Summary
Clusia reginae (Clusiaceae) is described using an integrative taxonomy approach. Field observations, discriminant analyses of morphological characters and phylogenetic inference based on molecular data led to the recognition of a new species of tree. This new taxon is distinct for having broadly obovate leaves, white petals, bright yellow flower resin and relatively large fruits. Clusia reginae is known from the foothills and valleys of the Venezuelan Andes and is here assessed using IUCN criteria as Vulnerable.
Resumen
Se describe Clusia reginae (Clusiaceae) usando métodos de taxonomía integrativa. Observaciones de campo, análisis discriminante de caracteres morfológicos y reconstrucciones filogenéticas basadas en datos moleculares permitieron reconocer esta nueva especie de árbol. El nuevo taxón se diferencia por sus hojas anchamente obovadas, pétalos blancos, resina floral amarilla brillante, y frutos relativamente grandes. Clusia reginae se conoce del piedemonte y valles de los Andes de Venezuela y siguiendo los criterios de evaluación de la UICN se estima que se encuentra en estado Vulnerable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The practice of delimiting and classifying species has changed fundamentally over time. Given the complexity of clearly defining species boundaries, integrative methods that consider multiple sources of evidence such as comparative morphology, genetics, phylogeography, ecology and behavioural biology, are becoming more widely used in modern taxonomy (Dayrat 2005; De Queiroz 2007; Padial et al. 2010). Moreover, in the context of the current global biodiversity crisis, methods that significantly reduce the time required to describe and name new species, such as DNA barcoding, are deemed to be more efficient alternatives to scientifically document species before they are lost through extinction (Goulding & Dayrat 2016).
Clusia Plum. ex L. (Clusiaceae) includes almost 400 species of woody plants that are ecologically important in the Neotropics, particularly in montane forests of the Northern Andes and Central America, where they are often locally abundant and dominate the canopy of the treeline between forests and high elevation grasslands (Gustafsson et al. 2007). Seventy-eight native species of Clusia were recognised in the most recent checklist for Venezuela (Pipoly & Cuello 2008). In addition, at least six new taxa have been described in the country (Luján 2016; Nascimento Jr. et al. 2019). As part of an ongoing taxonomic monograph of the genus Clusia, field-collected and herbarium specimens are being used in morphological and phylogenetic comparative analyses to document the diversity of the genus across its range. Here we describe a new species of Clusia from the Andes of Venezuela and provide comments on its morphological affinities, evolutionary relationships and geographic range.
Materials and Methods
Plant specimens were collected in various localities in the states of Mérida and Táchira in western Venezuela. Herbarium specimens were studied at K, MER, MERC, MO, NY, PORT and VEN (herbarium acronyms follow Thiers 2022, continuously updated). We followed terminology from Beentje (2020) to describe morphological characters.
A discriminant analysis was performed, based on morphological characteristics of the newly described species and closely related and sympatric species Clusia minor L. and C. rosiflora Planch. & Linden. ex Planch. & Triana (published as C. rosaeflora). Related species that do not co-occur with the newly described taxon, and for which fresh plant material was not available for this study, were not included in the discriminant analysis (i.e. C. decussata Ruiz & Pav. ex Planch. & Triana, C. pratensis Seem.). The characters measured were maximum length and width of the lamina, and maximum length and diameter of mature fruits. Between seven and nine mature leaves and 10 – 26 fruits from three individuals of each of the species were measured. The probability distribution of the morphological variables was estimated using the bootstrap method (Efron 1979) which considers the 95% confidence interval of the mean values. Statistical significance was estimated using Wilks's Lambda statistic. Discriminant analyses were performed using the software SYSTAT v. 13.00.05 (Systat Software Inc., Chicago, Illinois).
Molecular data from the nuclear ribosomal internal transcribed spacer marker (nrITS), generated by Luján (2016, 2019) and available at the National Center of Biotechnology Information Species (NCBI) GenBank database (http://www.ncbi.nlm.nih.gov/genbank/), were used to perform phylogenetic reconstructions. A subset of 44 nrITS sequences from taxa representing most of the subgeneric sections within Clusia were assembled and aligned using the MUSCLE algorithm with default settings as implemented in Geneious Prime ver. 2023.10.11 (Biomatters Ltd., Auckland, New Zealand). Model of evolution was estimated using ModelTest in the R package phangorn (Schliep 2011). The Akaike information criterion (AIC; Akaike 1974) was used to choose among nucleotide substitution models, and model GTR + G was selected. Maximum likelihood (ML) analysis was conducted using PhyML (Guindon et al. 2010) with 1000 nonparametric bootstrap replicates to estimate support values. Molecular data for the newly described species is deposited in NCBI GenBank with accession number MF871671.1.
