Summary
We show that the two continental African species previously ascribed to the genus Anacolosa, differ in so many architectural, floral and vegetative characters from the remaining species of the genus (which occur from Madagascar to the western Pacific, including the type) that they clearly represent a separate genus. The African genus represented by these two species is unique within the Olacaceae s.l. (excluding Erythropalaceae) in being a climber (vs shrubs or trees in Anacolosa sensu stricto). Climbing in the two African species is achieved by perennial hook-like structures formed by a combination of five separate traits each of which is unknown elsewhere in the Olacaceae s.l. We formally delimit, describe and name this new African genus as Keita. Placement is tentatively in Aptandraceae but confirmation by molecular studies is required. We describe a new species from Guinea as Keita deniseae sp. nov. and transfer the Central African (Democratic Republic of Congo (DRC), Republic of Congo & Gabon) species from Anacolosa to the new genus as Keita uncifera comb. nov. Keita deniseae is assessed using the IUCN (2012) criteria as Endangered due to the threat of clearance or degradation of forest habitat for mining and agriculture, while K. uncifera is assessed as Least Concern in view of its large range, number of locations and low levels of threat. We review the discovery of Keita deniseae in the light of other recent discoveries of new taxa in both Simandou and the Republic of Guinea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2006 and 2007 the first author collected specimens of a liana with climbing hook-like petioles from submontane forest in the Simandou and Kourandou Ranges of the Loma-Man Highlands of the Republic of Guinea. The specimens were found by the third author to match a strange species of Aptandraceae (Olacaceae sensu lato) described from Gabon, Republic of Congo and the Democratic Republic of Congo (hereafter DRC), as Anacolosa uncifera Louis & Boutique (1947). In late 2021 the Guinean plant was found again at Simandou in full flower by the first and second authors (Fig. 1), permitting us to test the hypothesis that the Guinean material is a different taxon to that of Central Africa.
In the course of comparing these two African taxa with each other, we also compared them with the other 14 species placed in Anacolosa Blume, which extend from Madagascar to the western Pacific. Although there are similarities between the African and non-African species, there are so many points of difference in habit, foliar structures, indumentum and floral structures that we conclude that the African material represents a new genus to science, which we describe for the first time and formally name as Keita gen. nov., describing the new species as K. deniseae Cheek sp. nov., and transferring the Congolian taxon from Anacolosa as K. uncifera comb. nov.
The genus Anacolosa was erected for a small tree in Java, A. frutescens Blume (1851). Additional species were added to the genus from SW India (Beddome 1864), Indo-China and Myanmar (Kurz 1876; Pierre 1892; Gagnepain 1947; Masters 1875), the Western Pacific (Christophersen 1935; Gillespie 1932; Kanehira 1936), New Guinea and neighbouring areas (Sleumer 1980, 1984; Schellenberg 1923), and Madagascar (Cavaco & Kerauden 1963; Baillon 1862). Fifteen species are currently accepted, including the African species (Plants of the World Online, continuously updated).
The non-African Anacolosa species are shrubs or trees, usually of lowland evergreen forest, except Anacolosa pervilleana Baill., which occurs in semi-deciduous forest in western Madagascar, one of the two species in that island (Rogers & Malécot 2021). The centre of diversity of Anacolosa is Indo-China, where six species occur, all but one endemic. The western Pacific follows in species diversity, with four species. In contrast Malesia holds only a single widespread species, the type of the genus, apart from New Guinea with two other species (Sleumer 1980).
Anacolosa is characterised by the combination of (five-)six petals, stamens opposite to the petals, anthers bearded and placed in the concave lower (proximal) portion of the petals, the upper (distal) petal portions are not papillate (Kuijt & Hansen 2015).
The genus was placed in the Olacaceae tribe Anacoloseae by Engler (1897), together with Brachynema Benth., Strombosiopsis Engl., Tetrastylidium Engl., Scorodocarpus Becc., Cathedra Miers, Strombosia Blume, and Worcesterianthus Merr. (=Microdesmis Planch. now Pandaceae) (Sleumer 1935).
Olacaceae sensu lato was divided into eight families by Nickrent et al. (2010) based mainly on molecular evidence e.g. Malécot & Nickrent (2008), supported by morphological analysis (Malécot 2002; Malécot et al. 2004). Anacolosa was included by Nickrent et al. (2010) in the Aptandraceae, together with Aptandra Miers, Cathedra, Chaunochiton Benth., Harmandia Baill., Ongokea Pierre, Phanerodiscus Cavaco and Hondurodendron C.Ulloa et al.
However, Kuijt & Hansen (2015), disputed this classification, accepting Aptandraceae, but restricting it to including genera with anthers opening with flaps, and placing Anacolosa, which lacks such flaps, into Olacaceae sensu stricto, together with Brachynema, Dulacia Vell., Ptychopetalum Benth., Strombosiopsis, Olax L., Heisteria Jacq., Scorodocarpus, Maburea Maas, Tetrastylidium, Cathedra, Engomegoma Breteler and Strombosia.
Morphological data (analysed in Malécot et al. 2004) and molecular data (Malécot & Nickrent 2008) do not support such a grouping, but rather a close relationship of Anacolosa and Cathedra with Phanerodiscus, placed by Kuijt in Aptandraceae (see also Rogers & Malécot 2021).
Materials & Methods
Specimens were collected using the patrol method as documented in Cheek & Cable (1997). Herbarium material was examined with a Leica Wild M8 dissecting binocular microscope fitted with an eyepiece graticule measuring in units of 0.025 mm at maximum magnification. The drawing of Keita deniseae was made with the same equipment with a Leica 308700 camera lucida attachment. Specimens, or their high-resolution images studied through gbif.org, were inspected from the following herbaria: BR, HNG, K, P, WAG and YBI. Specimens cited have been seen unless indicated “n.v.”. Names of species and authors follow the International Plant Names Index (IPNI continuously updated). Nomenclature follows Turland et al. (2018). Technical terms follow Beentje & Cheek (2003). The conservation assessment follows the IUCN (2012) categories and criteria. Herbarium codes follow Index Herbariorum (Thiers continuously updated).
Taxonomic Treatment
Louis & Boutique (1947) described, and attributed to Anacolosa, A. uncifera, the first continental African species on the basis that the flowers are 6-merous (4-merous or 5-merous in other African genera of Olacaceae), the drupes are apparently enveloped by the accrescent disc and that the calyx is persistent in the fruit. However, we contend that this placement was erroneous and that this species, and the similar Guinean species, described as new to science below, represent a distinct genus of Olacaceae hitherto unknown to science.
The two African species differ from the other species ascribed to Anacolosa, including the type species, A. frutescens, in numerous characters including some which characterise other genera of Olacaceae (sensu Kuijt & Hansen 2015). For example, the corolla of the two African species is united into a tube in the proximal half, therefore showing similarities with the South American monotypic genus Brachynema. In the other genera of Olacaceae, including the other species of Anacolosa, the petals are either distinct (free) or only basally coherent, not forming a tube.
The two African species also differ from the other species ascribed to Anacolosa in that the inflorescence axis resembles that of the genus Strombosiopsis, in that the flowers are crowded on a swollen, thick inflorescence axis (Fig. 2E), the pedicels placed in individual depressions, each subtended by a small bract and two prophyllar bracteoles. Moreover, the anthers in both the African species have, like Strombosiopsis, a connectival projection (absent in Anacolosa sensu stricto). Although this structure is not “narrowly triangular” as in Strombosiopsis (Kuijt & Hansen 2015), but instead rounded, and resembling an additional, fifth anther theca (Fig. 2K – M). The African species also have characters otherwise unknown in the Olacaceae, and even in the Santalales. For example, they are lianas, climbing by means of specialised hook-like structures that are developed at nodes that subtend lateral branches. In these petioles (differing from other petioles on the plant), the articulation (the abscission zone between petiole and stem), is displaced from the junction with the primary stem (Fig. 2C) towards the distal part of the petiole. The subtending petiole is elongated and reflexes and curves around supports, while its blade is usually caducous (Fig. 2A), but can sometimes persist, although is then often reduced (Hallé 1973). Remarkably, at the nodes bearing these recurved petioles, the axillary bud and subtending leaf are projected laterally on a 5 – 8 mm stalk (Fig. 2A). Lianas are otherwise unknown in the Olacaceae in the broad sense excepting the monotypic climbing genus Erythropalum Blume of SE Asia which has tendrils, and which Kuijt & Hansen exclude from Santalales, although Nickrent et al. (2010) differ, and include as the monotypic Erythropalaceae. The two Anacolosa of Africa are further discordant in the Olacaceae in having multicellular, brown hairs on the stems, petioles and midribs (Fig. 2B), while Santalales generally are glabrous or only papillate, excepting e.g. Coula Baill. (Coulaceae) and Octoknema Pierre (Octoknemaceae, Gosline & Malécot 2011).
