaceae — Dichanthelium lanuginosum (Ell.) Gould. {Panicum pubescens}, Panicgrass. D. Atha & M. Nee 7980; R. Naczi 12479; R. Naczi, A. Litt, M. Pace, T. Lane, D. Kaiser 12557; G. Nash 217, 252, 277, 328, 360,497, s.n.; M. Nee & K Cameron 54485; J. Schuler & C. Zhou 4. Frequent.
Poaceae — Dichanthelium latifolium L., {Panicum macrocarpon}, Broadleaved Panicgrass. G. Nash 270, 275, 284, 492. Historic.
Poaceae — Dichanthelium lindheimeri (Nash) Gould., Lindheimer Panicgrass. G. Nash 329, 361, s.n. Historic.
Poaceae — Dichanthelium linearifolium (Scribn.) Gould, {Panicum enslini}, Slimleaf Panicgrass. G. Nash 326, 327, 331. Historic.
Poaceae — Dichanthelium microcarpon (Muhl. ex Elliott) Mohlenbr. {Panicum barbulatum}, Smallfruited Panicgrass. J. Monachino 27; R. Naczi 12533; G. Nash 278. Rare.
Poaceae — Dichanthelium sphaerocarpon (Ell.) Gould, {Panicum sphaerocarpon}, Roundfruited Panicgrass. G. Bicknell 10893; G. Nash 288, 332, 419, 485, 488. Historic.
Poaceae — Dichanthelium villosissimum (Nash) Freckmann, {Panicum atlanticum}, Whitehair Panicgrass. G. Nash s.n. Historic.
Poaceae — Digitaria filiformis (L.) Koeler, {Syntherisma filiformis}, Slender Crabgrass. W. Bastedo s.n.; G. Nash 504. Historic.
Poaceae — Digitaria ischaemum (Schreb.) Schreb. ex Muhl., {Syntherisma linearis}, Smooth Crabgrass. G. Nash 541. Not Native. Historic.
Poaceae — Digitaria sanguinalis (L.) Scop., {Syntherisma sanguinalis}, Hairy Crabgrass. D. Atha 7842; D. Atha & M. Nee 7981; G. Nash 501; M. Nee 54718. Not Native. Frequent.
Poaceae — Echinochloa crusgalli (L.) P. Beauv., {Panicum crus-galli}, Barnyardgrass. D. Atha 7765; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12576; J. Schuler & M. Wolf 5. Not Native. Infrequent.
Poaceae — Echinochloa muricata (P. Beauv.) Fernald. Rough Barnyardgrass. G. Nash 458, s.n. Historic.
Poaceae — Echinochloa walteri (Pursh) Heller, {Panicum walteri}, Walter's Barnyardgrass. D. Atha 9030; N. Holmgren 430 (UTC). Rare.
Poaceae — Eleusine indica (L.) Gaertn., Goose Grass. D. Atha & M. Nee 7997; C. Gilly 70; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12570. Not Native. Frequent.
Poaceae — Elymus canadensis L., Canada Wild Rye. A. Hollick s.n.; P. Wilson 4. Historic.
Poaceae — Elymus hystrix L., {Hystrix hystrix}, Bottlebrush Grass. G. Nash 467. Historic.
Poaceae — Elymus repens (L.) Gould, {Agropyron repens}, Quackgrass. G. Nash 246, 271, 312. Not Native. Historic.
Poaceae — Elymus riparius Wiegand, River Wild Rye. E. Bicknell 9951; G. Nash 456. Historic.
Poaceae — Elymus virginicus L., Virginia Wild Rye. D. Atha 14069; J. Schuler & C. Zhou 1; P. Wilson 5. Infrequent.
Poaceae — Eragrostis capillaris (L.) Nees, Lace Grass. G. Nash 554. Historic.
Poaceae — Eragrostis cilianensis (All.) Vign. ex Janchen, Stinkgrass. D. Atha & M. Nee 8007; C. Gilly 155. Not Native. Rare.
Poaceae — Eragrostis hypnoides (Lam.) Britton, Sterns & Poggenb., Teal Lovegrass. J. Monachino s.n. Historic.
Poaceae — Eragrostis pectinacea (Michx.) Nees ex Jedw., {Eragrostis purshii}, Carolina Lovegrass. D. Atha & M. Nee 7998; W. Clute & P. Wilson s.n.; T. Kearney s.n.; H. Moldenke 8665, 11277; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12566; G. Nash 439, 440, 441. Rare.
Poaceae — Eragrostis spectabilis (Pursh) Steud., Purple Lovegrass. D. Atha 8075; E. Bicknell 10029; C. Gilly 74; G. Nash 452. Rare.
Poaceae — Festuca ovina L., Sheep Fescue. N. Holmgren 657 (UTC); P. Wilson s.n. Historic.
Poaceae — Festuca rubra L., Red Fescue. F. Pennell s.n. Not Native. Historic.
Poaceae — Glyceria acutiflora Torr., {Panicularia acutiflora}, Creeping Mannagrass. G. Nash s.n. Not Native. Historic.
Poaceae — Glyceria canadensis (Michx.) Trin., {Panicularia canadensis}, Rattlesnake Mannagrass. J. Monachino 28; G. Nash 344. Historic.
Poaceae — Glyceria septentrionalis Hitch., {Panicularia fluitans}, Floating Mannagrass. G. Nash 319. Historic.
Poaceae — Glyceria striata (Lam.) Hitchc., {Panicularia nervata}, Fowl Mannagrass. H. Moldenke 5059; G. Nash 212; F. Pennell s.n; R. Stewart s.n. Historic.
Poaceae — Holcus lanatus L., Common Velvetgrass. E. Yarrow s.n. Not Native. Historic.
Poaceae — Leersia oryzoides (L.) Sw., {Homalocenchrus oryzoides}, Cutgrass. D. Atha 8135; G. Nash 461. Rare.
Poaceae — Leersia virginica Willd., {Homalocenchrus virginicus}, Whitegrass. D. Atha & M. Nee 7974; A. Hollick s.n.; G. Nash 470. Rare.
Poaceae — Lolium perenne L. var. aristatum Willd., {Lolium italicum}, Italian Ryegrass. J. Monachino 557, 557a, 557b. Not Native. Historic.
Poaceae — Lolium perenne L. var. perenne, {Lolium perenne}, Perennial Ryegrass. G. Nash 214, 258; J. Monachino 35. Not Native. Historic.
Poaceae — Microstegium vimineum (Trin.) A. Camus, Japanese Stiltgrass. M. Nee 41826. Not Native. Infrequent.
Poaceae — Miscanthus sinensis Andersson, Chinese Silvergrass. D. Atha 6060; A. Hollick s.n. Not Native. Waif. Rare.
Poaceae — Muhlenbergia mexicana (L.) Trin., Mexican Muhly. G. Nash 492. Historic.
Poaceae — Muhlenbergia schreberi J. F. Gmel., {Muhlenbergia diffusa}, Schreber Muhly. G. Nash s.n.; P. Wilson s.n. Historic.
Poaceae — Muhlenbergia sobolifera (Muhl. ex Willd.) Trin., Rock Muhly. G. Nash 465. Historic.
Poaceae — Muhlenbergia tenuiflora (Willd.) Britton, Stearns & Poggenb., Slender Muhly. E. Bicknell 10260; G. Nash 479. Historic.
Poaceae — Panicum capillare L., Witch Panicgrass. G. Nash 453, 497. Historic.
Poaceae — Panicum dichotomiflorum Michx., {Panicum proliferum}, Fall Panicgrass. D. Atha 8105; J. Monachino s.n.; G. Nash 459. Infrequent.
Poaceae — Panicum miliaceum L., Proso Millet. G. Nash 473. Not Native. Historic.
Poaceae — Panicum philadelphicum Bernh. ex Trin., {Panicum minus}, Philadelphia Panicgrass. G. Nash 499. Historic.
Poaceae — Panicum rigidulum Bosc ex Nees, {Panicum agrostoides}, Redtop Panicgrass. G. Nash 538. Historic.
Poaceae — Paspalum laeve Michx., Smooth Paspalum. E. Bicknell s.n.; J. Monachino 359; G. Nash 489. Historic.
Poaceae — Paspalum setaceum Michx. var. muhlenbergii (Nash) Fernald, {Paspalum pubescens}, Thin Paspalum. D. Atha & M. Nee 7976; A. Bastedo s.n.; G. Nash 454, 500, 526, 528, 539, 542, 563, 564, s.n., s.n. Rare.
Poaceae — Paspalum setaceum Michx. var. setaceum. {Paspalum setaceum}, Thin Paspalum. E. Bicknell 11143; G. Nash 502. Historic.
Poaceae — Phalaris arundinacea L., Reed Canarygrass. N. Taylor s.n. Historic.
Poaceae — Phleum pratense L., Timothy. P. Wilson 123. Not Native. Historic.
Poaceae — Poa annua L., Annual Bluegrass. D. Atha 2611, 6965; J. Knowles 1; G. Nash 74. Not Native. Frequent.
Poaceae — Poa compressa L., Canada Bluegrass. G. Nash 307; R. Stewart s.n. Not Native. Historic.
Poaceae — Poa palustris L., Fowl Bluegrass. G. Nash 394. Historic.
Poaceae — Poa pratensis L., Kentucky Bluegrass. D. Atha 7439; G. Nash 126, s.n.; M. Nee & K. Cameron 54341. Not Native. Frequent.
Poaceae — Schedonorus giganteus (L.) Holub., Giant Fescue. J. Monachino 555. Not Native. Historic.
Poaceae — Schedonorus pratensis (Huds.) P. Beauv., {Festuca elatior}, Meadow Fescue. G. Nash 213; F. Pennell s.n.; R. Stewart s.n.; N. Taylor s.n. Not Native. Historic.
Poaceae — Secale cereale L., Cereal Rye. J. Monachino 32; P. Wilson 121. Not Native. Waif. Historic.
Poaceae — Setaria faberi R. A. W. Herrm., Chinese Foxtail. D. Atha 7928; J. Monachino 582, s.n.; M. Nee 43621. Not Native. Frequent.
Poaceae — Setaria pumila (Poir.) Roem. & Schult., {Chaetochloa glauca}, Yellow Foxtail. D. Atha 8143; P. Wilson 126A. Not Native. Infrequent.
Poaceae — Setaria viridis (L.) P. Beauv., {Chaetochloa viridis}, Green Foxtail. D. Atha & M. Nee 8000; C. Gilly 72, 79, 205; K. Kimball s.n.; J. Monachino 588, 611A, 611B, 630; D. duMouchel s.n.; P. Wilson s.n. Not Native. Infrequent.
Poaceae — Sphenopholis intermedia (Rydb.) Rydb., {Eatonia pennsylvanica}, Slender Wedgescale. E. Bicknell s.n.; G. Nash 152, 200. Historic.
Poaceae — Sphenopholis nitida (Biehler) Scribn., {Eatonia nitida}, Shiny Wedgescale. G. Nash 132. Historic.
Poaceae — Tridens flavus (L.) Hitchc., {Poa flava, Tricuspis seslerioides}, Purpletop. D. Atha 8122; G. Nash 506. Rare.
Pontederiaceae — Pontederia cordata L., Pickerelweed. D. Atha 10732; H. Beck 1374; M. Nee & K. Cameron 54491. Infrequent.
Potamogetonaceae — Potamogeton crispus L., Curly Pondweed. D. Atha 8102. Not Native. Rare.
Potamogetonaceae — Potemogeton pusillus L., Small Pondweed. D. Atha, D. Stevenson & M. Thadeo 8108. Rare.
Potamogetonaceae — Zannichellia palustris L., Horned Pondweed. D. Atha 8104. Rare.
Smilacaceae — Smilax glauca Walter, Cat Greenbrier. R. Naczi, A. Litt, M. Pace, T. Lane, D. Kaiser 12561; M. Nee & K. Cameron 54538; A. Vail s.n. Rare.
Smilacaceae — Smilax herbacea L., Smooth Carrionflower. G. Nash 175, s.n. Historic.
Smilacaceae — Smilax rotundifolia L., Roundleaf Greenbrier. D. Atha 6837, 8083; W. Cahilly 35; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12562; G. Nash 168, 583; M. Nee & K. Cameron 54358, 54486; E. Roy 113. Infrequent.
Typhaceae — Sparganium eurycarpum Engelm., Giant Bur-reed. D. Atha & M. Nee 8026; C. Gilly 49; G. Nash 308; P. Wilson s.n. Rare.
Typhaceae — Typha latifolia L., Common Cattail. M. Nee & K. Cameron 54480. Rare.
Xanthorrhoeaceae — Hemerocallis fulva (L.) L., Daylily. W. Nieder 67. Not Native. Waif. Rare. Reported by Britton (1898).
Dicots
Adoxaceae — Sambucus canadensis L., Common Elderberry. C. Gilly 44; J. Luteyn & S. Mori 7948; M. Nee & D. McClelland 54442; C. Ruiz 3; P. Wilson 10845. Frequent.
Adoxaceae — Viburnum acerifolium L., Mapleleaved Viburnum. D. Atha 7657; W. Cahilly 23; C. Gilly 254, 441; J. Gunderson 3; G. Nash s.n.; E. Roy 64; P. Wilson 10823. Frequent.
Adoxaceae — Viburnum dilatatum Thunb., Linden Arrowwood. D. Atha 7369, 7563; D. Atha & M. Nee 7982; M. Nee 43520, 56511. Not Native. Frequent.
Adoxaceae — Viburnum lentago L., Nannyberry. G. Nash s.n.; P. Wilson 10835. Historic.
Adoxaceae — Viburnum plicatum Thunb., Japanese Snowball. D. Atha, D. Stevenson & M. Thadeo 8113; W. Cahilly 14, 15; C. Gilly 308; A. Hollick s.n., s.n.; M. Nee & K. Cameron 54311, 54336; W. Nieder 32, 50. Not Native. Rare.
Adoxaceae — Viburnum prunifolium L., Blackhaw Viburnum. D. Atha 2608, 14404; W. Cahilly 33; S. Clarke s.n.; T. Edmondson 1091; G. Nash 969, s.n.; M. Nee & K. Cameron 54301, 54335; W. Nieder 40; E. Roy 25; P. Wilson 102, 10837. Frequent.
Adoxaceae — Viburnum recognitum Fernald, {Viburnum dentatum}, Smooth Arrowwood. D. Atha 7577; A. Carvalho & W. Thomas 6888; C. Gilly 388; G. Nash s.n. Frequent.
Adoxaceae — Viburnum setigerum Hance, Tea Viburnum. D. Atha & M. Nee 7986; M. Nee & K. Cameron 54351. Not Native. Infrequent.
Adoxaceae — Viburnum sieboldii Miq., Siebold's Arrowwood. W. Cahilly 12; M. Nee & K. Cameron 54337; W. Nieder 44. Not Native. Infrequent.
Altingiaceae — Liquidambar styraciflua L., Sweet Gum. D. Atha 6737; W. Cahilly 16, 19, 37; D. du Mouchel s.n.; M. Nee 36727, 43572; M. Nee & K. Cameron 54321; P. Wilson 10901, s.n. Frequent.
Amaranthaceae — Amaranthus blitoides S. Watson, Mat Amaranth. C. Gilly 158. Not Native. Historic.
Amaranthaceae — Amaranthus blitum L., Livid Amaranth. C. Gilly 75; H. Moldenke 10354; R. Naczi & D. Atha 12679; M. Nee 30150, 43869. Not Native. Frequent.
Amaranthaceae — Amaranthus hybridus L., Green Amaranth. D. Atha 6798, 8604; D. Atha, D. Stevenson & M. Thadeo 8110, 8111; C. Gilly 138; R. Naczi & D. Atha 12682, 12683; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12574; M. Nee 33270. Not Native. Frequent.
Amaranthaceae — Amaranthus retroflexus L., Rough Amaranth. A. Foss s.n.; C. Gilly 137. Historic.
Amaranthaceae — Chenopodium album L., Lambsquarters. D. Atha 6838, 8458; A. Foss s.n.; C. Gilly 73; R. Naczi & D. Atha 12684; R. Naczi 12664; M. Nee & K. Cameron 54698. Not Native. Frequent.
Amaranthaceae — Chenopodium simplex (Torr.) Raf., Mapleleaved Goosefoot. C. Gilly 453; J. Monachino s.n.; G. Nash 570. Not Native. Historic.
Amaranthaceae — Chenopodium standleyanum Aellen, Standley's Goosefoot. R. Naczi & D. Atha 12685. Rare.