Keita deniseae A habit; B indumentum on stem; C flowering node showing petiole articulation; D flowering node with flower at anthesis; E inflorescence, globose with sunken sockets; F flower bud, side view; G flower at anthesis, side view (arrow indicates receptacle-disc); H flower, showing disc, stamens, style-stigma (half of corolla removed); J disc, plan view (arrow indicates calyx); K stamen (inner face); L stamen (side view); M stamen (outer face). A from Cheek 19148A; B & C from Cheek 13710; D – M from Molmou 1920 (HNG, K). drawn by andrew brown.
Additional features separating the two African species previously ascribed to Anacolosa from other species of the genus are outlined in Table 1 below, strongly supporting separate generic status.
Generic description
Keita Cheek gen. nov.
http://www.ipni.org/urn:lsid:ipni.org:names:77332882-1
Type species: Keita deniseae Cheek
Hermaphrodite, evergreen, canopy-flowering lianas, regenerating by root suckers. Stems often flexuose (zig-zagging). Indumentum dense, red-brown, hairs multicellular, patent, persistent on stems, petioles and midribs of leaves. Phyllotaxy distichous. Leaves simple, astipulate, petioles canaliculate, articulate, non-pulvinate, hairs as stems, dimorphic, a) petioles (at nodes of the primary axis subtending axillary stems) projected laterally together with the axillary bud on short stalks several mm long, elongated, reflexing, and articulated at apex, blade caducous; b) other petioles not so elongated, nor reflexing, articulated at base with stem; leaf-blade pinnately nerved, brochidodromous, margin entire, slightly revolute, domatia absent, sparsely hairy near petiole abaxially and on midrib.
Inflorescences on axillary stems only, axillary, one per node at multiple successive nodes, racemes condensed, cone-like, 6 – 30-flowered, axis swollen, subglobose to shortly ellipsoid, bracts and bracteole pair triangular, minute, caducous, pedicels inserted in individual shallow pits. Flower hermaphrodite. Calyx cupular, shallowly 5-lobed or 5-denticular, inserted at base of ovary-disc, outer surface papillate. Corolla inserted with androecium on rim of the combined ovary-disc, corolla tubular in proximal half, inner surface glossy, glabrous; distal part divided into (5 –) 6 valvate, triangular lobes, apex of lobes thickened and triangular in section, adaxial surface (at least part of the midline) long-hairy, otherwise papillate. Stamens 6, opposite to the petals, filaments dorsiventrally flattened, glabrous, appressed to the corolla tube their entire length, adnate to the base of the tube, included in the corolla tube; anthers introrse, inclined inwards, dithecal, the thecae each divided into two superposed, ellipsoid cells arranged around the terminal, distal, connective appendage. Connective appendage ellipsoid, exserted above and slightly larger than the four anther cells, glabrous or sometimes with a few apical hairs. Disc intrastaminal, concave, forming the ovary apex, 5-lobed, lobes broadly rounded, alternating with staminal filament bases, midline of each lobe slightly raised, puberulous. Ovary inferior to corolla + androecium, superior to calyx, 1- or 2- locular, placentation central, apical, ovules 2, pendulous. Style-stigma included within disc, stigma capitate. Fruit a 1-seeded berry, calyx and disc persistent, not conspicuously accrescent, ripening greenish-white, papillate-puberulous, longitudinally shallowly 12-ridged when dry, mesocarp c. 1 mm thick, fleshy; endocarp sclerified, with the seedcoat longitudinally invaginated (producing the surface ridges when dried). Seed with minute, orthotropic embryo, cotyledons minute, endosperm large.
recognition. Differs from Anacolosa Blume in being forest canopy lianas with principal nodes modified for climbing as clasping hooks, corolla tube subequal to corolla lobes in length, vs shrubs or trees, climbing modifications absent, corolla tube absent, lobes free (see additional diagnostic characters in Table 1).
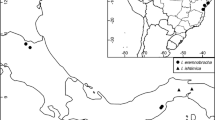
distribution. Two species, one throughout the Congo basin extending from Democratic Republic of Congo into Gabon, Republic of Congo, the other at (or near) Simandou, Guinea Highlands, Republic of Guinea.
etymology. Named for the late Professor Sékou Moussa Keita, Director of the Centre for Environmental Research (CERE), Université de Gamal Abdel Nasser-Conakry, Guinea, champion of plant species conservation in Guinea and of educating the next generation of Guinean botanists. In Mandinka, Keita is a clan name that can be interpreted as “blessing”.
notes. The precise phylogenetic placement of the new genus Keita remains to be resolved. The differences with Anacolosa sensu stricto (the species occurring from Madagascar to the western Pacific, Table 1) are so great that they may not have a sister relationship. The unique climbing mechanism of the African Keita species, with its five component traits each of which also appears to be unique in Olacaceae (see Table 1), speaks of long evolutionary isolation. It cannot be ruled out that phylogenomic analysis would support family level separation within Santalales for these two species. However, the presence of benzaldehyde, and pollen characters (see below), supports placement of Keita in Aptandraceae sensu Nickrent et al. (2010).
Pollen of Keita uncifera (as Anacolosa uncifera) has been studied by Lobreau-Callen (1980). The grains are triangular-obtuse with straight sides and triporate in polar view, 13 – 14 µm in diameter, rectangular obtuse, flattened in equatorial view, with psilate surface, lacking sculpture. These characters appear to match those of Malagasy and Asian Anacolosa (and also Phanerodiscus and Cathedra) according to Malécot et al. 2004).
The Guinean species produces a strong scent of benzaldehyde from roots and shoots when these are scraped. This information has not been recorded from the Congolian species but should be researched. It may be that this is an additional characteristic of the new genus. Benzaldehyde presence has also been reported in the genera Chaunochiton and Phanerodiscus (the scent of almonds from wood and bark, consistent with benzaldehyde), but not Anacolosa (Rogers & Malécot 2021).
A generic character stated for Anacolosa is the fruit enveloped by an accrescent disc (e.g. Rogers & Malécot 2021). However, recent observations on the fruit of Keita deniseae showed that the entire disc, shaped as in the flower, is restricted to the fruit apex and is only a little accrescent. Apart from the disc, the fruit is enveloped by a layer that appears to be an accrescent calyx, as seen in other genera of Olacaceae s.l., e.g. Olax itself. If confirmed, e.g. by anatomical investigation, this may represent a further generic character separating Keita from Anacolosa.
One hundred and seventeen records, of which 99 have images, were found on gbif.org (searched December 2022) searching under Anacolosa uncifera. These equate to about forty unique herbarium specimens, most of which are cited below.
Characters separating the Guinean taxon, described below as Keita deniseae, from the Central African species Keita uncifera, are documented in Table 2, below.
Key to the species of Keita (African former ‘Anacolosa’)
-
Leaf-blade base cuneate to rounded; abaxial surface when dried orange-brown; pedicel 1 – 1.8 (– 2.5) mm long; flower buds narrowly conical. DRC to Gabon…..….1. K. uncifera
-
Leaf-blade base rounded, truncate or cordate; abaxial surface drying green to black; pedicel 0.5 – 1 mm long; flower buds ovoid-globose. Republic of Guinea…….… 2. K. deniseae
1. Keita uncifera (Louis & Boutique) Cheek comb. nov.
http://www.ipni.org/urn:lsid:ipni.org:names:77332883-1
Anacolosa uncifera Louis & Boutique (1947: 256); Louis & Léonard (1948: 264); Villiers (1973: 104); Hallé (1973: 299); Cheek et al. 2022a: 8). Lectotype (selected here, see note below): Democratic Republic of Congo (formerly Congo Belge), District Forestier Central, Lumuna, Maniema, forêt, fleurs jaune, Aug. 1932, Lebrun 5908 (lectotype BR0000899393; isolectotype BR0000899361).
distribution. Democratic Republic of Congo, Gabon, and Republic of Congo.
selected specimens examined. democratic republic of congo. Orientale Prov., Isangi, Village Yabakondo, fl. Oct. 1952, Germain 8101 (P05327071); A 25 km en amont d'Isangi, le long du fleuve. Forêt marécageuse, fr. March 1949, Germain 4792 (BR0000017032391); Orientale, Isangi Terr., Ibila-Bolea, fl. 19 March 1957, Evrard 2240 (WAG.1121159); Terr. Ingende, Bolengambi (Ingembi), piste vers Kiri, fl. 12 April 1959, Evrard 6091 (YBI137140433); Yambao, rivière Eleo. Forêt ripicole, fr. 27 Dec. 1951, Germain 7282 (BR0000017032414, BR0000025317961V); Vallée Mbi mbungu, fl. 26 Nov. 1951, Devred 979 (BR0000017032179); Haut-Uele, Asonga Hill, 75 km S of Isiro, outskirts of Ituri Forest, fr. 1 Aug. 2011, Bujo 3159 (EPU n.v., K000024628); Maneima Prov. Namoya, Exploration camp to Kibiswa, fl. 9 Aug. 2008, Q. Luke 12351 (BR0000015261625V, EA n.v., K, MO n.v.); S Bank of Congo R., st. 8 Nov. 2004, J. P. & Q. Luke 10697Z (BR0000005031801, EA n.v., K, MO n.v.); Sankuru, fl. July 1947, Luja s.n. (BR0000017032216; Ligasa, R. Lokombe, fr. 30 Nov. 1948, Germain 4515 (BR0000017032384, BR0000017032407); Yangole, 20 km W of Yangambi, fl. Feb. 1939, Louis 13598 (syntype BR0000899364, BR00008992628, BR0000008993946); Kivu, Walikale Terr., Kabunga, fr. 19 Nov. 1958, Léonard 1783 (L.1665122); Walikale fl. 18 June 1959, Léonard 4647 (WAG.1121162); Bulumbu Terr., Schabunda, fl. buds 9 April 1954, Léonard 3760 (WAG.1121161); Yangambi, route D’Isangi, marais de la Lilanda, fl.14 Aug. 1947, Léonard 1373 (BR0000017032537, P05327070); Baringa/S/Maringa (Terr. Befale), foret rivulaire de la Maringa, fl. 25 Oct. 1958 (YBI166807454); Haut-Zaïre, S-Rég. Tshopo, Zone Banalia, Coll. Popoy, km 139 route Banalia-Panga, près dernier pont avant Panga. Près de la route, fr. 20 May 1987, Szafranski 1271(BR0000017032636); Eala, fl. June 1906, Laurent 1983 (BR0000017032438, BR0000017032445); Basankusu,1906, Bruneel s.n. (BR0000017032322; BR0000017032315); Dundusana, fl. Dec. 1913, Mortehan 1005 (BR0000017032629); Bas Congo, Kimayala, fl. June 1926, Vanderyst 14701 (BR0000017032193); Kasai, Terr. Luisa, R. Kateba, fl. 16 May 1957, Liben 3307 P05327069, WAG.1121163); Kasai, Kanda-kanda, Gandjika,, fr. 7 Nov. 1957, Risopoulos 682 (WAG.1121160); Kasai, Terr. Barumbu, fl. 12 Nov. 1913, Bequaert 1156 (BR0000017032292; BR0000017032308); Environs de Bomboie, fr. Sept. 1909, Claessens 169 (BR0000006868055, BR0000006867720. gabon. Région de Lastoursville, Gnyenzé fl. fl. 5 Sept. 1920, Le Testu 8300 (BR0000017032124; BR0000017032155, K, P05327073, P05327074); ibid., Moughamou sur Ogooué, 1929 – 1931, Le Testu 8293 (P05327072; P05327075); Ngounié, 15 km along the route Mimongo to Koulamoutou, fl. 10 Feb. 1983, J. J. F. E. De Wilde et al. 504 (BR0000017032131, LBV n.v., WAG 1121166, WAG 1121164, WAG 1121165); Belinga, st. 3 June 1966, Hallé 3174 (BR0000017032148, P05327087); ibid., fr. 6 June 1966, Hallé 3750 (P05327086); ibid., st. 6 June 1966, Hallé 3671 (P05327085). republic of congo. Alima-Likouala, Environs d’Edou, route pour le bac d’Alima, fl. 13 July 1961, Descoings 7673 (P05327079; P05327082); Alima-Likouala, Region de Fort Rousset, rives de Kouyou, fl. 17 Dec. 1970, Sita 3002 (P05327083).
habitat. Predominantly lowland swamp forest 470 – 700 (– 1000) m alt.
conservation status. Least Concern. The species has a vast range approximating to the extent of the Congolian forest in DRC, extending over the border into Gabon and Republic of Congo. The extent of occurrence is calculated as 1,277,662 km2, and the number of locations as over 20, while area of occupancy is estimated as 200 km2 using the 4 km2 cells preferred by IUCN. Threats to some sites exist e.g. in Gabon (future iron ore mining at the Belinga site), but through most of the range the habitat of this species is not thought to be under pressure (unlike in West Africa, the Congolian forests have mostly not been subjected to wholesale clearance for agriculture) and the species is not known to be targeted for exploitation.
etymology. Bearing hooks (from the Latin).
local name & uses. Kombe Ndjala I Fufow (Turumbu, Louis s.n. BR), Inaolo a Inongo (Turumbu, Germain 4792, BR), and Nginko E Likebe E Fufow (Turumbu). Uses, if any, are not recorded.
notes. In the protologue the authors indicate two specimens as types, one fruiting, Louis 13598, the other flowering, Lebrun 5908. Of these we here select as lectotype one of the two sheets of Lebrun 5908, indicated by barcode mentioned above. This is because one of the sheets of this syntype, that chosen as lectotype, bears a floral dissection that is indicated as having been used in the drawing of the protologue, and the metadata agrees with the protologue. The other sheet of this syntype does not, and so becomes the isolectotype. Since there are some differences in morphology (fruit stipitate vs sessile; connective appendage present vs absent; disc deep vs shallow) between the specimens from Gabon, as depicted in Villiers (1973) and those of DRC as featured in Louis & Boutique (1947), further studies to test the possibility that there may be two taxa rather than one in Keita uncifera are suggested. In addition, it is notable that while the DRC material described in the protologue derives from inundated and swamp lowland forest, that of Gabon comes from higher, apparently well-drained altitudes. We had insufficient material available from Gabon to investigate this matter during the current study.
2. Keita deniseae Cheek sp. nov. Type: Republic of Guinea, Guinée-Forestière, Simandou Range, Forêt Classée de Pic de Fon, Boyboyba Forest, c. 750 m alt., fl. 20 Nov. 2021, Molmou 1920 (holotype HNG; isotypes EA, K000593345, P, US). (Figs 1, 2, 3, 4, and 5).
http://www.ipni.org/urn:lsid:ipni.org:names:77332884-1
Anacolosa deniseae nom. nud. Cheek et al. (2022a: 9).