Amaranthaceae — Dysphania ambrosioides (L.) Mosyakin & Clemants, {Chenopodium ambrosioides}, Wormseed. R. Abbott 26827; D. Atha 6794; A. Foss s.n.; C. Gilly 167; H. Moldenke 10353; R. Naczi 12669; M. Nee & K. Cameron 54695. Not Native. Frequent.
Amaranthaceae — Dysphania pumilio (R. Br.) Mosyakin & Clemants, Clammy Goosefoot. D. Atha & M. Nee 8030; R. Naczi & D. Atha 12676; M. Nee 57173. Not Native. Frequent.
Anacardiaceae — Rhus copallinum L., Winged Sumac. P. Wilson 10804. Historic.
Anacardiaceae — Rhus glabra L., Smooth Sumac. D. Atha 8118; P. Wilson 10862. Rare.
Anacardiaceae — Rhus typhina L., Staghorn Sumac. M. Nee & K. Cameron 54462; P. Wilson 10863. Rare.
Anacardiaceae — Toxicodendron radicans (L.) Kuntze, {Rhus radicans}, Poison Ivy. D. Atha & M. Nee 8011; D. McClelland 332; E. Roy 66; P. Wilson 10813. Frequent.
Apiaceae — Aegopodium podagraria L., Goutweed. D. Atha & R. Naczi 7579; C. Gilly 326; M. Nee & K. Cameron 54463. Not Native. Infrequent.
Apiaceae — Anthriscus sylvestris (L.) Hoffm., Wild Chervil. D. Atha 14618. Not Native. Infrequent.
Apiaceae — Astrodaucus orientalis (L.) Drude., Astrodaucus. M. Nee & K. Cameron 54459. Not Native. Waif. Rare.
Apiaceae — Carum carvi L., Caraway. E. Bicknell 6670; G. Nash 269. Not Native. Historic.
Apiaceae — Cicuta maculata L., Common Waterhemlock. M. Nee & K. Cameron 54481. Infrequent.
Apiaceae — Cryptotaenia canadensis (L.) DC., {Deringa canadensis}, Canadian Honewort. D. Atha 7756; A. Foss s.n.; J. Gowdey s.n.; S. Hill 1168; G. Nash 469; M. Nee & K. Cameron 54490; E. Roy 99. Frequent.
Apiaceae — Daucus carota L., Queen Anne's Lace. D. Atha 7758, 7940; C. Gilly 104; D. MacDougal E270. Not Native. Infrequent.
Apiaceae — Heracleum maximum W. Bartram, Common Cowparsnip. J. Monachino 22; P. Wilson s.n. Historic.
Apiaceae — Osmorhiza claytonii (Michx.) C. B. Clarke, {Washingtonia claytoni}, Clayton's Sweetroot. G. Nash 236. Historic.
Apiaceae — Sanicula odorata (Raf.) K. M. Pryer & L. R. Phillippe, {Sanicula gregaria}, Clustered Black Snakeroot. G. V. Nash 305. Historic.
Apocynaceae — Amsonia tabernaemontana Walt., Bluestar. S. Clarke s.n.; C. Gilly 311; H. Moldenke 20593. Not Native. Historic.
Apocynaceae — Apocynum cannabinum L., Hemp Dogbane. C. Gilly 77; D. McClelland 341; M. Nee 43569; M. Nee & D. McClelland 54433. Infrequent.
Apocynaceae — Asclepias incarnata L. subsp. incarnata, Swamp Milkweed. M. Nee & K. Cameron 54541. Rare.
Apocynaceae — Asclepias incarnata L. subsp. pulchra (Ehrh. ex Willd.) Woodson, {Asclepias pulchra}, Swamp Milkweed. D. Atha & M. Nee 8013; C. Gilly 132; J. Monachino 577. Rare.
Apocynaceae — Asclepias syriaca L., Common Milkweed. C. Gilly 46; D. MacDougal s.n. Historic.
Apocynaceae — Asclepias tuberosa L., {Asclepias decumbens}, Butterfly Milkweed. G. Nash s.n. Historic.
Apocynaceae — Vinca minor L., Common Periwinkle. D. Atha 6938. Not Native. Frequent.
Apocynaceae — Vincetoxicum hirundinaria Medik., White Swallowwort. C. Gilly 380; J. Monachino 576. Not Native. Waif. Historic.
Apocynaceae — Vincetoxicum nigrum (L.) Moench, Black Swallowwort. Nee 43576. Not Native. Rare.
Apocynaceae — Vincetoxicum rossicum (Kleopow) Barbar., European Swallowwort. D. Atha 7946; J. Monachino 575; M. Nee & D. McClelland 54434. Not Native. Infrequent.
Aquifoliaceae — Ilex crenata Thunb., Japanese Holly. D. Atha 6928; M. Nee, D. Atha & A. Bardet 56453; P. Wilson 10807. Not Native. Waif. Rare.
Aquifoliaceae — Ilex opaca Aiton, American Holly. M. Nee, K. Cameron & D. McClelland 54689; P. Wilson 10808. Waif. Infrequent.
Aquifoliaceae — Ilex verticillata (L.) A. Gray, Common Winterberry. W. Cahilly 36; C. Gilly 260. Historic.
Araliaceae — Aralia elata (Miq.) Seem., Japanese Angelica Tree. R. Naczi 12600; M. Nee 41822, 43858; M. Nee & K. Cameron 54484, 54526; E. Roy 29. Not Native. Frequent.
Araliaceae — Aralia nudicaulis L., Wild Sarsaparilla. D. Atha 7660; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12558; E. Yarrow s.n. Rare.
Araliaceae — Aralia racemosa L., Spikenard. D. Atha 9037; C. Gilly 381; M. Nee & K. Cameron 54535; E. Roy 70. Infrequent.
Araliaceae — Hedera helix L., English Ivy. D. Atha & W. Cahilly 6929. Not Native. Waif. Rare.
Araliaceae — Panax trifolius L., Dwarf Ginseng. C. Gilly 283; J. Monachino s.n.; G. Nash 66; M. Nee & K. Cameron 54319; W. Nieder 28. Rare.
Asteraceae — Achillea millefolium L., Common Yarrow. W. Clute s.n.; A. Foss s.n.; C. Gilly 56; A. Hollick s.n. Not Native. Historic.
Asteraceae — Ageratina altissima (L.) King & H. Rob., {Eupatorium ageratoides},White Snakeroot. D. Atha 6796, 6826; R. Dragonetti 17; A. Foss s.n.; J. Furlaud 72; C. Gilly 439; A. Hollick s.n.; J. Monachino 662; M. Nee 54711; M. Petrino 2; J. Pruski 3551; E. Roy 7; C. Ruiz 16. Frequent.
Asteraceae — Ambrosia artemisiifolia L., Annual Ragweed. D. Atha 7955; C. Gilly 129; E. Roy 111. Frequent.
Asteraceae — Ambrosia trifida L., Giant Ragweed. A. Foss s.n.; C. Gilly 147; M. Nee & K. Cameron 54697; E. Roy 106; P. Wilson 82. Infrequent.
Asteraceae — Antennaria neglecta Greene, Field Pussytoes. T. Edmondson 1097. Historic.
Asteraceae — Antennaria plantaginifolia (L.) Hook., Plantainleaved Pussytoes. T. Edmondson 1072; J. Knowles 11. Historic.
Asteraceae — Anthemis arvensis L., Corn Chamomile. C. Gilly 14. Historic.
Asteraceae — Arctium minus (Hill) Bernh., Lesser Burdock. D. Atha 8565; D. Atha & M. Nee 7975; C. Gilly 136; T. Kearney s.n; P. Wilson 70. Not Native. Frequent.
Asteraceae — Artemisia vulgaris L., Common Wormwood. D. Atha 6800; C. Gilly 204; H. Moldenke 10744; M. Nee 37817, 43856; M. Nee, K. Cameron & D. McClelland 54699; E. Roy 115. Not Native. Frequent.
Asteraceae — Baccharis halimifolia L., Groundsel Tree. C. Gilly 255. Historic.
Asteraceae — Bellis perennis L., English Daisy. D. Atha 14746. Not Native. Rare.
Asteraceae — Bidens bipinnata L., Spanish Needles. T. Edmondson 1851. Historic.
Asteraceae — Bidens cernua L., Nodding Beggarticks. M. Kearns 26. Rare.
Asteraceae — Bidens connata Muhl. ex Willd., Purplestem Beggarticks. M. Petrino 34. Historic.
Asteraceae — Bidens frondosa L., Devil's Beggarticks. A. Foss s.n.; M. Petrino 11, 22, 35. Historic.
Asteraceae — Bidens vulgata Greene, Tall Beggarticks. D. Atha 8053; C. Gilly 206. Rare.
Asteraceae — Cichorium intybus L., Chicory. D. Atha 14832; A. Hollick s.n. Not Native. Infrequent.
Asteraceae — Cirsium arvense (L.) Scop., {Carduus arvensis}, Canada Thistle. D. Atha 10608; C. Gilly 345; M. Nee & K. Cameron 54475. Not Native. Infrequent.
Asteraceae — Cirsium muticum Michx., {Carduus muticus}, Swamp Thistle. W. Clute & P. Wilson s.n. Historic.
Asteraceae — Cirsium vulgare (Savi) Ten., Bull Thistle. D. Atha 7561; D. Atha & M. Nee 7996; C. Gilly 419; M. Petrino 12; E. Roy 107. Not Native. Infrequent.
Asteraceae — Conyza canadensis (L.) Cronq., {Leptilon canadense}, Horseweed. D. Atha 7938; C. Gilly 107. Frequent.
Asteraceae — Coreopsis lanceolata L., Lanceleaved Tickseed. A. Hollick s.n.; P. Wilson s.n. Not Native. Historic.
Asteraceae — Crepis capillaris (L.) Wallr., Smooth Hawksbeard. W. Clute s.n. Not Native. Historic.
Asteraceae — Eclipta prostrata (L.) L., False Daisy. D. Atha, R. Naczi & M. Nee 9015. Infrequent.
Asteraceae — Erechtites hieraciifolius (L.) Raf., Fireweed. D. Atha 7935; C. Gilly 191; M. Nee 59446. Frequent.
Asteraceae — Erigeron annuus (L.) Pers., Annual Fleabane. D. Atha 7443, 7651; C. Gilly 106; M. Nee & D. McClelland 43565; E. Roy 96. Frequent.
Asteraceae — Erigeron strigosus Muhl. ex Willd., {Erigeron ramosus}, Prairie Fleabane. G. Nash s.n. Historic.
Asteraceae — Eupatorium hyssopifolium L., Hyssopleaved Thoroughwort. C. Gilly 174. Historic.
Asteraceae — Eupatorium perfoliatum L., Common Boneset. D. Atha & M. Nee 8012; C. Gilly 421; M. Kearns 25. Rare.
Asteraceae — Eupatorium serotinum Michx., Lateflowering Thoroughwort. M. Nee & D. Atha 56504. Not Native. Rare.
Asteraceae — Eurybia divaricata (L.) G. L. Nesom, {Aster divaricatus}, White Wood Aster. D. Atha 6824; R. Dragonetti 18; J. Fargion 22; E. Roy 1. Frequent.
Asteraceae — Eurybia macrophylla (L.) Cass., {Aster macrophyllus}, Bigleaf Aster. C. Gilly 450. Historic.
Asteraceae — Euthamia graminifolia (L.) Nutt., Grassleaved Goldenrod. D. Atha 7954, 8087; C. Gilly 179; D. McClelland 360; M. Nee & K. Cameron 54536; E. Roy 88. Infrequent.
Asteraceae — Eutrochium dubium (Willd. ex Poir.) E. E. Lamont, Coastal Plain Joepyeweed. W. Clute & P. Wilson s.n.; C. Gilly 144; M. Nee & K. Cameron 54539. Rare.
Asteraceae — Eutrochium purpureum (L.) E. E. Lamont var. purpureum, {Eupatorium purpureum}, Sweetscented Joepyeweed. M. Petrino s.n. Historic.
Asteraceae — Galinsoga parviflora Cav., Lesser Quickweed. C. Gilly 201. Not Native. Historic.
Asteraceae — Galinsoga quadriradiata Ruiz & Pav., Common Quickweed. D. Atha 6732, 10600; E. Bicknell 9030; J. Furlaud 78; J. Gowdy s.n.; J. Monachino 52; M. Nee 43604, 54716; W. Nieder 68; E. Yarrow s.n. Not Native. Frequent.
Asteraceae — Gamochaeta pensylvanica (Willd.) Cabrera, Pennsylvania Cudweed. C. Gilly 385. Not Native. Historic.
Asteraceae — Gnaphalium uliginosum L., Low Cudweed. Anonymous s.n. (BKL). Not Native. Historic.
Asteraceae — Helianthus decapetalus L., Thinleaved Sunflower. C. Gilly 368. Historic.
Asteraceae — Helianthus giganteus L., Tall Sunflower. C. Gilly 447; W. Clute & P. Wilson s.n. Historic.
Asteraceae — Helianthus tuberosus L., Jerusalem Artichoke. C. Gilly 202. Not Native. Historic.
Asteraceae — Heliopsis helianthoides (L.) Sweet, Oxeye. C. Gilly 376. Historic.
Asteraceae — Hieracium umbellatum L., Canada Hawkweed. D. Atha 11489; D. Atha & M. Nee 7988; J. Monachino 366; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12563; M. Wolf & J. Schuler 27. Infrequent.
Asteraceae — Hypochaeris radicata L., Hairy Cat's Ear. D. Atha 7943; N. Britton s.n.; H. Moldenke 4674. Not Native. Rare.
Asteraceae — Krigia virginica (L.) Willd., {Adopogon carolinianum}, Virginia Dwarf Dandelion. T. Edmondson 3279. Historic.
Asteraceae — Lactuca canadensis L., Tall Lettuce. D. Atha 7934. Infrequent.
Asteraceae — Lactuca serriola L., Prickly Lettuce. D. Atha 7918; D. Atha & M. Nee 7994. Not Native. Frequent.
Asteraceae — Matricaria chamomilla L., German Chamomile. M. Nee 43582. Not Native. Waif. Rare.
Asteraceae — Mikania scandens (L.) Willd., {Willoughbaea scandens}, Climbing Hempvine. W. Clute & P. Wilson s.n. Historic.
Asteraceae — Nabalus trifoliolatus Cass., Threeleaved Rattlesnakeroot. C. Gilly 452. Historic.
Asteraceae — Petasites japonicus (Siebold & Zucc.) Maxim., Japanese Sweet Coltsfoot. D. Atha 7050. Not Native. Waif. Rare.
Asteraceae — Pluchea odorata (L.) Cass., Saltmarsh Fleabane. C. Gilly 417. Not Native. Historic.
Asteraceae — Pseudognaphalium obtusifolium (L.) Hillier & B. L. Burtt, Fragrant Cudweed. D. Atha 8116. Rare.
Asteraceae — Rudbeckia hirta L. var. pulcherrima Farw., {Rudbeckia hirta}, Blackeyed Susan. C. Gilly 355. Not Native. Historic.
Asteraceae — Rudbeckia laciniata L., Cutleaf Coneflower. D. Atha 6806; W. Clute & P. Wilson s.n.; C. Gilly 181; M. Nee & K. Cameron 54540; E. Roy 77. Frequent.
Asteraceae — Senecio vulgaris L., Common Groundsel. D. Atha 6909, 6967; M. Nee 43583; M. Nee & K. Cameron 54260. Not Native. Frequent.
Asteraceae — Silphium perfoliatum L., Cupplant. D. Atha 8091; R. Cowan s.n.; C. Gilly 340; A. Hollick s.n.; H. Moldenke 20255; M. Nee & K. Cameron 54497. Not Native. Infrequent.
Asteraceae — Solidago altissima L., {Solidago canadensis}, Tall Goldenrod. C. Gilly 210; N. Taylor 15720. Historic.
Asteraceae — Solidago bicolor L., White Goldenrod. W. Clute & P. Wilson s.n. Historic.
Asteraceae — Solidago caesia L., Wreath Goldenrod. D. Atha 6825; J. Fargion 20; C. Gilly 458; D. McClelland 352; J. Monachino 365; M. Nee, K. Cameron & D. McClelland 54690; E. Roy 89. Frequent.
Asteraceae — Solidago juncea Aiton, Early Goldenrod. W. Clute & P. Wilson s.n.; C. Gilly 186; G. Nash s.n. Historic.
Asteraceae — Solidago nemoralis Aiton, Grey Goldenrod. C. Gilly 208. Historic.
Asteraceae — Solidago odora Aiton, Anisescented Goldenrod. D. Atha 8505; M. Pace 503. Infrequent.
Asteraceae — Solidago patula Muhl., Roughleaved Goldenrod. W. Clute & P. Wilson s.n. Historic.
Asteraceae — Solidago rugosa Mill. var. rugosa, {Solidago rugosa}, Wrinkleleaved Goldenrod. D. Atha 6797; W. Clute & P. Wilson s.n.; G. Nash s.n.; M. Nee 44069; M. Petrino 5; M. Pace 502; E. Roy 82; N. Taylor 15634. Frequent.
Asteraceae — Solidago speciosa Nutt., Showy Goldenrod. C. Gilly 222; J. Monachino s.n. Historic.
Asteraceae — Sonchus asper (L.) Hill, Prickly Sowthistle. M. Nee 43580; M. Nee & D. McClelland 54425. Not Native. Frequent.
Asteraceae — Sonchus oleraceus L., Common Sowthistle. D. Atha 7373; W. Clute & P. Wilson s.n.; C. Gilly 60; N. Holmgren 460 (UTC); M. Nee, K. Cameron & D. McClelland 54702. Not Native. Frequent.
Asteraceae — Symphyotrichum cordifolium (L.) G. L. Nesom, {Aster cordifolius polycephalus}, Heartleaved Aster. C. Gilly 450; A. Hollick s.n., s.n.; R. Naczi 13396; G. Nash s.n., s.n.; M. Nee 56512; E. Roy 92; E. Yarrow s.n. Frequent.
Asteraceae — Symphyotrichum dumosum (L.) G. L. Nesom, Bushy Aster. C. Gilly 455; M. Petrino 9. Historic.
Asteraceae — Symphyotrichum lanceolatum (Willd.) G. L. Nesom., While Panicle Aster. C. Gilly 456; M. Nee 44068; M. Petrino 23, 33. Frequent.
Asteraceae — Symphyotrichum lateriflorum (L.) A. & D. Löve, {Aster lateriflorus, Aster vimineus}, Small White Aster. C. Gilly 451; G. Nash 577. Historic.
Asteraceae — Tanacetum parthenium (L.) Sch. Bip., {Chrysanthemum parthenium}, Feverfew. M. Nee & K. Cameron 54458. Not Native. Rare.
Asteraceae — Tanacetum vulgare L., Common Tansy. C. Gilly 420. Not Native. Historic.
Asteraceae — Taraxacum officinale F. H. Wigg., {Taraxacum taraxacum}, Common Dandelion. D. Atha 13365; W. Graham s.n.; J. Monachino s.n.; J. Small s.n.; M. Nee & K. Cameron 54259; W. Nieder 18; P. Wilson 305. Not Native. Frequent.
Asteraceae — Tragopogon pratensis L., Meadow Goat's Beard. A. Hollick s.n. Not Native. Historic.
Asteraceae — Tussilago farfara L., Colt's Foot. J. Knowles 8. Not Native. Historic.
Asteraceae — Verbesina alternifolia (L.) Britton ex Kearney, Wingstem. D. Atha 14895; C. Gilly 173; W. Graham s.n.; M. Nee 54727; J. Pruski 3082; E . Roy 83; P . Wilson 273. Not Native. Frequent.
Asteraceae — Vernonia noveboracensis (L.) Michx., New York Ironweed. D. Atha & M. Nee 8015; C. Gilly 371; P. Wilson 368. Infrequent.
Asteraceae — Xanthium strumarium L., Common Cocklebur. D. Atha 8088; N. Britton s.n.; C. Gilly 249; M. Nee 43855. Infrequent.
Balsaminaceae — Impatiens capensis Meerb., {Impatiens biflora}, Jewelweed. W. Cahilly 28; J. Gowdey s.n; M. Nee & K. Cameron 54455; M. Petrino 1. Frequent.
Balsaminaceae — Impatiens pallida Nutt., Pale Jewelweed. D. Atha 7759; E. Roy 87. Rare.
Berberidaceae — Podophyllum peltatum L., Mayapple. M. Bennett s.n.; W Cahilly 9; A. Foss s.n.; D. duMouchel s.n.; M. Nee & K. Cameron 54310. Infrequent.
Betulaceae — Alnus glutinosa (L.) Gaertn., Black Alder. D. Atha 4977, 4978, 6742, 6904; J. Fargion 26; J. Gowdey s.n.; N. Holmgren 574 (UTC); B. Meurer-Grimes 225, 226; B. Meurer-Grimes & H. Liao 9, 10, 11, 15; R. Naczi 12671; M. Nee 43615; M. Nee & K. Cameron 54245; P. Wilson 10910. Not Native. Infrequent.
Betulaceae — Betula alleghaniensis Britton, Yellow Birch. C. Gilly 263; P. Wilson 10928. Historic.
Betulaceae — Betula lenta L., Sweet Birch. E. Alexander s.n.; W. Cahilly 22; C. Gilly 269; D. McClelland 319; G. Nash 472; M. Nee 43859; M. Nee & K. Cameron 54284; E. Roy 35; P. Wilson 10844. Frequent.
Betulaceae — Betula populifolia Marshall, Gray Birch. C. Gilly 266; G. Nash 443; E. Roy 45; P. Wilson 10941. Rare.
Betulaceae — Carpinus caroliniana Walter, American Hornbeam. D. Atha 6832, 6991, 7386; D. Atha & M. Nee 6809; W. Gonzalez 5; C. Gilly 256, 267; G. Nash s.n.; M. Nee 43617; M. Nee & K. Cameron 54252, 54266; E. Roy 23. Infrequent.
Betulaceae — Corylus americana Walter, American Hazelnut. D. Atha 8134; P. Wilson 10851. Rare.
Betulaceae — Ostrya virginiana (Mill.) K. Koch, Hophornbeam. W. Cahilly 11; C. Gilly 268; M. Nee, D. Atha, & A. Bardet 56451; W. Nieder 33; P. Wilson 10953. Infrequent.
Bignoniaceae — Catalpa speciosa (Warder) Warder ex Engelm., Northern Catalpa. M. Nee & K. Cameron 54457. Not Native. Rare.
Boraginaceae — Heliotropium europaeum L., European Heliotrope. C. Gilly 362. Not Native. Historic.
Boraginaceae — Myosotis arvensis (L.) Hill, Field Forget-me-not. E. Bicknell 7318; G. Nash 232. Not Native. Historic.
Boraginaceae — Myosotis discolor Pers., Yellow and Blue Forget-me-not. M. Nee 59128. Not Native. Rare.
Boraginaceae — Myosotis scorpioides L., True Forget-me-not. D. Atha 532, 7755, 10616; C. Gilly 88; C. Morenberg, J. Cordero & M. Singer 92; M. Nee & D. McClelland 54446. Not Native. Frequent.
Boraginaceae — Symphytum officinale L., Comfrey. D.S. 187; D. Atha 2202; M. Nee 43528. Not Native. Waif. Rare.
Brassicaceae — Alliaria petiolata (M. Bieb.) Cavara & Grande, Garlic Mustard. M. Bennett s.n.; N. Holmgren 11818; S. Mori & C. Gracie 18836; M. Nee & K. Cameron 54268; W. Nieder 31; E. Wolfson 4. Not Native. Frequent.
Brassicaceae — Arabidopsis thaliana (L.) Heynh., Mouseear Cress. D. Atha 6943, 6957; M. Nee 54713, 59040; M. Nee & K. Cameron 54242; W. Nieder 19. Not Native. Frequent.
Brassicaceae — Barbarea verna (Mill.) Asch., {Barbarea praecox}, Early Wintercress. C. Curtis s.n.; T. Edmondson 1070. Not Native. Historic.
Brassicaceae — Barbarea vulgaris R. Br., {Barbarea barbarea}, Garden Yellowrocket. D. Atha 7025; S. Clarke s.n.; C. Gilly 299; S. Mori & C. Gracie 18835; G. Nash 56; M. North 146. Not Native. Infrequent.
Brassicaceae — Brassica rapa L. var. rapa, Field Mustard. M. Nee 54263. Not Native. Rare.
Brassicaceae — Capsella bursa-pastoris (L.) Medik., {Bursa bursa-pastoris}, Shepherd's Purse. D. Atha 6942; M. Bennett s.n.; J. Knowles 15; J. Luteyn 8081; M. Nee 31268; W. Nieder 45. Not Native. Frequent.
Brassicaceae — Cardamine bulbosa (Schreb. ex Muhl.) Britton, Sterns & Poggenb., Bulbous Bittercress. G. Nash 100. Historic.
Brassicaceae — Cardamine concatenata (Michx.) Sw., {Dentaria laciniata}, Fiveparted Toothwort. D. Atha & M. Nee 8524; W. Nieder 29. Rare.
Brassicaceae — Cardamine diphylla (Michx.) Alph. Wood, Crinkleroot. C. Gilly 276; M. Nee & K. Cameron 54323; W. Nieder 39. Rare.
Brassicaceae — Cardamine flexuosa With., Woodland Bittercress. D. Atha 6966, 11536; D. Atha & A. Rafalko 11546; M. Nee 58134, 59039. Not Native. Frequent.
Brassicaceae — Cardamine hirsuta L., Hoary Bittercress. D. Atha 6968; D. Atha & M. Nee 8526. Not Native. Frequent.
Brassicaceae — Cardamine parviflora L., Dryland Bittercress. E. Bicknell 4454. Historic.
Brassicaceae — Cardamine pensylvanica Muhl. ex Willd., Pennsylvania Bittercress. P. Wilson s.n. Historic.
Brassicaceae — Cardamine pratensis L. var. pratensis, Cuckooflower. D. Atha 7018; M. Nee 52612. Rare.
Brassicaceae — Diplotaxis tenuifolia (L.) DC., Slimleaf Wallrocket. D. Atha 7442, 7925; M. Nee 59129. Not Native. Waif. Rare.
Brassicaceae — Draba verna L., Whitlow Grass. D. Atha 6910, 6964; G. Nash s.n.; M. Nee & K. Cameron 54312. Not Native. Frequent.
Brassicaceae — Hesperis matronalis L., Damesrocket. S. Clarke s.n.; D. duMouchel s.n. Not Native. Historic.
Brassicaceae — Lepidium campestre (L.) W. T. Aiton., Field Pepperweed. M. Bennett s.n. Not Native. Historic.
Brassicaceae — Lepidium densiflorum Schrad., Prairie Pepperweed. E. Bicknell s.n.; F. Pennell 7008. Not Native. Historic.
Brassicaceae — Lepidium didymum L., Lesser Swinecress. D. Atha 7634, 7741, 8588. Not Native. Frequent.
Brassicaceae — Lepidium virginicum L. var. virginicum, {Lepidium virginicum}, Poorman's Pepper. S. Clarke s.n.; C. Gilly 124; J. Gowdey s.n.; M. Nee 43609; E. Roy 105. Infrequent.
Brassicaceae — Rorippa indica (L.) Hiern., Indian Yellowcress. H. Moldenke 18653, 19437, 19976; J. Monachino 516. Not Native. Waif. Historic.
Brassicaceae — Rorippa palustris (L.) Besser subsp. palustris, {Rorippa palustris}, Common Yellowcress. D. Atha 7748, 7922; D. Atha & M. Nee 8031; C. Curtis s.n.; C. Gilly 48; J. Monachino 65; M. Nee 43581. Frequent.
Brassicaceae — Rorippa sylvestris (L.) Besser, Creeping Yellowcress. C. Gilly 41. Not Native. Historic.
Brassicaceae — Sisymbrium officinale (L.) Scop., Hedgemustard. D. Atha 7441; J. Gowdey s.n.; M. Nee & D. McClelland 54430. Not Native. Rare.
Brassicaceae — Thlaspi arvense L., Field Pennycress. D. Atha 7931; H. Moldenke 4585. Not Native. Rare.
Campanulaceae — Campanula aparinoides Pursh, Marsh Bellflower. G. Nash s.n. Historic.
Campanulaceae — Lobelia cardinalis L., Cardinal Flower. G. Nash s.n. Historic.
Campanulaceae — Lobelia inflata L., Indian Tobacco. D. Atha 7933; D. Atha & M. Nee 7978; C. Gilly 133, s.n. Infrequent.
Campanulaceae — Lobelia siphilitica L., Great Blue Lobelia. D. Atha & M. Nee 8016; G. Nash s.n. Rare.
Campanulaceae — Triodanis perfoliata (L.) Nieuwl., {Specularia perfoliata}, Venus' Looking Glass. C. Gilly 38. Historic.
Cannabaceae — Celtis occidentalis L., Common Hackberry. D. Atha 6831, 6972; D. Atha & J. Schuler 6998, 7444; M. Nee & K. Cameron 54349; R. Naczi et al. 12565; G. Nash 183; P. Wilson 89, 10925. Rare.
Cannabaceae — Humulus japonicus Siebold & Zucc., Japanese Hops. N. Britton s.n.; C. Gilly 442; M. Nee 43857, 43868; M. Nee, K. Cameron & D. McClelland 54696; E. Roy 71. Not Native. Frequent.
Caprifoliaceae — Lonicera gracilipes Miq., Honeysuckle. D. Atha 6927; M. Nee & K. Cameron 54271. Not Native. Waif. Rare.
Caprifoliaceae — Lonicera japonica Thunb. ex Murray, Japanese Honeysuckle. M. Nee & D. McClelland 54419; R. Schneider 1407. Not Native. Frequent.
Caprifoliaceae — Lonicera maackii (Rupr.) Herder, Amur Honeysuckle. M. Balick & H. Martin 3713; M. Nee 43567; M. Nee & K. Cameron 54308. Not Native. Infrequent.
Caprifoliaceae — Lonicera morrowii A. Gray, Morrow's Honeysuckle. M. Nee & K. Cameron 54293. Not Native. Waif. Rare.
Caryophyllaceae — Agrostemma githago L., Common Corncockle. C. Gilly 78. Not Native. Historic.
Caryophyllaceae — Cerastium arvense L., Field Mouseear Chickweed. A. Hollick, s.n.; H. House 3520. Not Native. Historic.
Caryophyllaceae — Cerastium fontanum Baumg. ssp. vulgare (Hartm.) Greuter & Burdet, Big Chickweed. S. Clarke s.n.; C. Curtis s.n.; A. Hollick s.n.; M. Nee 59131. Not Native. Rare.
Caryophyllaceae — Paronychia canadensis (L.) Alph. Wood, {Anychia canadensis}, Forked Chickweed. A. Hollick s.n.; G. Nash 334. Historic.
Caryophyllaceae — Sagina japonica (Sw.) Ohwi., Japanese Pearlwort. D. Atha 7720. Not Native. Rare.
Caryophyllaceae — Sagina procumbens L., Pearlwort. D. Atha & R. Naczi 8514; E. Roy 95. Not Native. Infrequent.
Caryophyllaceae — Saponaria officinalis L., Bouncing Bet. C. Gilly 111. Not Native. Historic.
Caryophyllaceae — Scleranthus annuus L., Annual Knawel. D. Atha 7051, 8117; G. Nash 438; M . Nee 54712; J. Small s.n. Not Native. Infrequent.
Caryophyllaceae — Silene caroliniana Walter subsp. pensylvanica (Michx.) R. T. Clausen, {Silene caroliniana}, Pennsylvania Catchfly. T. Edmondson 1071; G. Nash 113; A. Vail s.n. Historic.
Caryophyllaceae — Silene latifolia Poir., White Campion. D. Atha 7944; C. Gilly 59, 322; J. Luteyn & S. Mori 7953; M. Nee & D. McClelland 54428; W. Nieder 41. Not Native. Infrequent.
Caryophyllaceae — Silene stellata (L.) W. T. Aiton, Starry Campion. D. Atha 14837; G. Nash 399; M. Nee & K. Cameron 54523; E. Roy 79. Infrequent.
Caryophyllaceae — Spergularia rubra (L.) J. Presl & C. Presl, Roadside Sandspurrey. D. Atha & N. Smith 8045. Not Native. Rare.
Caryophyllaceae — Stellaria graminea L., {Alsine graminea}, Common Stitchwort. C. Gilly 13; R. Schneider s.n; P. Wilson s.n. Not Native. Historic.
Caryophyllaceae — Stellaria longifolia Muhl., {Alsine longifolia}, Longleaved Stitchwort. G. Nash 177. Historic.