Canopy flowering liana, flowering on stems 5 – 20 m long. Stems up to 12 cm diam. at base, older plants with multiple stems arising at ground level, and separate plants arising several metres from the parent from horizontal roots running a few cm below the surface, orangish-white, 1.5 – 2 cm diam.; stems woody, cylindrical, bark dark grey-brown, lenticels inconspicuous. Roots and shoots smelling strongly of benzaldehyde when scraped. Leafy stems with principal axis terete, 2.5 – 4 mm diam., internodes (30 –) 38 – 50 (– 55) mm long; petiolar tendrils (18 –) 20 – 25 mm long, nodal protrusions (4 –) 6 – 8 mm long; axillary stems up to 38 cm long, 1 – 1.2 mm diam., internodes 9 – 17 mm long, densely dark brown hairy, hairs multicellular, (0.25 –) 0.5 mm long, patent, often with 6 – 7 hairs leaning together, cohering at apex, forming cone-like structures, intermixed with sparse translucent to white papillae 0.05 mm long. Leaves drying mid green, concolorous, obovate-elliptic or elliptic 42 – 65 (– 82) × (20 –) 25 – 36 (– 39) mm, acumen triangular (3 –) 4 (– 6) mm long, base cordate, truncate, or rounded, slightly asymmetric, margin slightly revolute when dried, midrib (and secondary nerves) impressed on upper surface, raised, broad and white on lower surface. Secondary nerves 3 – 5 on each side of the midrib, arising at 45 – 70° from midrib, brochidodromous, abruptly angled upwards at junction with secondary nerve below, forming a part looping, part angular infra-marginal nerve 2 – 5 mm from the margin; tertiary and quaternary nerves forming a reticulum with cells isodiametric, c. 1 mm diam. Indumentum of abaxial surface: proximal part as petiole, steadily becoming sparser distally, secondary nerves very sparsely hairy, adaxial surface glabrous. Petiole canaliculate (4 –) 5 – 8 (– 10) mm long, 1.1 mm wide, densely brown hairy as the axillary stems. Inflorescences on axillary branches, single in successive nodes, raceme contracted, 10 – 30-flowered, axis swollen, oblate, 1 – 1.5 × 1.5 – 2 (– 2.25) mm, bracts caducous, broadly triangular-pentagonal, to 0.5 × 0.75 mm, brown puberulous, bracteole pair triangular 0.3 × 0.2 mm, brown puberulous; rhachis brown hairy (Fig. 4). Flower pedicel 0.5 – 1 (– 1.2) mm long, 0.3 mm diam., densely white papillate. Calyx broadly cupular, 0.6 – 0.75 × 1.75 (– 2.2) mm, margin with (5 –) 6 shallow lobes up to 0.3 × 0.75 mm, or 5 – 6-denticulate, teeth 0.1 × 0.1 mm long, dense brown hairy, indumentum of calyx otherwise densely white papillate; bud broadly ovoid-globose, 2 (– 2.5) mm diam. Receptacle-disc united at base to calyx, nested within it, cupular, 0.5 – 0.7 × 1.7 – 2 mm, c. 0.5 mm thick, glabrous, outer surface smooth, distal, inner surface of disc with 18 radiating ridges, margin with 6 circular notches, each 0.1 – 0.2 mm diam. (Fig. 2H & J). Corolla inserted on rim of receptacle-disc, white, outer surface moderately densely papillate (50% cover) tube 1 – 1.2 × 2.3 – 2.5 mm, constricted at base and apex; lobes 6, reflexed-patent at anthesis, triangular 1 (– 1.5) × 1 – 1.1 mm, midrib raised, lobe base densely long hairy, hairs translucent, moniliform, spreading, 0.4 – 0.5 mm long; remainder of lobe densely papillate, papillae 0.025 – 0.05 mm long, translucent-white. Stamens 6, inserted in marginal notches of the disc, opposite to petals and united to corolla tube at base (only), filaments appressed to corolla tube, centripetally flattened, 0.5 × 0.25 – 0.3 mm, glabrous; anthers (inclined centrally Fig. 2L), cordate (Fig. 2M), 0.5 × 0.75 mm, thecae 2, collateral, each divided into two, subequal, ellipsoid, superposed cells (Fig. 2K & L); uppermost cells of the two thecae separated by the similarly shaped connective appendage resembling a fifth anther cell (Fig. 2K – M); anther cells glabrous, or with c. 6 erect hairs 0.35 mm long from the uppermost cells. Style subcylindric 0.3 – 0.55 × 0.2 mm, longitudinally ridged, widest at base; stigma capitate, 0.3 × 0.25 – 0.35 mm, minutely pitted (Fig. 2H). Fruit and seed reported to be produced in June, the fruits green, changing to brown when dried, slightly obovoid, c. 18 × 10 mm, all but apex enveloped in accrescent calyx (?), apex flattened, disc persistent, concave, 6-lobed at fruit apex, barely accrescent (Fig. 5). Seed single, ovoid, dull white in colour (pers. comm. Tokpa Seny Doré, see below).
recognition. Differing from Keita uncifera Louis & Boutique in the leaf base cordate or truncate, rarely rounded (vs cuneate to rounded); pedicels 0.5 – 1 mm long (vs 1 – 1.8 (– 2.5) mm long); flower bud broadly ovoid to globose (vs narrowly conical). Additional characters are provided in Table 2.
distribution. Republic of Guinea: Guinée-Forestière, Beyla Prefecture, Southern Simandou Range, Forêt Classée de Pic de Fon, Kankan Prefecture, Northern Simandou Range and Kourandou Mts.
specimens examined. republic of guinea. Guinée-Forestière. Southern Simandou Range, Forêt Classée de Pic de Fon, « Massif de Fon”, st. Aug. 1947 Schnell 3307 (P05327084); ibid., N of Pic de Fon, c. 1.5 km N of Dabatini, fl. 1 Dec. 2008, van der Burgt with Pepe Haba and Boubacar Diallo 1336 (HNG, K000460586, MO, P05183769, SERG, WAG); ibid., Moribadou to Canga East, 1st. 8 July 2006, Cheek with Tchiengue 13326 (HNG, K000437339); ibid., above Canga East, st. 17 Nov. 2021, Cheek with Tchiam, Molmou, Soropogui 19110 (BR, HNG, K, US); ibid., st. 5 March 2022, Cheek with Tchiam, Molmou, Princée 19213 (HNG, K); ibid., Oueleba, Boyboya Forest, st. 20 Nov. 2021, Cheek et al. 19148 (HNG, K); ibid., fl. 20 Nov. 2021, Molmou 1920 (holotype HNG, isotypes EA, K, P, US); gallery submontane forest, Oueleba, eastern side, 14 – 20 Dec. 2021 Molmou with Thiam & Soropogui, sight record; Oueleba, northern side, Siatoro forest, Dec. 2021 Molmou with Thiam, sight record; N of Moribadou, fr. 08.62236°N, 008.85732°W, fr. 19 June 2022, Doré s.n. (photo record); Mafindou, NE of Moribadou, 08.60537°N, 008.79357°W st. 24 June 2022, Doré s.n., sight record. Kourandou Mts, Sinko to Seberendou path, fl. buds 17 Nov. 2007, Cheek with Seydou Cissé, P. Haba & Condé 13710 (HNG, K000615587): Northern Simandou range, around Damaro, Plot 9, st. 13 Feb. 2012, Jongkind 14483 (WAG.1977189).
habitat. Canopy-flowering liana of submontane gallery forest, often rooting near streams; 750 – 1300 m alt.
Keita deniseae has been collected in flower at the forest-grassland edge (van der Burgt 1336). The type material was also collected at the forest edge (Molmou 1920). However, the origin of the stems in this last case was found to be downslope, towards a stream, the stems having spread along the canopy to its edge, where (at eye-level) it was more easily detected than high in the forest canopy. Elsewhere in the same forest patch (the Boyboyba forest) more juvenile (non-flowering) plants were seen at the edge of, or near, streams and springs.
conservation status. Keita deniseae is known from only three IUCN threat-based locations, one at the Kouroundou Mts (Cheek 13710, HNG, K) with a single site and individual recorded, where it grows close to a footpath in a farming area near a major town and is at risk of habitat clearance for charcoal production. At the second location, in the northern Simandou range (Jongkind 14483, WAG) again only a single site and collection is known 43 km north of the major southern subpopulation, and imminent iron ore mining and infrastructure is a major threat. At the third location, the Forêt Classée de Pic de Fon, Simandou Range, eight sites are recorded each with a single individual (including offset suckers, see Notes below) except two, the Boyboyba Forest, with ten widely separated individuals recorded including both the largest known individual of the species, and the type collection, and a site N of Moribadou with eight individuals (however these may all represent a single individual). The Pic de Fon location is set to host a major open pit iron ore mine. While the pit is not expected to directly impact sites for this species, there is a risk that despite best efforts to protect threatened species, nevertheless there will be negative impacts linked with the activities and infrastructure associated with the expected ore extraction, such as dumping of waste rock, alteration of hydrology and construction of new roads. The extent of occurrence is calculated as 216 km2, and the estimated area of occupancy as 32 km2 using the IUCN required 4 km2 cell-size. There has been a decline in quality of habitat e.g. due to interventions for environmental surveys (Cheek pers. obs. 2021 and 2022). Only six mature (flowering-sized, reaching canopy) individuals are known, which would qualify the species as Critically Endangered under Criterion D. However, estimating the numbers of individuals of large lianas is difficult, so here we employ only Criterion B. Accordingly we assess Keita deniseae as Endangered EN B1a,b(iii)+B2a,b(iii). It is to be hoped that this species will be searched for and found at other locations which would allow a lower extinction risk assessment than that made here. However, since even at a juvenile stage this species is so distinctive and easily recognised, it is strange that it has not been detected elsewhere before now if it indeed has a wider range.
etymology. Named for Denise Molmou, leading Guinea field botanist with the National Herbarium of Guinea and Simfer S.A. who discovered and collected the flowering type specimen material in November 2021. Her commitment and actions for the conservation of threatened species of Guinean plants as part of the Guinea TIPAs project (Couch et al. 2019) has been exceptional. She is also commemorated by the endemic, Critically Endangered (Possibly Extinct) Saxicolella deniseae Cheek (Podostemaceae, Cheek et al. 2022b) for which she collected the type and sole specimen.
local names and uses. According to Soumayila Condé, of Moribadou village, a local guide for the Southern Simandou Range where the species is most plentiful, no uses or names are known for this species (Tokpa Seny Doré pers. comm. 24 June 2022). The fruit was found to taste slightly sweet, with a hint of resin when tasted by Tokpa Seny Doré (pers. comm. June 2022).
notes. Keita deniseae is immediately recognisable in Guinea in the field, even as juvenile plants only 1 – 4 m tall, due to the conspicuous and unusual climbing stems (Fig. 1). Seedlings less than c. 60 cm tall lack climbing hooks.
Two seedlings less than 1 m tall, separated by c. 100 m, and two distantly separated larger juvenile plants 1 – 4 m tall were found in the Boyboyba forest of the Simandou range in late 2021, suggesting that natural regeneration from seed is occurring. The means of seed dispersal is unknown. Fruit was recently found in June 2022, early wet season (Fig. 5).
Researching this species in the field at two sites in March 2022, we found that full-sized plants at both sites had several (3 – 7) satellite plantlets arising from roots that we traced from the full-size parent. Identifying and tracing the roots of this species is facilitated by their strong scent of benzaldehyde, unusual in the plant world. Vegetative apomixis such as we observed is not often recorded in Olacaceae in Africa. The juveniles were all within 4 m of the parent and mostly about 1 – 2 m tall.
Keita deniseae at first sight is closely similar to K. uncifera, differing in the features detailed in Table 2, supporting the large geographical disjunction of 2600 km between these two species. The absence from the Cross-Sanaga Interval, in western Cameroon and SE Nigeria, which has the highest species and generic diversity for plants per degree square in tropical Africa (Cheek et al. 2001; Barthlott et al. 1996; Dagallier et al. 2020) is difficult to explain since many, if not most of the recently discovered Guinea Highland endemics with disjunctions to the east occur in the highlands of the Cross-Sanaga Interval (e.g. in the genera Brachystephanus Nees, Isoglossa Oerst., Talbotiella Baker f., Ternstroemia Mutis ex L.f., respectively Darbyshire et al. 2012; Champluvier & Darbyshire 2009; van der Burgt et al. 2018; Cheek et al. 2019a). This is the first case that we are aware of a far wider disjunction in this context.
The decision to formally designate a new generic name arose from the recommendations of an anonymous reviewer. It was first intended to name Keita deniseae as Anacolosa deniseae and this name was used in the bioRxiv pre-print (Cheek et al. 2022a: 9). However, such pre-prints, because they are not intended by the authors as the final publication, have no standing as publications for nomenclatural purposes according to the Code (Turland et al. 2018).
Therefore, Anacolosa deniseae is classed as a nominum nudum.
Discussion
Threatened plant species of the Simandou range
Keita deniseae is the latest in a line of species new to science discovered from or at the Simandou Range, all of which are threatened with extinction and constitute the highest priorities for plant conservation within that range. The species are either from the submontane bowal grassland on ferralitic (iron-rich) substrate (Xysmalobium samoritourei Goyder (Apocynaceae, Goyder 2008), Coleus ferricola Phillipson, O.Hooper & A.J. Paton (Lamiaceae, Phillipson et al. 2019), Polystachya orophila Stévart & E.Bidault (Orchidaceae, Bidault et al. 2016) or from adjacent submontane forest: Brachystephanus oreacanthus Champl. (Acanthaceae, Champluvier & Darbyshire 2009), Isoglossa dispersa I.Darbysh. (Acanthaceae, Darbyshire et al. 2012), Gymnosiphon samoritoureanus Cheek (Burmanniaceae, Cheek & van der Burgt 2010), Allophylus samoritourei Cheek (Sapindaceae, Cheek & Haba 2016a) and Psychotria samoritourei Cheek (Rubiaceae, Cheek & Williams 2016), or restricted to the interface (or transition) between these two habitats: Hibiscus fabiana Cheek (Malvaceae, Cheek et al. 2020a), while one occurs in mainly lowland woodland: Striga magnibracteata Eb.Fisch. & I.Darbysh. (Orobanchaceae, Fischer et al. 2011).
Most of these species have since been found in one or more other locations outside the Simandou Range, but other discoveries, (Eriosema triformum Burgt (Leguminosae, van der Burgt et al. 2012) and Keetia futa Cheek (Rubiaceae, Cheek et al. 2018a)), remain globally restricted to the Simandou Range on current evidence and are Critically Endangered. The last species formerly occurred at two other sites far from southern Simandou where it has been lost. Keita deniseae will also become restricted to southern Simandou and need re-assessment, likely as Critically Endangered, if it is lost at its other two, unprotected locations near Sinko and Damaro, both outside of southern Simandou.
New plant discoveries elsewhere in the Republic of Guinea
The Simandou Range is not exceptional in Guinea for conservation importance and recent discovery of new, range-restricted species. The Kounounkan area of the Fouta Djalon range, with habitats based on table mountains of Ordovician sandstone is far more exceptional in terms of historically known endemic taxa, such as the monotypic genus Caillella Jacq.-Fél. (Melastomataceae, Veranso-Libalah et al. 2021), Mesanthemum bennae Jacq.-Fél. (Eriocaulaceae, Phillips et al. 2018), Impatiens bennae Jacq.-Fél. (Balsaminaceae) and Rhytachne perfecta Jacq.-Fél. (Gramineae, both Couch et al. 2019). Surveys for the Guinea Tropical Important Plant areas programme have recently resulted in discovery of the following range-restricted species, all also threatened: Keetia susu Cheek (Rubiaceae, Cheek et al. 2018a), Gladiolus mariae Burgt (Iridaceae, van der Burgt et al. 2019), Ternstroemia guineensis Cheek (Ternstroemiaceae, Cheek et al. 2019a), Trichanthecium tenerium Xanthos (Gramineae, Xanthos et al. 2020), Ctenium bennae Xanthos (Gramineae, Xanthos et al. 2021) and even the Kounounkan endemic new genus Benna alternifolia Burgt (Melastomataceae, van der Burgt et al. 2022), with additional endemic range-restricted species to be described of Virectaria (Rubiaceae) and Hibiscus (Malvaceae).
Elsewhere in Guinea range-restricted new species to science have been found in numerous provinces and habitats, from surviving fragments of lowland forest, to cliffs, flat, bare sandstone, and waterfalls and rapids: Eriocaulon cryptocephalum S.M.Phillips & Mesterházy (Eriocaulaceae, Phillips & Mesterházy 2015), Napoleona alata Jongkind (Lecythidaceae, Prance & Jongkind 2015), Inversodicraea pepehabai Cheek (Podostemaceae, Cheek & Haba 2016b; Cheek et al. 2017), Karima Cheek (Euphorbiaceae, Cheek et al. 2016), Kindia gangan Cheek (Rubiaceae, Cheek et al. 2018b), Talbotiella cheekii Burgt (Leguminosae, van der Burgt et al. 2018), Inversodicraea koukoutamba Cheek and I. tassing Cheek (Podostemaceae, Cheek et al. 2019b) and Vepris occidentalis Cheek & Onana (Rutaceae, Cheek et al. 2019c).