Caryophyllaceae — Stellaria media (L.) Vill., {Alsine media}, Common Chickweed. D. Atha 6908, 6923, 7645. Not Native. Frequent.
Celastraceae — Celastrus orbiculatus Thunb., Oriental Bittersweet. D. Atha 7639; A. Doody s.n.; D. McClelland 315, 370; S. Mori & C. Gracie 23702; M. Nee 43608; E. Roy 65; P. Wilson 10817; E. Yarrow s.n. Not Native. Infrequent.
Celastraceae — Celastrus scandens L., American Bittersweet. D. Atha 10599, P. Wilson 10903. Rare.
Celastraceae — Euonymus alatus (Thunb.) Siebold, Burning Bush. D. Atha & M. Nee 7983; A. Doody s.n.; M. Nee & K. Cameron 54291; W. Nieder 48; P. Wilson 10830. Not Native. Rare.
Celastraceae — Euonymus fortunei (Turcz.) Hand.-Maz. Climbing Euonymus. D. Atha & M. Nee 8523; H. Forgione 2; P. Wilson 10799. Not Native. Waif. Rare.
Celastraceae — Euonymus hamiltonianus Wall. subsp. sieboldianus (Blume) H. Hara, Hamilton's Spindletree. M. Nee & D. McClelland 54438. Not Native. Waif. Rare.
Cistaceae — Crocanthemum canadense (L.) Britton, {Helianthemum canadense}, Longbranch Frostweed. E. Bicknell s.n. Historic.
Cistaceae — Lechea intermedia Legg., Largepod Pinweed. E. Bicknell 5938. Historic.
Cistaceae — Lechea pulchella Raf., {Lechea leggettii}, Leggett's Pinweed. E. Bicknell 5985. Historic.
Cleomaceae — Cleome hassleriana Chod., Pink Queen. D. Atha 15279; M. Nee 43611. Not Native. Waif. Infrequent.
Clethraceae — Clethra alnifolia L., Coastal Sweetpepperbush. W. Cahilly 20; C. Gilly 258, 363; J. Gunderson 2; G. Nash 760; M. Nee 41823, 43620; M. Nee & K. Cameron 54275; E. Roy 8, 27; P. Wilson 10855. Infrequent.
Convolvulaceae — Calystegia sepium (L.) R. Br., {Convolvulus sepium}, Hedge Bindweed. D. Atha 7919; C. Gilly 54, 96; M. Nee & K. Cameron 54524; E. Roy 102; P. Wilson 135. Not Native. Infrequent.
Convolvulaceae — Convolvulus arvensis L., Field Bindweed. T. White s.n. Not Native. Historic.
Convolvulaceae — Cuscuta gronovii Willd., Common Dodder. D. Atha & M. Nee 8017, 8021; D. Atha, D. Stevenson and M. Thadeo 8114; N. Britton s.n.; R. Dragonetti 14; C. Gilly 154. Infrequent.
Convolvulaceae — Cuscuta pentagona Engelm., Field Dodder. R. Naczi 13397; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12568. Infrequent.
Convolvulaceae — Ipomoea hederacea Jacq., Ivyleaf Morning-glory. D. Atha 14192. Rare.
Convolvulaceae — Ipomoea purpurea (L.) R. Br., Tall Morning-glory. D. Atha 7929; D. Atha & L. Vargues 11488; M. Nee, K. Cameron & D. McClelland 54701. Not Native. Infrequent.
Cornaceae — Cornus alternifolia L. f., Pagoda Dogwood. D. Atha & M. Nee 7984; G. Nash 123, 442. Rare.
Cornaceae — Cornus amomum Mill., Silky Dogwood. D. Atha 7585; D. Atha & M. Nee 8018, 8019; N. Britton s.n.; G. Nash 365, 509; M. Nee & D. McClelland 54443. Infrequent.
Cornaceae — Cornus florida L., Flowering Dogwood. D. Atha 8608; W. Cahilly 24; G. Nash 106, 591; M. Nee 37825; W. Nieder 23; E. Roy 22; P. Wilson 86. Rare.
Cornaceae — Cornus racemosa Lam., {Cornus candidissima}, Gray Dogwood. G. Nash 204. Historic.
Cucurbitaceae — Citrullus lanatus (Thunb.) Matsum., Watermelon. D. Atha 14193. Not Native. Waif. Rare.
Cucurbitaceae — Cucurbita pepo L., Summer Squash. J. Schuler 19. Not Native. Waif. Rare.
Cucurbitaceae — Echinocystis lobata (Michx.) Torr. & A. Gray, Wild Cucumber. D. Atha 8595; C. Curtis s.n.; A. Foss s.n.; C. Gilly 145; R. Hill 1606; D. McClelland 361, 371; D. du Mouchel s.n.; G. Nash s.n. Infrequent.
Cucurbitaceae — Sicyos angulatus L., Bur Cucumber. D. Atha 8471, 8596; H. Cross & T. Motley 43; C. Gilly 182; R. Hill 1607; D. du Mouchel s.n.; G. Nash s.n.; M. Nee 55705, 54710; E. Roy 72, 86. Infrequent.
Elaeagnaceae — Elaeagnus umbellata Thunb., Autumn Olive. M. Nee & D. McClelland 54440. Not Native. Rare.
Ericaceae — Chimaphila maculata (L.) Pursh, Spotted Wintergreen. S. Clarke s.n; J. Schuler 6. Rare.
Ericaceae — Epigaea repens L., Trailing Arbutus. G. Nash 21. Historic.
Ericaceae — Gaultheria procumbens L., Wintergreen. D. Atha & B. Torke 15360. Rare.
Ericaceae — Gaylussacia baccata (Wangenh.) K. Koch, Black Huckleberry. D. Atha & M. Nee 7989. Rare.
Ericaceae — Kalmia latifolia L., Mountain Laurel. D. Atha 7656; J. Gunderson 1; P. Wilson 10800. Rare.
Ericaceae — Monotropa uniflora L., Indian Pipe. D. Atha & M. Nee 7985; J. Gunderson 6; G. Nash 434; M. Pace 459; E. Roy 67. Infrequent.
Ericaceae — Pyrola americana Sweet, American Wintergreen. J. Schuler 18. Rare.
Ericaceae — Rhododendron periclymenoides (Michx.) Shinners, {Azalea nudiflora}, Pink Azalea. D. Atha 6840; T. Edmondson 1094; A. Vail s.n. Rare.
Ericaceae — Rhododendron schlippenbachii Maxim., Rhododendron. D. Atha 7658. Not Native. Waif. Rare.
Ericaceae — Vaccinium angustifolium Aiton, Common Lowbush Blueberry. R. Naczi 13684. Rare.
Ericaceae — Vaccinium corymbosum L., Highbush Blueberry. W. Nieder 37. Rare.
Ericaceae — Vaccinium pallidum Aiton, {Vaccinium vacillans}, Hillside Blueberry. M. Nee & K. Cameron 54287; R. Naczi 12994; W. Nieder 38. Rare.
Eucommiaceae — Eucommia ulmoides Oliv., Eucommia. D. Atha & M. Nee 8010; M. Nee 54706, 58200. Not Native. Waif. Rare.
Euphorbiaceae — Acalypha australis L., Asian Copperleaf. D. Atha & M. Nee 7977; D. Atha 8080, 8081; R. Naczi & D. Atha 12674, 12680. Not Native. Frequent.
Euphorbiaceae — Acalypha gracilens A. Gray, Slender Copperleaf. D. Atha 7937; D. Atha & M. Nee 8008; W. Clute & P. Wilson s.n.; C. Gilly 170; J. Monachino 343, s.n. Rare.
Euphorbiaceae — Acalypha rhomboidea Raf., Common Copperleaf, D. Atha 6792, 7743, 9020; D. Atha & M. Nee 7999, 8002, 8005; C. Gilly 103; M. Nee & K. Cameron 54530; R. Naczi 12670; A. Neill 3303; E. Yarrow s.n. Frequent.
Euphorbiaceae — Euphorbia cyparissias L., Cypress Spurge. C. Gilly 312. Not Native. Historic.
Euphorbiaceae — Euphorbia maculata L., Spotted Sandmat. D. Atha 7642, 8603; D. Atha & M. Nee 8213; C. Gilly 71; R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12569; R. Naczi & D. Atha 12678; M. Nee 43864, 59407, 59408; M. Nee & K. Cameron 54519. Frequent.
Euphorbiaceae — Euphorbia nutans Lag., Eyebane. W. Clute & P. Wilson s.n. Historic.
Euphorbiaceae — Euphorbia oblongata Griseb., Eggleaf Spurge. E. Roy 104. Not Native. Waif. Rare.
Euphorbiaceae — Euphorbia peplus L., Petty Spurge. M. Nee 54715. Not Native. Waif. Rare.
Euphorbiaceae — Phyllanthus urinaria L., Chamber Bitter. D. Atha 8479; R. Naczi & D. Atha 12762. Not Native. Waif. Rare.
Fabaceae — Chamaecrista nictitans (L.) Moench, {Cassia nictitans}, Wild Sensitiveplant. Bicknell s.n. Historic.
Fabaceae — Desmodium canadense (L.) DC., {Meibomia canadensis}, Canadian Ticktrefoil. P. Wilson 138. Historic.
Fabaceae — Desmodium perplexum B. G. Schub. {Meibomia dillenii, Meibomia nudiflora }, Perplexed Ticktrefoil. E. Bicknell s.n, s.n, s.n. Historic.
Fabaceae — Gleditsia triacanthos L., Honeylocust. P. Wilson 10889. Not Native. Historic.
Fabaceae — Gymnocladus dioicus (L.) K. Koch, Kentucky Coffeetree. D. Atha 6507; M. Nee & D. McClelland 54423. Waif. Infrequent.
Fabaceae — Lespedeza cuneata (Dum. Cours.) G. Don, Chinese Lespedeza. A. Foss s.n. Not Native. Historic.
Fabaceae — Lespedeza procumbens Michx., Downy Trailing Lespedeza. W. Clute s.n. Historic.
Fabaceae — Lespedeza repens (L.) Barton, Creeping Bushclover. E. Bicknell 5170, s.n. Historic.
Fabaceae — Lespedeza violacea (L.) Pers., {Lespedeza frutescens}, Wand Bushclover. E. Bicknell 5132, 5180, s.n.; W. Clute & P. Wilson s.n. Historic.
Fabaceae — Medicago lupulina L., Black Medick. C. Gilly 47; M. Nee & D. McClelland 54429. Not Native. Infrequent.
Fabaceae — Melilotus albus Medik., White Sweetclover. D. Atha 14873; E. Roy 68; E. Yarrow s.n. Not Native. Rare.
Fabaceae — Robinia pseudoacacia L., Blacklocust. M. Nee 43600; E. Roy 120. Not Native. Rare.
Fabaceae — Strophostyles helvola (L.) Ell., Trailing Fuzzybean. C. Gilly 415; W. Clute & P. Wilson s.n.; H. Moldenke 11281. Historic.
Fabaceae — Trifolium arvense L., Rabbitfoot Clover. M. Nee 54483. Not Native. Rare.
Fabaceae — Trifolium hybridum L., Alsike Clover. D. Atha & M. Nee 7995. Not Native. Rare.
Fabaceae — Trifolium pratense L., Red Clover. H. Ahles s.n.; D. Atha & M. Nee 8003; D. Ellington 1; C. Gilly 58; J. Luteyn & S. Mori 7955. Not Native. Infrequent.
Fabaceae — Trifolium repens L., White Clover. C. Gilly 34; M. Nee & D. McClelland 54418. Not Native. Infrequent.
Fabaceae — Vicia cracca L., Bird Vetch. C. Gilly 339. Not Native. Historic.
Fabaceae — Vicia sativa L., Narrowleaved Vetch. S. Clarke s.n. Not Native. Historic.
Fagaceae — Castanea dentata (Marshall) Borkh., American Chestnut. W. Cahilly 19; C. Curtis s.n.; G. Nash 321, s.n.; P. Wilson 10907. Historic.
Fagaceae — Fagus grandifolia Ehrh., {Fagus americana}, Beech. D. Atha 6980, 7019, 8121, 9039; W. Cahilly 40; A. Carvalho & W. Thomas 6890; C. Gilly 270; G. Nash 71; M. Nee 54674; M. Nee & K. Cameron 54270; E. Roy 13, 60; P. Wilson 10913. Frequent.
Fagaceae — Quercus alba L., White Oak. D. Atha 9017; W. Cahilly 17; D. McClelland 359; G. Nash 69, 585; M. Nee 37824, 56463; P. Wilson s.n, 10926. Frequent.
Fagaceae — Quercus bicolor Willd., {Quercus platanoides}, Swamp White Oak. C. Gilly 303; B. Meurer-Grimes 216; G. Nash 83, 546; M. Nee 54318; P. Wilson 10934. Rare.
Fagaceae — Quercus coccinea Münchh., Scarlet Oak. P. Wilson 10909. Historic.
Fagaceae — Quercus macrocarpa Michx., Bur Oak. E. Roy 114. Rare.
Fagaceae — Quercus palustris Münchh., Pin Oak. D. Atha 6791, 8085, 8600; N. Britton s.n; W. Cahilly 32; B. Meurer-Grimes & H. Liao 42; G. Nash 57, 84, 547; M. Nee 43616; P. Wilson 185, 10946. Frequent.
Fagaceae — Quercus rubra L., Red Oak. D. Atha 6987, 7020, 7027, 8084, 8606; R. Benedict s.n.; W. Cahilly 12; B. Meurer-Grimes 211; B. Meurer-Grimes & H. Liao 32; M. Nee 36726, 43860; M. Nee & K. Cameron 54285; E. Roy 61; P. Wilson 903, 10824, s.n. Frequent.
Fagaceae — Quercus velutina Lam., Black Oak. C. Gilly 301, 302; B. Meurer-Grimes & H. Liao 43; G. Nash 85, 584; M. Nee 33268, 59510; M. Nee & K. Cameron 54313; E. Roy 21; P. Wilson 10908. Frequent.
Geraniaceae — Erodium cicutarium (L.) L'Hér. ex Aiton, Redstem Filaree. D. Atha 6978; M. Nee & K. Cameron 54258; P. Wilson s.n. Not Native. Infrequent.
Geraniaceae — Geranium carolinianum L., Carolina Cranesbill. H. Moldenke 18739. Historic.
Geraniaceae — Geranium maculatum L., Spotted Geranium. C. Gilly 307; G. Nash 182, 183. Historic.
Geraniaceae — Geranium robertianum L., Herb Robert. J. Schuler, M. Martello & E. Deluca 14. Rare.
Geraniaceae — Geranium thunbergii Siebold & Zucc. ex Lindl. & Paxton, Thunberg's Geranium. D. Atha 7841, 8052; D. Atha & M. Nee 8025; M. Eaton s.n.; C. Gilly 343; A. Hollick s.n.; J. Monachino s.n.; E. Roy 78. Not Native. Infrequent.
Grossulariaceae — Ribes rubrum L., Garden Currant. Atha 6952; M. Nee & K. Cameron 54276; E. Roy 58. Not Native. Rare.
Hamamelidaceae — Hamamelis virginiana L., American Witchhazel. E. Roy 3, 9; G. Nash 70, 594; M. Nee & K. Cameron 54294; A. Vail s.n.; P. Wilson 10812. Infrequent.
Hydrangeaceae — Hydrangea heteromalla D. Don. Himalayan Hydrangea. M. Nee & A. Bardet 56454. Not Native. Waif. Rare.
Hydrangeaceae — Hydrangea paniculata Sieb., Panicle Hydrangea. D. Atha 7578; W. Cahilly 29, 39; H. Forgione 1; A. Foss s.n. Not Native. Waif. Rare.
Hydrangeaceae — Philadelphus coronarius L., European Mockorange. W. Cahilly 25; M. Nee & D. McClelland 54420; W. Nieder 56. Not Native. Waif. Rare.
Hydrophyllaceae — Hydrophyllum canadense L., Bluntleaf Waterleaf. M. Nee & D. McClelland 54441. Not Native. Waif. Rare.
Hypericaceae — Hypericum gentianoides (L.) Britton, Sterns & Poggenb., {Sarothra gentianoides}, Orangegrass. D. Atha 8082. Rare.
Hypericaceae — Hypericum mutilum L., Dwarf St. Johnswort. D. Atha 7752, 8561, 8985; C. Gilly 148. Infrequent.
Hypericaceae — Hypericum perforatum L., Common St. Johnswort. D. Atha 8556. Not Native. Infrequent.