Conclusions
About 2000 new flowering plant species are described each year (Cheek et al. 2020b), adding to the estimated 369,000 (but number debated) already known to science (Nic Lughadha et al. 2016; 2017). Widespread species tend to have already been discovered, although there are exceptions, such as Vepris occidentalis (cited above) that occurs from Guinea to Ghana. More usually, newly discovered species are those that are range-restricted and so are much more likely to be threatened, such as Keita deniseae published here. Until new species are formally named and known to science, it is much more difficult to assess them for their IUCN conservation status and so the possibility of protecting them is reduced (Cheek et al. 2020b). The majority of plant species still lack such assessments (Nic Lughadha et al. 2020). Documented extinctions of plant species are increasing (Humphreys et al. 2019) and recent estimates suggest that as many as two fifths of the world’s plant species are now threatened with extinction (Nic Lughadha et al. 2020). This makes it imperative to discover and publish such species so that they can be assessed, and, if merited, conservation actions taken to avoid the risk of becoming, like Guinea’s Inversodicraea pygmaea G.Taylor, globally extinct (Cheek et al. 2017; Cheek 2018; Cheek & Magassouba 2018). Designating and implementing Important Plant Areas (Darbyshire et al. 2017; continuously updated) is key to in situ conservation of plant species. For this reason, the Important Plant Areas (TIPAs) of Guinea have been recently designated (Couch et al. 2019) and accepted for incorporation into the national protected area network by the Government of Guinea (Col. Seyba, head of Oguipar (protected areas) pers. comm. 2019). Fortunately, the major population of Keita deniseae occurs within the newly designated Southern Simandou TIPA (TIPAs Guinea-Conakry (2016 – 2019); Couch et al. (2019). Making a conservation action plan (e.g. Couch et al. 2022) for Keita deniseae is now a priority.
References
Barthlott, W., Lauer, W. & Placke, A. (1996). Global distribution of species diversity in vascular plants: towards a world map of phytodiversity. Erdkunde 50: 317 – 327. https://doi.org/10.3112/erdkunde.1996.04.03
Baillon, H. (1862). Anacolosa pervilleana pp. 118 – 119 in Deuxième Mémoire sur les Loranthacées. Adansonia 3: 50 – 128. https://www.biodiversitylibrary.org/item/10558#page/52/mode/1up
Beddome, R. D. (1864). Contributions to the botany of southern India. Madras J. Lit. Sci., ser. 3, 1: 38. https://www.biodiversitylibrary.org/item/164965#page/50/mode/1up
Beentje, H. & Cheek, M. (2003). Glossary. In: H. Beentje (ed.), Flora of Tropical East Africa. Balkema, Lisse.
Bidault, E., Lowry, P. P. & Stevart, T. (2016). Polystachya orophila (Orchidaceae, Polystachynae), a new species from tropical West Africa, and clarification on the nomenclature and taxonomy of P. microbambusa. Phytotaxa 260 (3): 247 – 257. https://phytotaxa.mapress.com/pt/article/view/phytotaxa.260.3.4
Blume, C. L. (1851). Anacolosa frutescens. In: Museum botanicum Lugduno-Batavum 1: 250 – 251 & t. 46. E. J. Brill, Lugduni-Batavorum. https://www.biodiversitylibrary.org/page/50106757#page/260/mode/1up
Cavaco, A. & Kerauden, M. (1963). Nouvelles Olacacées de Madagascar. Bull. Soc. Bot. France 110: 245 – 248. https://doi.org/10.1080/00378941.1963.10838160
Champluvier, D. & Darbyshire I. (2009). A revision of the genera Brachystephanus and Oreacanthus (Acanthaceae) in tropical Africa. Syst. Geogr. Pl. 79: 115 – 192. https://www.jstor.org/stable/25746605
Cheek, M. (2018). Inversodicraea pygmaea. The IUCN Red List of Threatened Species 2018: e.T98569037A100439967. https://doi.org/10.2305/IUCN.UK.2018-1.RLTS.T98569037A100439967.en. [Downloaded 02 May 2022].
____ & Cable, S. (1997). Plant Inventory for conservation management: the Kew-Earthwatch programme in Western Cameroon, 1993 – 96, pp. 29 – 38. In: S. Doolan (ed.), African Rainforests and the Conservation of Biodiversity. Earthwatch Europe, Oxford.
____ & Haba P. M. (2016a). Spiny African Allophylus (Sapindaceae): a synopsis. Kew Bull. 71: 57. https://doi.org/10.1007/s12225-016-9672-3
____ & ____ (2016b). Inversodicraea Engl. resurrected and I. pepehabai sp. nov. (Podostemaceae), a submontane forest species from the Republic of Guinea. Kew Bull. 71: 55. https://doi.org/10.1007/s12225-016-9673-2
____ & Magassouba, S. (2018). The Importance of Plant Conservation in Guinea. A Guide for Secondary School Teachers. Royal Botanic Gardens, Kew.
____, Molmou, D., Gosline, G. & Magassouba, S. (2022a). The generic status of Anacolosa (Olacaceae) in Africa with A. deniseae a new species to science of Endangered submontane forest liana from Simandou, Republic of Guinea. bioRxiv https://doi.org/10.1101/2022.05.30.493947
____ & van der Burgt, X. (2010). Gymnosiphon samoritoureanus (Burmanniaceae) a new species from Guinea, with new records of other achlorophyllous heteromycotrophs. Kew Bull. 65: 83 – 88. https://doi.org/10.1007/s12225-010-9180-9
____ & Williams, T. (2016). Psychotria samoritourei (Rubiaceae), a new liana species from Loma-Man in Upper Guinea, West Africa. Kew Bull. 71: 19. https://doi.org/10.1007/s12225-016-9638-5
____, Challen, G., Lebbie, A., Banks, H., Barberá, P. & Riina, R. (2016). Discovering Karima (Euphorbiaceae) a New Crotonoid Genus from West Tropical Africa long hidden within Croton. PLoS one 11: (4). https://doi.org/10.1371/journal.pone.0152110
____, Feika, A., Lebbie, A., Goyder, D., Tchiengue, B., Sene, O., Tchouto, P. & van der Burgt, X. (2017). A synoptic revision of Inversodicraea (Podostemaceae). Blumea 62: 125 – 156. https://doi.org/10.3767/blumea.2017.62.02.07
____, Haba, P. K. & Cisse, S. (2020a). Hibiscus fabiana sp. nov. (Malvaceae) from the Guinea Highlands (West Africa). Blumea 65: 69 – 74. https://doi.org/10.3767/blumea.2020.65.01.08
____, ____, Konomou, G. & Van Der Burgt, X. (2019a). Ternstroemia guineensis (Ternstroemiaceae), a new endangered cloudforest shrub with neotropical affinities from Kounounkan, Guinea, W Africa. Willdenowia 49 (3): 351 – 360. https://doi.org/10.3372/wi.49.49306
____, Mackinder, B., Gosline, G., Onana, J.-M. & Achoundong, G. (2001). The phytogeography and flora of western Cameroon and the Cross River-Sanaga River interval. Syst. Geogr. Pl. 71: 1097 – 1100. https://doi.org/10.2307/3668742
____, Magassouba, S., Howes, M.-J. R., Doré, T., Doumbouya, S., Molmou, D., Grall, A., Couch, C. & Larridon, I. (2018b). Kindia (Pavetteae, Rubiaceae), a new cliff-dwelling genus with chemically profiled colleter exudate from Mt Gangan, Republic of Guinea. PeerJ 6: e4666. https://doi.org/10.7717/peerj.4666
____, ____, Molmou, D., Doré, T. S., Couch, C., Yasuda, S., Gore, C., Guest, A., Grall, A., Larridon, I., Bousquet, I. H., Ganatra, B. & Gosline, G. (2018a). A key to the species of Keetia (Rubiaceae - Vanguerieae) in West Africa, with three new, threatened species from Guinea and Ivory Coast. Kew Bull. 73: 56. https://doi.org/10.1007/s12225-018-9783-0
____, ____, Jennings, L., Magassouba, S. & van der Burgt, X. (2019b). Inversodicraea koukoutamba and I. tassing (Podostemaceae), new waterfall species from Guinea, West Africa. Blumea 64: 216 – 224. https://doi.org/10.3767/blumea.2019.64.03.03
____, Molmou, D., Magassouba, S. & Ghogue, J.-P. (2022b). Taxonomic revision of Saxicolella (Podostemaceae), African waterfall plants highly threatened by Hydro-Electric projects. Kew Bull. 77: 403 – 433. https://doi.org/10.1007/s12225-022-10019-2
____, Nic Lughadha, E., Kirk, P., Lindon, H., Carretero, J., Looney, B., Douglas, B., Haelewaters, D., Gaya, E., Llewellyn, T., Ainsworth, M., Gafforov, Y., Hyde, K., Crous, P., Hughes, M., Walker, B. E., Forzza, R. C., Wong, K. M. & Niskanen, T. (2020b). New scientific discoveries: plants and fungi. Plants, People Planet 2: 371 – 388. https://doi.org/10.1002/ppp3.10148
____, Onana, J-M., Yasuda, S., Lawrence, P., Ameka, G. & Buinovskaja G. (2019c). Addressing the Vepris verdoorniana complex (Rutaceae) in West Africa, with two new species. Kew Bull. 74: 53. https://doi.org/10.1007/S12225-019-9837-Y
Christophersen, E. (1935). Anacolosa insularis pp. 80 – 81. In: Flowering Plants of Samoa V. Bull. Bernice P. Bishop Museum 128: 1 – 221.