Hypericaceae — Hypericum stragulum P. Adams & N. Robson, St. Andrew's Cross. D. Atha 7843. Rare.
Juglandaceae — Carya cordiformis (Wangenh.) K. Koch, {Hicoria minima}, Bitternut Hickory. D. Atha 6738; D. Atha & M. Nee 6834; A. Doody s.n.; G. Nash 202, s.n.; M. Nee & K. Cameron 54348; E. Roy 36; P. Wilson 169, 10939. Frequent.
Juglandaceae — Carya glabra (Mill.) Sweet, {Hicoria microcarpa, Hicoria glabra}, Pignut Hickory. D. Atha 6736; M. Nee & H. Bardet 56444, 56447, 56448, 56456; M. Nee, D. Atha & A. Bardet 56450; E. Roy 16; P. Wilson 10942. Frequent.
Juglandaceae — Carya ovata (Mill.) K. Koch, {Hicoria ovata}, Shagbark Hickory. J. Schuler 20; P. Wilson 10930. Rare.
Juglandaceae — Carya tomentosa (Poir.) Nutt., {Hicoria alba}, Mockernut Hickory. D. Atha 8076, 10596; M. Nee & K. Cameron 54322; E. Roy 31; P. Wilson 10927. Frequent.
Juglandaceae — Juglans cinerea L., Butternut. P. Wilson 5, 10860, s.n. Historic.
Juglandaceae — Juglans nigra L., Black Walnut. P. Wilson 10935. Historic.
Lamiaceae — Ajuga reptans L., Carpet Bugle. D. Atha 2607; G. Nash 139, s.n. Not Native. Rare.
Lamiaceae — Collinsonia canadensis L., Canada Horsebalm. C. Gilly 438. Historic.
Lamiaceae — Glechoma hederacea L., Gill-over-the-ground. D. Atha 6944; T. Edmondson 1064; A. Foss s.n.; C. Gilly 279; J. Knowles 6; M. Nee & K. Cameron 54298; J. Walker 680; P. Wilson s.n.; E. Yarrow s.n. Not Native. Frequent.
Lamiaceae — Hedeoma pulegioides (L.) Pers., American Pennyroyal. G. Nash 478. Historic.
Lamiaceae — Lamium amplexicaule L., Henbit. D. Atha 6936. Not Native. Frequent.
Lamiaceae — Lamium hybridum Vill., Hybrid Deadnettle. M. Nee & K. Cameron 54243. Not Native. Rare.
Lamiaceae — Lamium purpureum L., Red Deadnettle. D. Atha 6937; S. Mori & C. Gracie 18833A; J. Walker 679. Not Native. Frequent.
Lamiaceae — Leonurus cardiaca L., Motherwort. J. Monachino 34. Not Native. Historic.
Lamiaceae — Lycopus americanus L., American Bugleweed. C. Gilly s.n.; G. Nash s.n., s.n. Historic.
Lamiaceae — Lycopus uniflorus Michx., Northern Bugleweed. C. Gilly 150. Historic.
Lamiaceae — Lycopus virginicus L., Virginia Bugleweed. D. Atha 8562, 8605, 9021, 9028; C. Gilly 127; G. Nash s.n.; M. Nee 59450; E. Roy 90. Frequent.
Lamiaceae — Monarda fistulosa L., Wild Bergamot. C. Gilly 109. Historic.
Lamiaceae — Nepeta cataria L., Catmint. D. Atha 7921. Not Native. Waif. Rare.
Lamiaceae — Origanum vulgare L., Wild Marjoram. G. Nash 421. Not Native. Historic.
Lamiaceae — Prunella vulgaris L., Common Selfheal. R. Abbott 26869; C. Gilly 386. Not Native. Infrequent.
Lamiaceae — Pycnanthemum muticum (Michx.) Pers., {Koellia mutica}, Clustered Mountainmint. G. Nash 431; P. Wilson 136. Historic.
Lamiaceae — Scutellaria lateriflora L., Blue Skullcap. G. Nash s.n.; D. Atha 8566. Rare.
Lamiaceae — Trichostema dichotomum L., Forked Bluecurls. G. Nash 505. Historic.
Linaceae — Linum usitatissimum L., Common Flax. E. Schoefield s.n.; G. Nash s.n. Not Native. Historic.
Lythraceae — Decodon verticillatus (L.) Elliott, Waterwillow. D. Atha & M. Nee 8024. Rare.
Lythraceae — Lythrum salicaria L., Purple Loosestrife. D. Atha 8602; D. Atha & M. Nee 8024; D. Atha & R. Naczi 7679; C. Gilly 44; J. Gowdey s.n.; M. Nee 43561; M. Nee & K. Cameron 54474; E. Roy 73. Not Native. Infrequent.
Malvaceae — Abutilon theophrasti Medik., Velvetleaf. D. Atha 15179. Not Native. Rare.
Malvaceae — Hibiscus moscheutos L., Rosemallow. D. Atha & L. Vargues 11467; G. Nash s.n.; M. Nee & K. Cameron 54527; M. Petrino 19. Frequent.
Malvaceae — Tilia americana L., American Basswood. D. Atha 7654; N. Holmgren 802 (UTC); H. Moldenke 9793; M. Nee 31285; E. Roy 48; P. Wilson 10899. Rare.
Mazaceae — Mazus pumilus (Burm. f.) Steenis, Japanese Mazus. D. Atha 8046; M. Nee 59406. Not Native. Frequent.
Menispermaceae — Menispermum canadense L., Moonseed. D. Atha 6356; D. McClelland 337. Rare.
Molluginaceae — Mollugo verticillata L., Carpetweed. D. Atha 7643; C. Gilly 102; M. Nee & K. Cameron 54525. Not Native. Frequent.
Montiaceae — Claytonia virginica L., Springbeauty. D. Atha 6912; T. Edmondson 1066; N. Holmgren 635 (UTC); G. Nash 17; M. Nee 55582; M. Nee & K. Cameron 54279; W. Nieder 25. Frequent.
Moraceae — Broussonetia papyrifera (L.) Vent., Paper Mulberry. D. Atha 9035. Not Native. Rare.
Moraceae — Morus alba L., White Mulberry. D. Atha 7368, 7375, 7384, 7446; C. Gilly 318; M. Nee 36722-a, 43532; M. Nee & K. Cameron 54317; E. Roy 42. Not Native. Frequent.
Moraceae — Morus rubra L., Red Mulberry. E. Roy 46; G. Nash 98, 335; M. Nee & K. Cameron 54473. Rare.
Myricaceae — Morella carolinensis (Mill.) Small, {Myrica carolinensis}, Southern Bayberry. G. Nash 79, 95. Historic.
Nyctaginaceae — Mirabilis nyctaginea (Michx.) MacMillan, Heartleaved Umbrellawort. D. Atha 7449; C. Gilly 329; M. Nee 43870. Not Native. Rare.
Nyssaceae — Nyssa sylvatica Marshall, Black Gum. D. Atha 6735; M. Nee 43636; M. Nee & K. Cameron 54333; E. Roy 15. Infrequent.
Oleaceae — Chionanthus virginicus L., Fringe Tree. D. Atha 7655; M. Nee & D. McClelland 54435. Not Native. Waif. Infrequent.
Oleaceae — Fraxinus americana L., White Ash. D. Atha 7026, 7572; C. Gilly 274; G. Nash s.n.; M. Nee 38598, 38615, 38616, 55895; M. Nee & K. Cameron 54353; P. Wilson 10891, s.n. Frequent.
Oleaceae — Fraxinus ornus L., Flowering Ash, D. Atha 14191. Not Native. Waif. Rare.
Oleaceae — Fraxinus pennsylvanica Marshall var. pensylvanica, Red Ash. D. Atha 6787; 6811, 6829, 6830, 8599; D. Atha & M. Nee 6812; N. Britton s.n., s.n.; G. Nash s.n.; M. Nee 38597, 58128; M. Nee & K. Cameron 54334; P. Wilson 10890. Frequent.
Oleaceae — Fraxinus profunda (Bush) Bush, Pumpkin Ash. D. Atha 8599; D. Atha & M. Nee 6810; N. Britton s.n.; C. Curtis 67. Rare.
Onagraceae — Circaea canadensis (L.) Hill, {Circaea lutetiana}, Enchanter's Nightshade. D. Atha 7574, 7661; W. Gonzalez 6; D. McClelland 336; G. Nash 315; W. Nieder 70. Frequent.
Onagraceae — Epilobium coloratum Biehler, Eastern Willowherb. Infrequent. M. Nee 59447; M. Nee & K. Cameron 54534. Infrequent.
Onagraceae — Ludwigia palustris (L.) Elliott, Common Waterpurslane. D. Atha & M. Nee 8029; C. Gilly 156; M. Nee & K. Cameron 54528. Infrequent.
Onagraceae — Oenothera biennis L., {Onagra biennis}, Common Evening-primrose. D. Atha 7950, 8585; G. Nash 457; E. Roy 108. Infrequent.
Onagraceae — Oenothera fruticosa L. {Kneiffia fruticosa}, Narrowleaved Evening-primrose. G. Nash 301, 302. Historic.
Onagraceae — Oenothera laciniata Hill., Cutleaf Evening-primrose. C. Gilly 324. Historic.
Onagraceae — Oenothera parviflora L. Smallflowered Evening-primrose. D. Atha 533. Rare.
Onagraceae — Oenothera perennis L., {Kneiffia pumila}, Little Sundrops. G. Nash 316. Historic.
Orobanchaceae — Agalinis tenuifolia (Vahl) Raf., {Garardia tenuifolia}, Slenderleaf False Foxglove. W. Clute & P. Wilson s.n. Historic.
Orobanchaceae — Conopholis americana (L.) Wallr., Squawroot. W. Nieder 54; M. Pace 553. Infrequent.
Orobanchaceae — Epifagus virginiana (L.) W. Bartram, {Leptamnium virginianum}, Beechdrops. D. Atha 8128; R. Brand, S. Canham & M. Choi 1a; C. Gilly 454; D. McClelland 351; G. Nash s.n.; M. Nee 56500; E. Roy 81. Frequent.
Orobanchaceae — Melampyrum lineare Desr. var. latifolium Farw., Narrowleaf Cowwheat. G. Nash s.n. Historic.
Orobanchaceae — Orobanche uniflora L., {Thalesia uniflora}, Cancerroot. W. Nieder 52; M. Pace 532. Rare.
Orobanchaceae — Pedicularis canadensis L., Canadian Lousewort. C. Gilly 298; G. Nash s.n. Historic.
Oxalidaceae — Oxalis corniculata L., Creeping Yellow Woodsorrel. D. Atha 7747; G. Eiten 546, 547, 548, 549, 648, 649, 650, 652, 917, 918, 919a; N. Holmgren 700 (UTC); M. Nee 59448. Not Native. Frequent.
Oxalidaceae — Oxalis dillenii Jacq., Southern Yellow Woodsorrel. D. Atha 7636, 7742; G. Eiten 321, 529, 624, 625, 919b. Infrequent.
Oxalidaceae — Oxalis stricta L., {Oxalis cymosa}, Common Yellow Woodsorrel. D. Atha 7744, 10598; G. Eiten 530, 626, 651; C. Gilly 378; A. Hollick s.n.; D. McClelland 334; J. Monachino s.n.; W. Nieder 66; C. Ruiz 6; J. Small s.n. Frequent.
Papaveraceae — Capnoides sempervirens (L.) Borkh., Rock Harlequin. C. Gilly 327; G. Nash 289. Historic.
Papaveraceae — Chelidonium majus L., Celandine. D. Atha 2162, 6985, 13389; M. Nee & K. Cameron 54352. Not Native. Frequent.
Papaveraceae — Corydalis incisa (Thunb.) Pers., Incised Fumewort. D. Atha 6925, 7021, 14402, 14403, 14406, 14407. Not Native. Waif. Infrequent.
Papaveraceae — Dicentra cucullaria (L.) Bernh., {Bicuculla cucullaria}, Dutchman's breeches. G. Nash 16; T. Edmondson 1068. Historic.
Papaveraceae — Sanguinaria canadensis L., Bloodroot. D. Atha 11550. Rare.
Papaveraceae — Stylophorum diphyllum (Michx.) Nutt., Wood Poppy. D. Atha 11552. Not Native. Waif. Rare.
Paulowniaceae — Paulownia tomentosa (Thunb.) Siebold & Zucc. ex Steud., Empress Tree. E. Alexander 303; M. Nee 38599, 43570, 58199; M. Nee, K. Cameron & D. McClelland 54691; Wilson 10843, 10861. Not Native. Infrequent.
Penthoraceae — Penthorum sedoides L., Ditch Stonecrop. D. Atha 7753; C. Gilly 342; G. Nash 496. Infrequent.
Phrymaceae — Mimulus ringens L., Allegheny Monkeyflower. C. Gilly 361. Historic.
Phrymaceae — Phryma leptostachya L., Lopseed. G. Nash s.n. Historic.
Phytolaccaceae — Phytolacca americana L., {Phytolacca decandra}, Pokeweed. D. Atha 1911; D. Atha & L. Vargues 11485; C. Gilly 65; D. McClelland 367; M. Nee 37818, 43614, 43876; M. Nee & K. Cameron 54476, 54522; E. Roy 6; C. Ruiz 8; P. Wilson s.n. Frequent.
Plantaginaceae — Callitriche stagnalis Scop., Pond Waterstarwort. D. Atha & M. Nee 8022. Not Native. Rare.
Plantaginaceae — Chelone glabra L., White Turtlehead. F. Pennell 6745. Historic.
Plantaginaceae — Gratiola neglecta Torrey, {Gratiola virginiana}, Clammy Hedgehyssop. G. Nash s.n. Historic.
Plantaginaceae — Linaria vulgaris Hill, {Linaria linaria}, Butter and Eggs. D. Atha 7939; C. Gilly 130. Not Native. Infrequent.
Plantaginaceae — Penstemon calycosus Small., Longsepal Beardtongue. C. Gilly 330. Not Native. Historic.
Plantaginaceae — Penstemon digitalis Nutt ex Sims, Foxglove Beardtongue. C. Gilly 332; P. Wilson s.n. Historic.
Plantaginaceae — Plantago lanceolata L., Narrowleaved Plantain. D. Atha 7652; P. Wilson 118. Not Native. Frequent.
Plantaginaceae — Plantago major L., Common Plantain. D. Atha 7745, 8555; P. Wilson 20. Not Native. Frequent.
Plantaginaceae — Plantago rugelii Decne., Blackseed Plantain. D. Atha 7567, 7649, 7746, 7768; P. Wilson 71. Frequent.
Plantaginaceae — Veronica arvensis L., Corn Speedwell. M. Nee & D. McClelland 54432. Not Native. Frequent.
Plantaginaceae — Veronica chamaedrys L., Germander Speedwell. F. Pennell 6768. Not Native. Historic.
Plantaginaceae — Veronica hederifolia L., Ivyleaved Speedwell. D. Atha 6982. Not Native. Infrequent.
Plantaginaceae — Veronica officinalis L., Common Speedwell. G. Nash s.n. Not Native. Historic.
Plantaginaceae — Veronica peregrina L., Purslane Speedwell. D. Atha 2609, 15003. Frequent.
Plantaginaceae — Veronica persica Poir., Birdeye Speedwell. D. Atha 6988; M. Nee 59130. Not Native. Frequent.
Plantaginaceae — Veronica serpyllifolia L., Thymeleaved Speedwell. R. Abbott 26868. Not Native. Infrequent.
Platanaceae — Platanus occidentalis L., Sycamore. T. Edmondson 1098; C. Gilly 315; D. McClelland 322; M. Nee & K. Cameron 54316; P. Wilson 10900. Infrequent.
Polygalaceae — Polygala verticillata L., {Polygala ambigua}, Whorled Milkwort. C. Gilly 112. Historic.
Polygonaceae — Persicaria careyi (Olney) Greene, Carey's Smartweed. C. Gilly 403. Historic.
Polygonaceae — Persicaria extremiorientalis (Vorosch.) Tzelev., Fareastern Smartweed. D. Atha 6839, 7647, 7763, 7764, 8089, 8092, 8093, 8094, 8095, 8098, 8459, 8480, 8511, 8515, 8516, 8568, 8594; D. Atha & R. Naczi 8510; R. Naczi 12667; M. Nee 54709; M. Nee & D. Atha 55985. Not Native. Frequent.