Couch, C., Cheek, M., Haba, P. M., Molmou, D., Williams, J., Magassouba, S., Doumbouya, S. & Diallo, Y. M. (2019). Threatened habitats and Important Plant Areas (TIPAs) of Guinea, west Africa. Royal Botanic Gardens, Kew. https://kew.iro.bl.uk/concern/books/1554f509-3e22-453c-9fab-51b45722d250
____, Molmou, D., Magassouba, S., Doumbouya, S., Diawara, M., Diallo, M. Y., Keita S. M., Koné, F., Diallo, M. C., Kourouma, S., Diallo, M. B., Keita, M. S., Oularé, A., Darbyshire, I., Gosline, G., Nic Lughadha, E., van der Burgt, X., Larridon, I. & Cheek, M. (2022). Piloting development of species conservation action plans in Guinea. Oryx 57: 497 – 506. https://doi.org/10.1017/s0030605322000138
Dagallier, L. P., Janssens, S. B., Dauby, G., Blach-Overgaard, A., Mackinder, B. A., Droissart, V., Svenning, J. C., Sosef, M. S., Stévart, T., Harris, D. J. & Sonké, B. (2020). Cradles and museums of generic plant diversity across tropical Africa. New Phytol. 225: 2196 – 2213. https://doi.org/10.1111/nph.16293
Darbyshire, I. (continuously updated). Tropical Important Plant Areas. https://tipas.kew.org
____, Anderson, S., Asatryan, A., Byfield, A., Cheek, M., Clubbe, C., Ghrabi, Z., Harris, T., Heatubun, C. D., Kalema, J., Magassouba, S., McCarthy, B., Milliken, W., Montmollin, B. de, Nic Lughadha, E., Onana, J-M., Saıdou, D., Sârbu, A., Shrestha, K. & Radford, E. A. (2017). Important Plant Areas: revised selection criteria for a global approach to plant conservation. Biodivers. Conserv. 26: 1767 – 1800. https://doi.org/10.1007/s10531-017-1336-6
____, Pearce, L. & Banks, H. (2012). The genus Isoglossa (Acanthaceae) in west Africa. Kew Bull. 66: 425 – 439. https://doi.org/10.1007/s12225-011-9292-x
Engler, A. (1897). Olacaceae. Pp. 144 – 149. In: A. Engler & K. Prantl (eds), Natürlichen Pflanzenfamilien Nachtrӓge zu Teil II – IV. W. Engelmann, Leipzig.
Fischer, E., Darbyshire, I. & Cheek, M. (2011). Striga magnibracteata (Orobanchaceae) a new species from Guinée and Mali. Kew Bull. 66: 441 – 445. https://doi.org/10.1007/s12225-011-9296-6
Gagnepain, F. (1947). Quelques espèces nouvelles des Olacacées (sensu lato). Notul. Syst. (Paris) 13: 131 – 137. https://www.biodiversitylibrary.org/page/7774310#page/413/mode/1up
Gillespie, J. W. (1932). Anacolosa lutea pp. 5 & Fig. 3. In: New Plants from Fiji – III. Bull. Bernice P. Bishop Mus. 91: 1 – 38, Fig. 1 – 43.
Gosline, G. & Malécot, V. (2011). A monograph of Octoknema (Octoknemaceae—Olacaceae s.l.). Kew Bull. 66: 367 – 404. https://doi.org/10.1007/s12225-011-9293-9
Goyder, D. J. (2008). Xysmalobium samoritourei (Apocynaceae: Asclepiadoideae), a new species from the Guinea Highlands of West Africa. Kew Bull. 63: 473 – 475. https://doi.org/10.1007/s12225-008-9059-1
Hallé, N. (1973). Crochets des lianes du Gabon: Ancistrocladus et Anacolosa (Ancistrocladacées et Olacacées). Adansonia Sér. 2, 13 (3): 299 – 306. https://www.biodiversitylibrary.org/item/281076#page/51/mode/1up
Humphreys, A. M., Govaerts, R., Ficinski, S. Z., Lughadha, E. N. & Vorontsova, M. S. (2019). Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nature Ecol. Evol. 3.7: 1043 – 1047. https://doi.org/10.1038/s41559-019-0906-2
IPNI (continuously updated). The International Plant Names Index. http://ipni.org/ [Accessed 25 April 2022].
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1. Second edition. IUCN, Gland, Cambridge. https://portals.iucn.org/library/node/10315
Kanehira, R. (1936). Anacolosa glochidiiformis pp. 601 – 604, Fig. 8. In: New or Noteworthy Trees from Micronesia XVIII. Bot. Mag. (Tokyo) 50: 599 – 607. https://doi.org/10.15281/jplantres1887.50.599
Kuijt, J. & Hansen, B. (2015). Olacaceae pp. 127 – 136. In: K. Kubitzki, The Families & Genera of Vascular Plants. XII Flowering Plants. Eudicots. Santalales, Balanophorales. Springer, Cham. https://link.springer.com/book/https://doi.org/10.1007/978-3-319-09296-6
Kurz, S. (1876). Anacolosa. J. Asiatic Soc. Bengal, Pt. 2, Nat. Hist. 44 (3): 153. https://www.biodiversitylibrary.org/item/114410#page/163/mode/1up
Lobreau-Callen, D. (1980). Caractѐres comparés du pollen des Icacinaceae et des Olacaceae. Adansonia, ser. 2, 20 (1): 29 – 89. https://www.biodiversitylibrary.org/item/281377#page/31/mode/1up
Louis, J. & Boutique, R. (1947). Une espèce nouvelle d’Anacolosa au Congo Belge. Bull. Jard. Bot. Etat. Bruxelles 18: 255 – 258.
____ & Léonard, J. (1948). Anacolosa pp. 264 – 266. In: Flore du Congo Belge 1. I.N.E.A.C., Bruxelles.
Malêcot, V. (2002). Histoire, classification et phylogénie des Olacaceae Brown (Santalales). (Doctoral dissertation, Paris 6).
____ & Nickrent, D. L. (2008). Molecular Phylogenetic relationships of Olacaceae and related Santalales. Syst. Bot. 33 (1): 97 – 106. https://doi.org/10.1600/036364408783887384
____, ____, Baas, P., van den Oever, L. & Lobreau-Callen, D. (2004). A morphological cladistic analysis of Olacaceae. Syst. Bot. 29: 569 – 586. https://doi.org/10.1600/0363644041744301
Masters, M. (1875). Anacolosa. In: J. D. Hooker (ed.), Flora of British India 1 (3): 580 – 581. Reeve & Co., London. https://www.biodiversitylibrary.org/item/61976#page/594/mode/1up
Nickrent, D. L., Malécot, V., Videl-Russell, R. & Der, J. P. (2010). A revised classification of Santalales. Taxon 59: 538 – 558. https://doi.org/10.1002/tax.592019
Nic Lughadha, E., Bachman, S. P. & Govaerts, R. (2017). Plant fates and states: Response to Pimm & Raven. Trends Ecol. Evol. 32: 887 – 889. https://doi.org/10.1016/j.tree.2017.09.005
____, ____, Leão, T. C., Forest, F., Halley, J. M., Moat, J., Acedo, C., Bacon, K. L., Brewer, R. F., Gâteblé, G. & Gonçalves, S. C. (2020). Extinction risk and threats to plants and fungi. Plants, People, Planet 2 (5): 389 – 408. https://doi.org/10.1002/ppp3.10146
____, Govaerts, R., Belyaeva, I., Black, N., Lindon, H., Allkin, R., Magill, R. E. & Nicoloson, N. (2016). Counting counts: revised estimates of numbers of accepted species of flowering plants, seed plants, vascular plants and land plants with a review of other recent estimates. Phytotaxa 272 (1): 82 – 88. https://doi.org/10.11646/phytotaxa.272.1.5
Phillips, S. M. & Mesterházy, A. (2015). Revision of small ephemeral species of Eriocaulon (Eriocaulaceae) in West Africa with long involucral bracts. Kew Bull. 70: 5. https://doi.org/10.1007/s12225-014-9557-2
____, Fofana, F. & Cheek, M. (2018). Mesanthemum tuberosum Lecomte resurrected from M. prescottianum (Bong.) Körn. (Eriocaulaceae), variation and lectotypification. Kew Bull. 73: 13. https://doi.org/10.1007/s12225-018-9744-7
Phillipson, P., Hooper, O., Haba, P., Cheek, M. & Paton, A. (2019). Three species of Coleus (Lamiaceae) from the Guinean Highlands: a new species, a new combination and clarification of Coleus splendidus. Kew Bull.74: 24. https://doi.org/10.1007/s12225-019-9812-7
Pierre, L. (1892). Anacolosa clarkii t. 266. In : Flore Forestière de la Cochinchine Vol. 3. Octave Doin, Paris. https://www.biodiversitylibrary.org/item/126010#page/168/mode/1up
Plants of the World Online (continuously updated). Facilitated by the Royal Botanic Gardens, Kew. http://www.plantsoftheworldonline.org/?f=accepted_names&q=Impatiens [Accessed 1 April 2022].
Prance, G. T. & Jongkind, C. C. H. (2015). A revision of African Lecythidaceae. Kew Bull. 70: 6. https://doi.org/10.1007/s12225-014-9547-4
Rogers, Z. S. & Malécot, V. (2021). Olacaceae and allies, pp. 215 – 338. In: J. S. Miller et al., Boraginales, Olacaceae and allies, Apiaceae. Flora of Madagascar and the Comoro Islands. Publications scientifiques du Muséum, Paris. IRD, Marseille.
Schellenberg, G. (1923). Die bis jeszt aus Neu-Guinea bekannt gewordenen Opiliaceae, Olacaceae, Icacinaceae. Bot. Jahrb. Syst. 58: 155 – 177. https://www.biodiversitylibrary.org/item/95492#page/165/mode/1up
Sleumer, H. (1935). Olacaceae. In: A. Engler & K. Prantl, Die Natürlichen Pflanzenfamilien, Ed. 2, 16b: 5 – 32. Wilhelm Engelmann, Leipzig.
____ (1980). A taxonomic account of the Olacaceae of Asia, Malesia, and the adjacent areas. Blumea 26: 145 – 168.
____ (1984). Olacaceae. In: C. G. G. J. van Steenis & W. J. J. O. de Wilde (eds), Flora Malesiana, I, 10: 1 – 29. Kluwer, Dordrecht, London.
Thiers, B. (continuously updated). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/
TIPAs Guinea-Conakry (2016 – 2019). https://www.kew.org/science/projects/tipas-guinea-conakry-2016-2019 [Accessed 30 April 2022].
Turland, N. J., Wiersema, J. H., Barrie, F. R., Greuter, W., Hawksworth, D. L., Herendeen, P. S., Knapp, S., Kusber, W-H., Li, D-Z., Marhold, K., May, T. W., McNeill, J., Monro, A. M., Prado, J., Price, M. J. & Smith, G. F. (eds) (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress. Shenzhen, China, July 2017. Regnum Veg. 159. Koeltz Botanical Books, Glashütten.
van der Burgt, X. M., Haba, P. K., Haba, P. M. & Goman, A. S. (2012). Eriosema triformum (Leguminosae: Papilionoideae), a new unifoliolate species from Guinea, West Africa. Kew Bull. 67: 263 – 271. https://doi.org/10.1007/s12225-012-9357-5
____, Haba, P. M., Magassouba, S. & Veranso-Libalah, M. C. (2022). Benna alternifolia (Melastomataceae: Sonerileae), a new herbaceous genus and species from Guinea, West Africa. Willdenowia 52: 25 – 37. https://doi.org/10.3372/wi.52.52102
____, Konomou, G., Haba, P. M. & Magassouba, S. (2019). Gladiolus mariae (Iridaceae), a new species from fire-free shrubland in the Kounounkan Massif, Guinea. Willdenowia 49: 117 – 126. https://doi.org/10.3372/wi.49.49112
____, Molmou, D., Diallo, A., Konomou, G., Haba, P. M. & Magassouba, S. (2018). Talbotiella cheekii (Leguminosae: Detarioideae), a new tree species from Guinea. Kew Bull. 73: 26. https://doi.org/10.1007/s12225-018-9755-4
Veranso-Libalah, M. C., Stone, R. D., Haba, P. M., Magassouba, S., Kadereit, G. & Van Der Burgt, X. M. (2021). Phylogenetic placement of Cailliella praerupticola (Melastomataceae), a rare, monospecific lineage from Guinea, West Africa. Willdenowia 51: 47 – 56. https://doi.org/10.3372/wi.51.51104
Villiers, J.-F. (1973). Olacaceae. In: A. Aubreville (ed.), Flore du Gabon 20: 101 – 162. Muséum national d’histoire naturelle, Paris. https://portal.cybertaxonomy.org/flore-gabon/cdm_dataportal/taxon/c20a1f4a-42f4-4418-924f-f58b7cdba103
Xanthos, M., Konomou, G., Haba, P. M. & van der Burgt, X. M. (2020). Trichanthecium tenerium (Poaceae: Panicoideae), a new species from Guinea-Conakry. Kew Bull. 75: 11. https://doi.org/10.1007/s12225-020-9864-8
____, ____, ____ & ____ (2021). Ctenium bennae (Poaceae; Chloridoideae), a new rheophytic species from Guinea-Conakry. Kew Bull. 76: 745 – 750. https://doi.org/10.1007/s12225-021-09989-6
Acknowledgements
This paper was completed as part of the Guinea Important Plant Areas programme managed by Charlotte Couch, currently supported by the Franklinia Foundation and the Critical Ecosystem Partnership Fund (CEPF).
Most of the specimens cited were collected with the support of Simfer S.A. and those collected in 2021 and 2022 under the framework of collaboration between RBG, Kew and Sylvatrop Consulting, and under the Memorandum of Collaboration between RBG, Kew and Université Gamal Abdel Nasser-Herbier National de Guinée (MINRESI), i.e. UGANC-HNG. We especially thank former Simfer Environment staff John Merry, Leon Payne, Thomas Williams, and current staff Mohamed Tahlaoui, Harry Nevard, M. Soumaoro and M. Diaby. At Sylvatrop, we thank Sylvain Dufour and especially Dr Eric Muller. The authors thank their colleagues at UGAN-C-HNG, for support, especially field assistant Aminata Thiam for her work supporting two of the co-authors at Simandou in 2021 and 2022 and Tokpa Seny Doré who provided important new data on local uses and collected fruit in June 2022 after this paper was submitted for publication. Carel Jongkind is thanked for providing his record of the species from northern Simandou.
The Bentham Moxon Trust through the efforts of Dr Isabel Larridon, supported Denise Molmou’s visit to RBG Kew in April – June 2022 during which time it was possible to complete the bioRxiv pre-print (Cheek et al. 2022) and submit the paper for publication. Two anonymous reviewers are thanked for constructive comments on an earlier draft of the paper, especially reviewer 2 who advanced many arguments that we should formally name the new genus, despite the lack of molecular data. As a result, we revised the paper to follow this guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheek, M., Molmou, D., Gosline, G. et al. Keita (Aptandraceae-Olacaceae s.l.), a new genus for African species previously ascribed to Anacolosa, including K. deniseae sp. nov., an Endangered submontane forest liana from Simandou, Republic of Guinea. Kew Bull 79, 317–332 (2024). https://doi.org/10.1007/s12225-024-10172-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-024-10172-w