Polygonaceae — Persicaria hydropiper (L.) Opiz, Waterpepper Smartweed. D. Atha 8127, 8460, 8507; D. Atha & M. Nee 8028; W. Cahilly 26; J. Furlaud 71, 76; C. Gilly 166; R. Naczi 12666; M. Nee 59449; M. Wolf 30. Not Native. Infrequent.
Polygonaceae — Persicaria lapathifolia L., Pale Smartweed. D. Atha 8054, 8096, 8097, 8107; E. Bicknell 3843; A. Foss s.n.; C. Gilly 66; R. Naczi et al. 12571; P. Wilson s.n. Infrequent.
Polygonaceae — Persicaria longiseta (Bruijn) Kitagawa, Bristly Smartweed. R. Abbott 26828; D. Atha 7566, 7575, 7576, 7646, 8050, 8057, 8077, 8079, 8100, 8101, 8136, 8138, 8139, 8457, 8461, 8462, 8500, 8501, 8502, 8513, 9443, 10603, 10604, 10605; A. Foss s.n.; J. Furlaud 70, 77, 81; C. Gilly 57, 57b; D. McClelland 335, 368; J. Monachino 190, 11747, 11811, 11812, 11813, 11892; R. Naczi 12668; M. Nee & K. Cameron 54694; W. Nieder 69; E. Roy 10, 116; C. Ruiz 5. Not Native. Frequent.
Polygonaceae — Persicaria maculosa Gray, {Polygonum persicaria}, Spotted Smartweed. D. Atha 7644, 7757, 8051, 8078, 8512, 8517, 8559; D. Atha & 2010 Class of Summer Intensive 8554; R. Naczi et al. 12573. Not Native. Frequent.
Polygonaceae — Persicaria pensylvanica (L.) M. Gómez, Pennsylvania Smartweed. D. Atha 8055, 8056, 8099, 8992, 14835; R. Dragonetti 13; C. Gilly 199; R. Naczi 12665; M. Petrino 10; E. Roy 76; M. Wolf 29. Infrequent.
Polygonaceae — Persicaria perfoliata (L.) H. Gross, Mile-a-minute Vine. D. Atha & J. Schuler 8560. Not Native. Rare.
Polygonaceae — Persicaria punctata (Elliott) Small, Dotted Smartweed. D. Atha et al. 8115; C. Gilly 194; J. Monachino 189; M. Nee & K. Cameron 54533; R. Naczi 13395. Frequent.
Polygonaceae — Persicaria sagittata (L.) H. Gross, {Polygonum sagittatum}, Arrowleaf Tearthumb. D. Atha & M. Nee 8014; C. Gilly 180; G. Nash 535; M. Petrino 20. Infrequent.
Polygonaceae — Persicaria virginiana (L.) Gaertn., {Polygonum virginianum}, Virginia Smartweed. C. Gilly 123; D. duMouchel s.n.; M. Nee & K. Cameron 54521; E. Roy 80. Frequent.
Polygonaceae — Polygonum aviculare L., Prostrate Knotweed. D. Atha 8140, 8141, 8456. Not Native. Frequent.
Polygonaceae — Reynoutria × bohemica Chrtek & Chrtková, Bohemian Knotweed. D. Atha 8124, 8126; D. McClelland 364; M. Nee & K. Cameron 54489. Not Native. Frequent.
Polygonaceae — Reynoutria japonica Houtt., {Polygonum cuspidatum}, Japanese Knotweed. D. Atha 8125, 14836; H. Moldenke 18447; E. Roy 74. Not Native. Infrequent.
Polygonaceae — Reynoutria sachalinensis (F. Schmidt) Nakai, Giant Knotweed. D. Atha 6799. Not Native. Rare.
Polygonaceae — Rumex acetosella L., Red Sorrel. D. Atha 7052, 7648; G. Nash 169. Not Native. Frequent.
Polygonaceae — Rumex crispus L., Curly Dock, D. Atha 7377; N. Holmgren 781; M. Nee 32535. Not Native. Frequent.
Polygonaceae — Rumex obtusifolius L., Bitter Dock. D. Atha 7374, 7438, 10744, 10745; M. Nee 43556; M. Nee & K. Cameron 54477; M. Nee & D. McClelland 54431; P. Wilson s.n. Not Native. Frequent.
Polygonaceae — Rumex patientia L., Patience Dock. D. Atha 7380; J. Monachino 64. Not Native. Rare.
Portulacaceae — Portulaca oleracea L., Purslane. D. Atha 7926. Not Native. Frequent.
Primulaceae — Lysimachia arvensis (L.) U. Manns & Anderb., Pimpernel. R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12567. Not Native. Rare.
Primulaceae — Lysimachia ciliata L., {Steironema ciliatum}, Fringed Loosestrife. C. Gilly 82; G. Nash s.n., s.n.; M. Nee & K. Cameron 54493. Rare.
Primulaceae — Lysimachia quadrifolia L., Whorled Loosestrife. D. Atha & M. Nee 7979; G. Nash s.n.; W. Nieder 30, 60. Rare.
Primulaceae — Lysimachia terrestris (L.) Britton, Sterns & Poggenb., Bulbil Loosestrife. M. Kearns 2; G. Nash s.n., s.n. Historic.
Ranunculaceae — Actaea pachypoda Ell., White Baneberry. S. Clarke s.n.; C. Gilly 444. Historic.
Ranunculaceae — Anemone americana (DC.) H. Hara, {Hepatica hepatica}, Roundleaved Liverleaf. G. Nash s.n. Historic.
Ranunculaceae — Anemone canadensis L., Canada Anemone. P. Wilson s.n. Historic.
Ranunculaceae — Anemone quinquefolia L., Wood Anemone. T. Edmondson 1067; G. Nash 154, 55; M. Pace & J. Schuler 531; A. Vail s.n. Rare.
Ranunculaceae — Anemone virginiana L., Tall Thimbleweed. G. Nash 398. Historic.
Ranunculaceae — Aquilegia canadensis L., Columbine. A. Vail s.n.; G. Nash 81. Historic.
Ranunculaceae — Clematis terniflora DC., Japanese Virgin's Bower. D. Atha 8986. Not Native. Infrequent.
Ranunculaceae — Clematis virginiana L., Virgin's Bower. G. Nash 445. Historic.
Ranunculaceae — Eranthis hyemalis (L.) Salisb., Winter Aconite. D. Atha 6969. Not Native. Waif. Rare.
Ranunculaceae — Ficaria verna Huds., Lesser Celandine. D. Atha 6917, 6924; M. Bennett s.n.; S. Mori & C. Gracie 18815; M. Nee & K. Cameron 54246, 54264; W. Nieder 1. Not Native. Frequent.
Ranunculaceae — Ranunculus abortivus L., Littleleaf Buttercup. G. Nash 92; P. Wilson s.n. Historic.
Ranunculaceae — Ranunculus acris L., Tall Buttercup. G. Nash s.n.; P. Wilson s.n. Not Native. Historic.
Ranunculaceae — Ranunculus ambigens S. Watson, {Ranunculus obtusiusculus}, Waterplantain Spearwort. G. Nash 429. Historic.
Ranunculaceae — Ranunculus bulbosus L., Bulbous Buttercup. C. Gilly 290. Not Native. Historic.
Ranunculaceae — Ranunculus caricetorum Greene., {Ranunculus septentrionalis}, Swamp Buttercup. D. Atha 14405; T. Edmondson 1068; C. Gilly 289; P. Wilson s.n. Rare.
Ranunculaceae — Ranunculus hispidus Michx., Hispid Buttercup. T. Edmondson 1062; G. Nash 33. Historic.
Ranunculaceae — Ranunculus recurvatus Poir., Hooked Crowfoot. G. Nash 130. Historic.
Ranunculaceae — Ranunculus repens L., Creeping Buttercup. D. Atha 14401; E. Britton s.n.; M. Nee & D. McClelland 54445. Not Native. Frequent.
Ranunculaceae — Ranunculus trichophyllus Chaix, {Batrachium trichophyllum}, White Watercrowfoot. W. Clute s.n. Historic.
Ranunculaceae — Thalictrum pubescens Pursh, {Thalictrum polygamum}, King-of-the-meadow. C. Gilly 344; G. Nash s.n. Historic.
Ranunculaceae — Thalictrum thalictroides (L.) A. J. Eames & B. Boivin, {Syndesmon thalictroides}, Rue Anemone. C. Gilly 295; G. Nash 35. Historic.
Rhamnaceae — Frangula alnus Mill., Glossy Buckthorn. W. Nieder 44, 59; M. Nee & K. Cameron 54451. Not Native. Infrequent.
Rhamnaceae — Rhamnus cathartica L., Common Buckthorn. D. Atha 8506; M. Nee & D. McClelland 54439; E. Roy 47; P. Wilson 92, 10797. Not Native. Rare.
Rosaceae — Agrimonia gryposepala Wallr., {Agrimonia hirsuta}, Common Agrimony. G. Nash 28. Historic.
Rosaceae — Agrimonia pubescens Wallr., {Agrimonia mollis}, Downy Agrimony. E. Bicknell 4621. Historic.
Rosaceae — Agrimonia rostellata Wallr., Beaked Agrimony. W. Clute & P. Wilson s.n. Historic.
Rosaceae — Agrimonia striata Michx., Roadside Agrimony. M. Nee & K. Cameron 54531. Rare.
Rosaceae — Amelanchier arborea (F. Michx.) Fernald, Common Serviceberry. D. Atha 6971, 6976. Infrequent.
Rosaceae — Amelanchier laevis Wiegand, Smooth Serviceberry. D. Atha 6986, 7383; W. Cahilly 21; C. Gilly 273; M. Nee 43490, 43490-a; M. Nee & K. Cameron 54267; E. Roy 59. Frequent.
Rosaceae — Crataegus monogyna Jacq., {Crataegus oxycantha}, Oneseeded Hawthorn. W. Eggleston s.n. Not Native. Historic.
Rosaceae — Crataegus pruinosa (Wendl. f.) K. Koch., Frosted Hawthorne. W. Eggleston 154. Historic.
Rosaceae — Fragaria virginiana Duch., Virginia Strawberry. G. Nash s.n.; T. Edmondson 1369. Historic.
Rosaceae — Geum canadense Jacq., White Avens. C. Gilly 62; G. Nash s.n.; M. Nee & K. Cameron 54496; E. Roy 100. Infrequent.
Rosaceae — Geum virginianum L., Creamcolored Avens. J. Monachino 162; G. Nash s.n. Historic.
Rosaceae — Malus floribunda Van Houtte, Japanese Flowering Crabapple. D. Atha & M. Nee 7987; M. Nee & D. Atha 56505. Not Native. Infrequent.
Rosaceae — Malus hupehensis (Pamp.) Rehder., Tea Crabapple. D. Atha 15042. Not Native. Frequent.
Rosaceae — Malus pumila Mill., {Malus malus}, Apple. T. Edmondson 1087; C. Gilly 285. Not Native. Waif. Historic.
Rosaceae — Potentilla anglica Laichard., English Cinquefoil. J. Monachino 622, s.n., s.n. Not Native. Historic.
Rosaceae — Potentilla indica (Andrews) T. Wolf, Indian Strawberry. R. Abbott 26867; D. Atha 7650, 11821. Not Native. Frequent.
Rosaceae — Potentilla norvegica L., Rough Cinquefoil. D. Atha 7923; C. Gilly 76. Not Native. Rare.
Rosaceae — Potentilla recta L., Sulfur Cinquefoil. D. Atha 7941. Not Native. Rare.
Rosaceae — Potentilla simplex Michx., Common Cinquefoil. Dragonetti 6. Rare.
Rosaceae — Prunus avium L., Sweet Cherry. D. Atha 13359; T. Edmondson 1073; G. Nash s.n.; J. Schuler & A. Aiello 9. Not Native. Waif. Infrequent.
Rosaceae — Prunus incisa Thunb., Fuji Cherry. J. Schuler & A. Aiello 13. Not Native. Waif. Infrequent.
Rosaceae — Prunus padus L. European Bird Cherry. D. Atha 6954. Not Native. Waif. Rare.
Rosaceae — Prunus persica L., Peach. C. Gilly 271. Not Native. Waif. Rare.
Rosaceae — Prunus sargentii Rehder., Sargent's Cherry. J. Schuler & A. Aiello 7. Not Native. Waif. Infrequent.
Rosaceae — Prunus serotina Ehrh., Black Cherry. W. Cahilly 10; J. Luteyn 8083; H. Moldenke 10911; M. Nee 43575; M. Nee & K. Cameron 54343; W. Nieder 53. Frequent.
Rosaceae — Prunus subhirtella Miq., Winterflowering Cherry. M. Nee & K. Cameron 54256; J. Schuler & A. Aiello 10, 11. Not Native. Waif. Infrequent.
Rosaceae — Prunus virginiana L., Choke Cherry. D. Atha 7024; D. duMouchel s.n.; M. Nee & K. Cameron 54307. Infrequent.
Rosaceae — Prunus x yedoensis Matsum., Hybrid Cherry. D. Atha 13350; M. Nee 59041; M. Nee & K. Cameron 54253; J. Schuler & A. Aiello 8. Not Native. Waif. Infrequent.
Rosaceae — Rhodotypos scandens (Thunb.) Makino, Jetbead. M. Nee & K. Cameron 54295; P. Wilson 88. Not Native. Rare.
Rosaceae — Rosa carolina L., Pasture Rose. G. Nash 12. Historic.
Rosaceae — Rosa multiflora Thunb., Multiflora Rose. D. Atha 7942; D. McClelland 324, 369; M. Nee 43043; E. Roy 94; C. Ruiz 4; P. Wilson 68. Not Native. Frequent.
Rosaceae — Rosa palustris Marsh., Swamp Rose. G. Nash s.n. Historic.
Rosaceae — Rosa wichuriana Crépin., Memorial Rose. R. Naczi, A. Litt, M. Pace, T. Lane & D. Kaiser 12560. Not Native. Waif. Rare.
Rosaceae — Rubus allegheniensis Porter, Allegheny Blackberry. D. Atha 7662; G. Nash s.n.; W. Nieder 51. Frequent.
Rosaceae — Rubus canadensis L., Smooth Blackberry. D. Atha 7562, 10606. Rare.
Rosaceae — Rubus discolor Weihe & Nees, Himalayan Blackberry. D. Atha 7948. Not Native. Waif. Rare.
Rosaceae — Rubus enslenii Tratt., Southern Dewberry. G. Nash s.n. Historic.
Rosaceae — Rubus flagellaris Willd., {Rubus villosus}, Northern Dewberry. D. Atha 7762; W. Blanchard s.n. Rare.
Rosaceae — Rubus frondosus Bigelow., Yankee Blackberry. W. Blanchard s.n. Historic.
Rosaceae — Rubus occidentalis L., Black Raspberry. G. Nash s.n. Historic.
Rosaceae — Rubus odoratus L., Flowering Raspberry. C. Gilly s.n. Historic.
Rosaceae — Rubus pensilvanicus Poir., Pennsylvania Blackberry. M. Nee & D. McClelland 54436; M. Wolf & C. Ruiz 14. Infrequent.
Rosaceae — Rubus phoenicolasius Maxim., Wineberry. M. Nee 54437. Not Native. Infrequent.
Rubiaceae — Cephalanthus occidentalis L., Buttonbush. D. Atha 8133; C. Gilly s.n.; M. Kearns 7; M. Nee & K. Cameron 54470; P . Wilson 192. Infrequent.
Rubiaceae — Galium album Mill., {Galium mollugo},White Bedstraw. S. Burnham s.n.; G. Nash s.n.; M. Nee & D. McClelland 54427. Not Native. Infrequent.
Rubiaceae — Galium aparine L., Annual Bedstraw. G. Nash s.n.; M. Nee & K. Cameron 54346. Infrequent.
Rubiaceae — Galium asprellum Michx., Rough Bedstraw. G. Nash s.n. Historic.
Rubiaceae — Galium circaezans Michx., Licorice Bedstraw. G. Nash s.n.; W. Wiegmann s.n. Historic.
Rubiaceae — Galium obtusum Bigelow, Bluntleaved Bedstraw. G. Nash s. n. Historic.
Rubiaceae — Mitchella repens L., Partridgeberry. D. Atha 7431. Infrequent.
Rutaceae — Phellodendron amurense Rupr., Amur Corktree. D. McClelland 321; M. Nee 43585, 52599, 52594, 52600; E. Roy 38. Not Native. Frequent.
Rutaceae — Tetradium daniellii (Hemsl.) Hartley, Evodia. D. duMouchel s.n.; M. Nee 58084; M. Nee & A. Bardet 56457. Not Native. Waif. Rare.
Rutaceae — Zanthoxylum simulans Hance, Chinese Pepper. M. Nee & K. Cameron 54456. Not Native. Waif. Rare.
Salicaceae — Idesia polycarpa Maxim., Idesia. D. Atha 15377. Not Native. Waif. Rare.
Salicaceae — Populus deltoides Marshall, Eastern Cottonwood. D. Atha 6962. Rare.
Salicaceae — Populus grandidentata Michx., Bigtooth Aspen. G. Nash 30, 107, 342,749; W. Nieder 15; E. Roy 50, 51. Historic.
Salicaceae — Populus tremuloides Michx., Quaking Aspen. D. Atha 6993, 7046; M. Nee & K. Cameron 54479. Rare.
Salicaceae — Salix discolor Muhl., Pussy Willow. G. Nash, 29, 89; M. Nee 57785, 58071. Rare.
Salicaceae — Salix fragilis L., Crack Willow. D. Atha 6995, 7022, 7382; S. Mori 24071; M. Nee 43496; M. Nee & K. Cameron 54269. Not Native. Rare.
Salicaceae — Salix petiolaris Sm., Meadow Willow. G. Nash 6. Historic.
Salicaceae — Salix sericea Marshall, Silky Willow. G. Nash 50, 51. Historic.
Santalaceae — Comandra umbellata (L.) Nutt., Bastard Toadflax. G. Nash 132. Historic.
Sapindaceae — Acer campestre L., Hedge Maple. D. Atha 8508; D. du Mouchel s.n. Not Native. Waif. Rare.
Sapindaceae — Acer negundo L., Boxelder. D. Atha 6789, 6790, 6984, 7448; C. Gilly 384; M. Nee 43571; M. Nee & K. Cameron 54289; E. Roy 43, 56; P. Wilson 10937. Infrequent.
Sapindaceae — Acer platanoides L., Norway Maple, D. Atha 6958, 7385; M. Nee 43606; M. Nee & K. Cameron 54272; E. Roy 18, 57; P. Wilson 10918. Not Native. Infrequent.
Sapindaceae — Acer pseudoplatanus L., Sycamore Maple. D. McClelland 318; M. Nee 43873; M. Nee & K. Cameron 54290; W. Nieder 46; P. Wilson 10917. Not Native. Infrequent.
Sapindaceae — Acer rubrum L., Red Maple. D. Atha 6916, 6949; N. Britton s.n., s.n.; M. Curtis s.n.; T. Delendick 76/178, 76/796, 76/882, 77/273, 78/215, 1097; G. Nash s.n., s.n., s.n.; M. Nee & K. Cameron 54244, 54250; W. Nieder 10; E. Roy 33, 52; P. Wilson 10906, s.n., s.n., s.n. Frequent.
Sapindaceae — Acer saccharum Marshall, Sugar Maple. D. Atha 6743, 6960, 6963, 7376, 7640, 7641; W. Cahilly 20; T. Delendick 76/1075; M. Nee 50113; M. Nee & K. Cameron 54278, 54282, 54487; M. Nee, D. Atha & A. Bardet 56452; P. Wilson 10905, 10920. Frequent.
Sapindaceae — Acer spicatum Lam. Mountain Maple. W. Wiegmann s.n. Not Native. Waif. Historic.
Sapindaceae — Aesculus glabra Willd., Ohio Buckeye. D. Atha 6940, 7445, 13361; R. Naczi et al. 12564; M. Nee 43618. Not Native. Waif. Rare.
Sapindaceae — Aesculus sylvatica W. Bartram, Painted Buckeye. D. Atha 6981, 6983, 7450, 7917, 7920; D. Atha & M. Nee 7993. Not Native. Waif. Rare.
Saxifragaceae — Heuchera americana L., American Alumroot. G. Nash 164, 306. Historic.
Saxifragaceae — Micranthes virginiensis (Michx.) Small, {Saxifraga virginiensis}, Early Saxifrage. G. Nash 23, 82. Historic.
Scrophulariaceae — Scrophularia lanceolata Pursh, {Scrophularia leporella}, American Figwort. G. Nash 420, s.n., s.n. Historic.
Scrophulariaceae — Scrophularia marilandica L., Eastern Figwort. G. Nash s.n. Historic.
Scrophulariaceae — Verbascum blattaria L., Moth Mullein. D. Atha 7584; C. Gilly 7; J. Luteyn & S. Mori 7956; M. Nee 50142. Not Native. Infrequent.
Scrophulariaceae — Verbascum thapsus L., Common Mullein. D. Atha 7956; P. Wilson 75. Not Native. Frequent.
Simaroubaceae — Ailanthus altissima (Mill.) Swingle, {Ailanthus glandulosa}, Tree of Heaven. D. Atha 7947; G. Nash 2672;M. Nee & J. Beitel 32572; M. Nee 43566; E. Roy 28; P. Wilson 10865, s.n. Not Native. Infrequent.
Solanaceae — Datura stramonium L., Jimsonweed. D. Atha 7930. Not Native. Waif. Rare.
Solanaceae — Physalis philadelphica Lam., Tomatillo. R. Abbott 26964; M. Nee 37823. Not Native. Waif. Rare.
Solanaceae — Solanum carolinense L., Carolina Horsenettle. D. Atha 7953; R. Meyer 395. Rare.
Solanaceae — Solanum dulcamara L., Bittersweet. C. Gilly 55; M. Kearns 9; J. Luteyn & S. Mori 7957; G. Nash 630, s.n.; M. Nee & D. McClelland 54448; M. Nee & S. Knapp 57200; E. Roy 112; P. Wilson 79, s.n., s.n. Not Native. Frequent.
Solanaceae — Solanum lycopersicum L., Tomato. D. Atha & M. Nee 8009; D. Atha, D. Stevenson and M. Thadeo 8112. Not Native. Waif. Infrequent.
Solanaceae — Solanum nigrum L., Black Nightshade. D. Atha 7932; J. Furlaud 79; C. Gilly 87; D. McClelland 365; R. Meyer 402, M. Nee 43613, 54714; M. Nee & S. Knapp 57198; M. Petrino 7; E. Yarrow s.n. Not Native. Frequent.
Solanaceae — Solanum ptychanthum Dunal, West Indian Nightshade. R. Meyer 403; M. Nee 53366, 57199. Infrequent.
Staphyleaceae — Staphylea trifolia L., Bladdernut. D. Atha 6827, 6828; W. Cahilly 17; S. Clarke s.n.; C. Gilly 15a, 309; D. McClelland 320, 363; D. du Mouchel s.n.; E. Roy 26; P. Wilson 166, 10832. Infrequent.
Ulmaceae — Ulmus americana L., American Elm. D. Atha 6975; H. T. Beck 1441; H. Moldenke 11341; G. Nash 10; M. Nee 43488, 43488-a; M. Nee & K. Cameron 54249; E. Roy 30; R. Schneider s.n.; P. Wilson 1, 2, 10912. Infrequent.
Ulmaceae — Ulmus parvifolia Jacq., Lacebark Elm. M. Nee & Cameron 54693. Not Native. Waif. Rare.
Ulmaceae — Ulmus rubra Muhl., Slippery Elm. W. Eggleston & R. Schneider s.n.; P. Wilson 10921. Historic.
Ulmaceae — Zelkova serrata (Thunb.) Makino, Japanese Zelkova. D. Atha 7766, 10602, 11513; M. Nee 58117; M. Nee & K. Cameron 54461. Not Native. Waif. Infrequent.
Urticaceae — Boehmeria cylindrica (L.) Sw., False Nettle. D. Atha 8563; C. Gilly 387; M. Nee & K. Cameron 54471; P. Wilson & E. Alexander 252. Rare.
Urticaceae — Laportea canadensis (L.) Weddell, {Urticastrum divaricatum}, Canadian Woodnettle. W. Gonzalez 4; M. Nee & K. Cameron 54495; E. Roy 69; P. Wilson 180. Rare.
Urticaceae — Parietaria pensylvanica Muhl. ex Willd., Pennsylvania Pellitory. D. Atha 7653. Rare.
Urticaceae — Pilea pumila (L.) A. Gray, Canadian Cleanweed. D. Atha 6793; C. Gilly 157; M. Nee & K. Cameron 54529. Rare.
Urticaceae — Urtica dioica L., Eurasian Stinging Nettle. D. Atha 7381; W. Gonzalez 8; J. Luteyn & S. Mori 7952; M. Nee & D. McClelland 54422; J. Walker 738. Not Native. Frequent.
Urticaceae — Urtica gracilis Aiton., American Stinging Nettle. J. Monachino 51. Historic.
Verbenaceae — Verbena bonariensis L., Purpletop Vervain. D. Atha & M. Nee 8004; M. Nee 47132. Not Native. Rare.
Verbenaceae — Verbena hastata L., Common Vervain. C. Gilly 105. Historic.
Verbenaceae — Verbena urticifolia L., White Vervain. D. Atha 7754; C. Gilly 110; M. Nee & K. Cameron 54488; G. Nash s.n.; R. Schneider 25417. Frequent.
Violaceae — Viola arvensis Murray, European Field Pansy. D. Atha 7053. Not Native. Rare.
Violaceae — Viola blanda Willd., {Viola blanda amoena}, Sweet White Violet. W. Nieder 13. Infrequent.
Violaceae — Viola fimbriatula Sm., {Viola ovata}, Arrowleaved Violet. J. Enquist 499. Historic.
Violaceae — Viola palmata L., {Viola palmata dilatata}, Wood Violet. T. White 13-2. Historic.
Violaceae — Viola pedata L., Birdsfoot Violet. S. Clarke s.n. Historic.
Violaceae — Viola pubescens Aiton var. pubescens, Yellow Forest Violet. C. Gilly 291. Historic.
Violaceae — Viola rotundifolia Michx., Roundleaved Yellow Violet. W. Wiegmann s.n. Historic.
Violaceae — Viola sororia Willd., Dooryard Violet. L. Andrews s.n.; D. Atha 6961, 7023, 11553, 11555, 11556, 11784, 13362; D. Atha & J. Arcate 6997; N. Britton s.n.; C. Gilly 286, 294; N. Holmgren 503 (UTC), 645 (UTC); C. Morenberg 89; S. Mori & C. Gracie 18822; M. Nee 57024; M. Nee & K. Cameron 54273; W. Nieder 27, 34; A. Vail s.n.; E. Wolfson 1. Frequent.
Violaceae — Viola striata Aiton, Striped Cream Violet. D. Atha 11561, 15093. Infrequent.
Vitaceae — Ampelopsis brevipedunculata (Maxim.) Trautv., Porcelainberry. D. Atha 534, 7372, 7750, 7751; H. Beck 1329; J. Gunderson 5; D. McClelland 366; M. Nee 43577; M. Nee & K. Cameron 54498. Not Native. Frequent.
Vitaceae — Parthenocissus quinquefolia (L.) G. Planch., Virginia Creeper. D. Atha 6731; W. Cahilly 31; C. Morenberg 88; M. Nee & K. Cameron 54482. Frequent.
Vitaceae — Parthenocissus tricuspidata (Siebold & Zucc.) G. Planch., Boston Ivy. D. Atha & M. Nee 7991. Not Native. Waif. Rare.
Vitaceae — Vitis aestivalis Michx., Summer Grape. N. Britton s.n; M. Nee & K. Cameron 54452. Rare.
Vitaceae — Vitis labrusca L., Fox Grape. D. Atha & R. Naczi 7582; N. Britton s.n.; P. Wilson 10816. Rare.
Vitaceae — Vitis riparia Michx., Riverbank Grape. R. Gunderson 4. Rare.
Appendix II. Native taxa named from Garden Specimens
The following taxa were named from plants collected on the grounds of The New York Botanical Garden or Bronx Park.
Malvaceae — Hibiscus oculiroseus Britton. G. Nash s.n. = Hibiscus moscheutos L.
Oleaceae — Fraxinus michauxii Britton. N. Britton s.n. = Fraxinus profunda (Bush) Bush.
Poaceae — Panicum atlanticum Nash. G. Nash s.n. = Dichanthelium villosissimum (Nash) Freckmann.
Poaceae — Panicum bicknellii Nash. E. Bicknell s.n. = Dichanthelium boreale (Nash) Freckmann.
Poaceae — Panicum tsugetorum Nash. G. Nash 287 = Dichanthelium columbianum (Scribn.) Freckmann.
Rosaceae — Crataegus bronxensis Sarg. W. Eggleston 154 = Crataegus pruinosa (Wendl. f.) K. Koch.
Scrophulariaceae — Scrophularia leporella E. P. Bicknell. = Scrophularia lanceolata Pursh.
Appendix III. New Records
The following 46 taxa are new records for Bronx County and/or New York State (Weldy et al., 2015). Not all are extant in 2015.
Adoxaceae — Viburnum plicatum Thunb.
Amaranthaceae — Amaranthus blitoides S. Watson
Amaranthaceae — Chenopodium standleyanum Aellen
Amaranthaceae — Dysphania pumilio (R. Br.) Mosyakin & Clemants
Apocynaceae — Asclepias incarnata L. subsp. incarnata
Araceae — Wolffia brasiliensis Wedd.
Asteraceae — Bellis perennis L.
Asteraceae — Bidens cernua L.
Asteraceae — Gamochaeta pensylvanica (Willd.) Cabrera, also new for New York State (Atha et al., 2016).
Asteraceae — Sonchus asper (L.) Hill
Balsaminaceae — Impatiens pallida Nutt.
Bignoniaceae — Catalpa speciosa (Warder) Warder ex Engelm.
Brassicaceae — Barbarea verna (Mill.) Asch.
Brassicaceae — Cardamine flexuosa With.
Campanulaceae — Lobelia cardinalis L.
Caryophyllaceae — Cerastium arvense L.
Cyperaceae — Carex aggregata Mack., was historic for New York State, but discovered at the Garden in 2009 and is now planted from local stock.
Cyperaceae — Carex communis Bailey var. communis
Cyperaceae — Carex debilis Michx. var. debilis
Cyperaceae — Carex digitalis Willd. var. digitalis
Cyperaceae — Carex gracillima Schwein.
Cyperaceae — Carex reznicekii Werier
Cyperaceae — Carex sprengelii Dewey ex Spreng.
Cyperaceae — Carex umbellata Schkuhr ex Willd.
Cyperaceae — Cyperus squarrosus L.
Ericaceae — Gaultheria procumbens L.
Fagaceae — Quercus macrocarpa Michx.
Lamiaceae — Ajuga reptans L.
Lamiaceae — Pycnanthemum muticum (Michx.) Pers.
Oleaceae — Fraxinus profunda (Bush) Bush, also new for New York State.
Orchidaceae — Corallorhiza maculata (Raf.) Raf. var. maculata
Oxalidaceae — Oxalis dillenii Jacq.
Plantaginaceae — Callitriche stagnalis Scop.
Poaceae — Bromus commutatus Schrad.
Poaceae — Dichanthelium boscii (Poir.) Gould & C. A. Clark
Poaceae — Festuca rubra L.
Polygonaceae — Persicaria careyi (Olney) Greene
Rosaceae — Agrimonia striata Michx.
Rosaceae — Potentilla anglica Laichard., also new for New York State.
Rosaceae — Prunus virginiana L.
Rosaceae — Rubus canadensis L.
Rubiaceae — Galium circaezans Michx.
Violaceae — Viola blanda Willd.
Violaceae — Viola pedata L.
Violaceae — Viola striata Aiton
Vitaceae — Vitis aestivalis Michx.
Appendix IV. Rare Plants
S1. Critically imperiled in New York State because of extreme rarity (five or fewer sites or very few remaining individuals) or extremely vulnerable to extirpation from New York State due to biological or human factors (DEC, 2016).
Cyperaceae — Carex amphibola Steud. S1.
Cyperaceae — Carex reznicekii Werier. S1S2.
Hypericaceae — Hypericum stragulum P. Adams & N. Robson, S1 (as Hypericum hypericoides subsp. multicaule in DEC, 2016).
S2. Imperiled in New York State because of rarity (6–20 sites or few remaining individuals) or highly vulnerable to extirpation from New York State due to biological or human factors (DEC, 2016).
Cyperaceae — Carex debilis Michx. var. debilis, S2.
Cyperaceae — Cyperus lupulinus (Spreng.) Marcks. S2 (as Cyperus lupulinus subsp. lupulinus in DEC, 2016).
SH. Historical. No existing sites known in New York State in the last 20–30 years but it may be rediscovered (DEC, 2016).
Cyperaceae — Carex aggregata Mack. SH. This species is extant on the grounds of NYBG and is being cultivated from local stock.
Appendix V. Sight Records
Included here are names reported by Britton (1898, 1899), Nash (1900) and observations by the authors for which no voucher specimen of a spontaneous occurrence was found. These names are not included in summaries of native and non-native taxa reported in the results.
Ferns
Cystopteridaceae — Cystopteris fragilis (L.) Bernh., Brittle Bladderfern. (Britton, 1899)
Dryopteridaceae — Dryopteris marginalis (L.) A. Gray, Marginal Woodfern. (Britton, 1899)
Osmundaceae — Osmunda regalis L., Royal Fern. (Britton, 1899)
Polypodiaceae — Polypodium virginianum L., {Polypodium vulgare}, Rock Polypody. (Britton, 1899)
Selaginellaceae — Selaginella apoda (L.) Spring, {Selaginella apus}, Meadow Spikemoss. (Britton, 1899)
Selaginellaceae — Selaginella rupestris (L.) Spring, Rock Spikemoss. (Britton, 1899)
Thelypteridaceae — Phegopteris connectilis (Michx.) Watt, {Phegopteris phegopteris}, Long Beechfern. (Britton, 1899)
Thelypteridaceae — Phegopteris hexagonoptera (Michx.) Fée, Broad Beechfern. (Britton, 1899)
Thelypteridaceae — Thelypteris palustris (A. Gray) Schott, {Dryopteris thelypteris}, Eastern Marsh Fern. (Britton, 1899)
Gymnosperms
Pinaceae — Larix laricina (Du Roi) K. Koch, Tamarack. (Britton, 1899)
Pinaceae — Picea abies (L.) H. Karst., {Picea excelsa}, Norway Spuce. (Britton, 1899)
Pinaceae — Picea mariana (Mill.) Britton, Sterns & Poggenb., Black Spruce. (Britton, 1899)
Monocots
Alliaceae — Allium canadense L., Wild Onion. (Britton, 1899)
Cyperaceae — Carex cristatella Britton, Crested Sedge. (Britton, 1899)
Cyperaceae — Carex laxiflora patulifolia. (Britton, 1899)
Cyperaceae — Carex sterilis Willd., Dioecious Sedge. (Britton, 1899)
Cyperaceae — Carex typhina Michx., {Carex typhinoides}, Cattail Sedge. (Britton, 1899)
Cyperaceae — Eleocharis tenuis (Willd.) Schult., Slender Spikerush. (Britton, 1899)
Juncaceae — Juncus bufonius L., Toad Rush. (Britton, 1899)
Juncaceae — Juncus effusus L., Common Rush. (Britton, 1899)
Liliaceae — Lilium canadense L., Wild Yellow Lily. (Britton, 1899)
Liliaceae — Medeola virginiana L., Indian Cucumberroot. (Britton, 1899)
Orchidaceae — Spiranthes cernua (L.) Rich., {Gyrostachys cernua}, Nodding Ladiestresses. (Britton, 1899)
Orchidaceae — Spiranthes lacera (Raf.) Raf. var. gracilis (Bigelow) Luer, {Gyrostachys gracilis}, Southern Slender Ladystresses. (Britton, 1899)
Orchidaceae — Spiranthes lacera (Raf.) Raf. var. lacera, {Habenaria lacera}, Northern Slender Ladiestresses. (Britton, 1899)
Poacaee - Agrostis hyemalis (Walt.) Britton, Stearns & Poggenb., Winter Bentgrass. (Observed by the authors)
Poaceae — Aristida longespica Poir. var. longespica, {Aristida gracilis}, Slimspike Threeawn. (Britton, 1899)
Poaceae — Avena sativa L., Oats. (Britton, 1899)
Poaceae — Bromus tectorum L., Cheatgrass. (Observed by the authors)
Poaceae — Cenchrus tribuloides L., Dune Sandbur. (Britton, 1899)
Poaceae — Glyceria fluitans (L.) R. Br., {Panicularia brachyphylla }, Water Mannagrass. (Britton, 1899)
Poaceae — Panicum gattingeri Nash, {Panicum capillare gattingeri}, Gattinger's Panicgrass. (Britton, 1899)
Poaceae — Panicum virgatum L., Switch Grass. (Observed by the authors)
Poaceae — Phragmites australis (Cav.) Trin. ex Steud., Common Reed. (Observed by the authors)
Poaceae — Schizachyrium scoparium (Michx.) Nash, {Andropogon scoparius}, Little Bluestem. (Britton, 1899)
Poaceae — Setaria italica (L.) P. Beauv., {Panicum elongatum}, Italian Foxtail. (Britton, 1899)
Poaceae — Sporobolus vaginiflorus (Torr.) A. Wood, Sheathed Dropseed. (Britton, 1899)
Poaceae — Torreyochloa pallida (Torr.) Church, {Panicularia pallida}, Pale Mannagrass. (Britton, 1899)
Poaceae — Vulpia octoflora (Walter) Rydb., {Festuca octoflora}, Sixweeks Fescue. (Britton, 1899)
Dicots
Apiaceae — Pastinaca sativa L., Wild Parsnip. (Britton, 1899)
Apiaceae — Sanicula canadensis L., Canadian Black Snakeroot. (Britton, 1899)
Apocynaceae — Asclepias exaltata L., Poke Milkweed. (Britton, 1899)
Araliaceae — Hydrocotyle americana L., Marsh Pennywort. (Britton, 1899)
Araliaceae — Kalopanax septemlobus (Thunb.) Koidz., Castor Aralia. (Observed by the authors)
Asteraceae — Bidens laevis (L.) Britton, Sterns & Poggenb., {Bidens helianthoides}, Smooth Beggarticks. (Britton, 1899)
Asteraceae — Cirsium discolor (Muhl. ex Willd.) Spreng., {Carduus discolor}, Field Thistle. (Britton, 1899)
Asteraceae — Erigeron pulchellus Michx., Robins Plantain. (Britton, 1899)
Asteraceae — Eutrochium maculatum (L.) E. E. Lamont, {Eupatorium maculatum}, Spotted Joepyeweed. (Britton, 1899)
Asteraceae — Helianthus annuus L., Sunflower. (Observed by the authors)
Asteraceae — Helianthus strumosus L., Roughleaved Sunflower. (Britton, 1899)
Asteraceae — Hieracium gronovii L., Hairy Hawkweed. (Britton, 1899)
Asteraceae — Hieracium paniculatum L., Panicled Hawkweed. (Britton, 1899)
Asteraceae — Hieracium scabrum Michx., Rough Hawkweed. (Britton, 1899)
Asteraceae — Hieracium venosum L., Rattlesnake Hawkweed. (Britton, 1899)
Asteraceae — Leucanthemum vulgare Lam., {Chrysanthemum leucanthemum}, Oxeye Daisy. (Britton, 1899)
Asteraceae — Matricaria discoidea DC., Pineapple Weed. (Observed by the authors)
Asteraceae — Packera aurea (L.) Á. Löve & D. Löve, {Senecio aureus}, Golden Ragwort. (Britton, 1899)
Asteraceae — Scorzoneroides autumnalis (L.) Moench, {Leontodon autumnale}, Fall Dandelion. (Britton, 1899)
Asteraceae — Solidago gigantea Aiton, {Solidago serotina}, Smooth Goldenrod. (Britton, 1899)
Asteraceae — Symphyotrichum ericoides (L.) G. L. Nesom, {Aster ericoides, Aster multiflorus}, White Heath Aster. (Britton, 1899)
Asteraceae — Symphyotrichum lowrieanum (Porter) G. L. Nesom, {Aster lowrieanus}, Fall Aster. (Britton, 1899)
Asteraceae — Symphyotrichum novae-angliae (L.) G. L. Nesom, {Aster novae-angliae}, New England Aster. (Britton, 1899)
Asteraceae — Symphyotrichum novi-belgii (L.) G. L. Nesom, New York Aster. (Observed by the authors)
Asteraceae — Symphyotrichum puniceum (L.) A. & D. Löve var. puniceum, {Aster puniceus}, Purplestem Aster. (Britton, 1899)
Asteraceae — Symphyotrichum tradescantii (L.) G. L. Nesom, {Aster tradescanti}, Shore Aster. (Britton, 1899)
Asteraceae — Symphyotrichum undulatum (L.) G. L. Nesom, {Aster undulatus}, Wavyleaf Aster. (Britton, 1899)
Asteraceae — Taraxacum laevigatum (Willd.) DC., {Taraxacum erythrospermum}, Rock Dandelion. (Britton, 1899)
Berberidaceae — Berberis thunbergii DC., Japanese Barberry. (Observed by the authors)
Betulaceae — Alnus incana (L.) Moench subsp. rugosa (Du Roi) Clausen, {Alnus rugosa}, Speckled Alder. (Britton, 1899)
Bignoniaceae — Campsis radicans (L.) Seem., {Tecoma radicans}, Trumpet Creeper. (Britton, 1899)
Boraginaceae — Myosotis laxa Lehm., Smaller Forget-me-not. (Britton, 1899)
Brassicaceae — Arabis canadensis L., Sicklepod. (Britton, 1899)
Brassicaceae — Arabis laevigata (Muhl. ex Willd.) Poir., Smooth Rockcress. (Britton, 1899)
Brassicaceae — Barbarea stricta Andrz., Yellow Rocket. (Britton, 1899)
Cactaceae — Opuntia humifusa (Raf.) Raf., {Opuntia opuntia}, Eastern Pricklypear. (Britton, 1899)
Campanulaceae — Campanulastrum americanum (L.) Small, American Bellflower. (Observed by the authors)
Caryophyllaceae — Arenaria serpyllifolia L., Thymeleaved Sandwort. (Britton, 1899)
Caryophyllaceae — Dianthus armeria L., Deptford Pink. (Britton, 1899)
Celastraceae — Euonymus americanus L., Strawberry Bush. (Britton, 1899)
Ericaceae — Pyrola elliptica Nutt., Elliptic Shinleaf. (Britton, 1899)
Euphorbiaceae — Acalypha virginica L., Virginia Copperleaf. (Britton, 1899)
Fabaceae — Albizia julibrissin Durazz., Mimosa. (Observed by the authors)
Fabaceae — Amphicarpaea bracteata (L.) Fernald, {Falcata comosa}, American Hogpeanut. (Britton, 1899)
Fabaceae — Apios americana Medik., {Apios apios}, Common Groundnut. (Britton, 1899)
Fabaceae — Crotalaria sagittalis L., Weedy Rattlebox. (Britton, 1899)
Fabaceae — Desmodium paniculatum (L.) DC., {Meibomia paniculata}, Narrowleaved Tick-trefoil. (Britton, 1899)
Fabaceae — Lathyrus palustris L., {Lathyrus palustris}, Marsh Pea. (Britton, 1899)
Fabaceae — Lotus corniculatus L., Birdsfoot Trefoil. (Observed by the authors)
Fabaceae — Medicago sativa L. Alfalfa. (Observed by the authors)
Fabaceae — Melilotus officinalis (L.) Pall., Yellow Sweetclover. (Britton, 1899)
Fabaceae — Securigera varia (L.) Lassen, Crownvetch. (Observed by the authors)
Fabaceae — Trifolium aureum Pollich., {Trifolium agrarium}, Palmate Hopclover. (Britton, 1899)
Fabaceae — Vicia tetrasperma (L.) Schreb., Lentil Vetch. (Britton, 1899)
Fabaceae — Wisteria floribunda (Willd.) DC., Japanese Wisteria. (Observed by the authors)
Fagaceae — Quercus cerris L., Turkey Oak. (Observed by the authors)
Fagaceae — Quercus montana Willd., Chestnut Oak. (Observed by the authors)
Gentianaceae — Gentiana andrewsii Griseb., Closed Bottle Gentian. (Britton, 1899)
Hypericaceae — Hypericum majus (A. Gray) Britton, St. Johnswort (Observed by the authors)
Lamiaceae — Lycopus rubellus Moench, Stalked Bugleweed. (Britton, 1899)
Linaceae — Linum virginianum L., Virginia Yellow Flax. (Britton, 1899)
Malvaceae — Hibiscus syriacus L., Rose of Sharon. (Britton, 1899)
Malvaceae — Malva neglecta Wallr., {Malva rotundifolia}, Common Mallow. (Britton, 1899)
Myricaceae — Comptonia peregrina (L.) J. M. Coult., Sweetfern. (Britton, 1899)
Myrsinaceae — Lysimachia nummularia L., Creeping Jenny. (Britton, 1899)
Nymphaeaceae — Nuphar microphylla (Pers.) Fernald, {Nymphaea kalmiana}, Yellow Pondlily. (Britton, 1899)
Nymphaeaceae — Nymphaea odorata Aiton, {Castalia odorata}, American White Waterlily. (Britton, 1899)
Oleaceae — Syringa vulgaris L., Lilac. (Britton, 1899)
Onagraceae — Epilobium ciliatum Raf., Fringed Willowherb. (Observed by the authors)
Onagraceae — Oenothera pilosella Raf., {Kneiffia fruticosa pilosella}, Midwestern Sundrops. (Britton, 1899)
Orobanchaceae — Pedicularis lanceolata Michx., Swamp Lousewort. (Britton, 1899)
Phrymaceae — Mimulus ringens L., Monkey Flower. (Observed by the authors)
Plantaginaceae — Callitriche palustris L., Vernal Waterstarwort. (Britton, 1899)
Plantaginaceae — Nuttalanthus canadensis (L.) D. A. Sutton, {Linaria canadensis}, Canada Toadflax. (Britton, 1899)
Polygonaceae — Fallopia convolvulus (L.) A. Löve, {Polygonum convolvulus}, Black Bindweed. (Britton, 1899)
Polygonaceae — Persicaria arifolia (L.) Haraldson, {Polygonum arifolium}, Triangleleaf Tearthumb. (Britton, 1899)
Polygonaceae — Rumex britannica L., Water Dock. (Britton, 1899)
Ranunculaceae — Thalictrum dioicum L. Early Meadow Rue. (Observed by the authors)
Rhamnaceae — Ceanothus americanus L., New Jersey Tea. (Britton, 1899)
Rosaceae — Potentilla argentea L., Silvery Cinquefoil. (Britton, 1899)
Rosaceae — Potentilla canadensis L., {Potentilla pumila}, Running Cinquefoil. (Britton, 1899)
Rosaceae — Pyrus communis L., Pear. (Britton, 1899)
Rosaceae — Rosa virginiana Mill., {Rosa humilis}, Virginia Rose. (Britton, 1899)
Rosaceae — Rubus setosus Bigelow, Bristly Blackberry. (Britton, 1899)
Rubiaceae — Galium tinctorium L., Stiff Bedstraw. (Britton, 1899)
Rubiaceae — Galium triflorum Michx., Fragrant Bedstraw. (Britton, 1899)
Rubiaceae — Oldenlandia uniflora L., Clustered Mille Graines. (Lamont et al., 2011, based on sight record)
Salicaceae — Populus alba L., White Poplar. (Britton, 1899)
Salicaceae — Salix alba L., {Salix alba vitellina}, White Willow. (Britton, 1899)
Salicaceae — Salix nigra Marshall, Black Willow. (Britton, 1899)
Sapindaceae — Acer saccharinum L., Silver Maple. (Britton, 1899)
Saxifragaceae — Chrysosplenium americanum Schwein. ex Hook., American Golden Saxifrage. (Britton, 1899)
Solanaceae — Physalis heterophylla Nees, Clammy Groundcherry. (Britton, 1899)
Solanaceae — Physalis longifolia Nutt., Longleaf Groundcherry. (Observed by the authors)
Ulmaceae — Ulmus pumila L. Siberian Elm. (Observed by the authors)
Violaceae — Viola cucullata Aiton, {Viola obliqua}, Marsh Blue Violet. (Britton, 1899)
Violaceae — Viola labradorica Schrank, Alpine Violet. (Britton, 1899)
Violaceae — Viola pubescens Aiton var. scabriuscula Schwein. ex Torr. & A. Gray, {Viola scabriuscula}, Yellow Forest Violet. (Britton, 1899)
Vitaceae — Parthenocissus inserta (A. Kern.) Fritsch, Woodbine. (Observed by the authors)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Atha, D.E., Forrest, T., Naczi, R.F.C. et al. The historic and extant spontaneous vascular flora of The New York Botanical Garden. Brittonia 68, 245–277 (2016). https://doi.org/10.1007/s12228-016-9417-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12228-016-9417-5