Conservation status for the new species was assessed following the IUCN Red List categories and criteria (IUCN 2019). The GeoCat tool (Bachman et al. 2011) with a cell size of 2 km2, was used, following IUCN guidelines to estimate extent of occurrence (EOO) and area of occupancy (AOO). A species distribution map was generated based on specimen locality information, using QGIS Development Team (2023).
Taxonomic Treatment
Clusia reginae Paolini-Ruiz sp. nov. Type: Venezuela. Mérida: Municipio Libertador, Vía Mérida-Jají, frente al Mirador cerca del Hotel Las Lomas, borde de carretera, 08°34.365'N, 71°12.265'W, [8.5727061°N, -71.206756°W], 1541 m, 10 Aug. 2014, Luján M. & G. Ghiselli 464 (holotype MERC!; isotype MER!).
http://www.ipni.org//urn:lsid:ipni.org:names:77327634-1
Tree 3 – 7 m; branches cylindrical, epidermis on young branches exfoliating in transverse strips; exudate white, abundant. Leaves with petiole 1.5 – 2.5 cm, unwinged, base excavated and forming a pit in the adaxial portion of the junction between the petiole and the stem; lamina drying dark brown adaxially and light brown abaxially, 10.5 – 12 × 6 – 8.5 cm, broadly elliptic, base attenuate, apex rounded to obtuse, margin slightly revolute; venation pinnate brochidodromous; primary vein evident along the first half to ¾ of the length of the lamina and thinning in the distal portion; principal secondary veins 22 – 24 pairs, 1.8 – 2 mm apart, forming a 45° angle to primary vein, prominent abaxially and flat to slightly prominent adaxially; intramarginal vein <1 mm from margin; resin canals visible when dry in both lamina surfaces as thin, continuous lines arising at a very narrow angle from, but eventually more or less parallel to the secondary veins. Staminate inflorescence and flowers not seen. Pistillate inflorescences dichasial, slightly deflexed, 3 – 5 × 2 – 3 cm, with 1 – 2 ramifications per node and a total of 3 – 5 flowers, sometimes single flowers, peduncles 1 – 1.5 cm, cylindrical; bracts (subtending inflorescence branches) and bracteoles (subtending flowers) paired, 2 – 3 mm, deltate, the apex acute. Pistillate flowers with pedicel 3 – 6 mm long, cylindrical; perianth 2 – 3 cm diam.; sepals 4 – 6, light green, 4 – 5 × 5 – 6 mm, broadly ovate; petals 5 – 6, white, 1 – 1.5 × 1.5 – 2 cm, obovate to reniform; modified staminodes forming a resin-secreting ring at the base of the ovary; resin bright yellow; ovary light green, 3 – 8 × 4 – 5 mm, ovate; stigmata 6 – 7, sessile, white, obtusely triangular, surface minutely papillose (Figs 1, 2). Fruits light green, smooth-surfaced, 1.8 – 2 × 2.2 – 2.4 cm, ovoid; sepals persistent; stigmata turning black, obtusely triangular, 4 × 3 mm, persistent on fruits. Seeds 6 – 8 per locule, covered with orange-red aril.
recognition. Clusia reginae resembles C. minor but differs in having broadly elliptic leaves with 22 – 24 pairs of secondary veins (vs leaves obovate to elliptic with 15 – 20 secondary veins), flower’s resinous ring without anthers (vs ring anther-bearing), petals white (vs petals mostly pink) and fruits ovate (vs fruits spheroidal). Clusia reginae is also similar to C. rosiflora, it can be distinguished by having leaves with the apex rounded to obtuse (vs acute), white petals (vs light pink and more or less hyaline petals [Fig. 3]), bright yellow flower resin (vs red to orange flower resin [Fig. 3]) and having relatively smaller, ovoid fruits (vs slightly longer, ellipsoid fruits).
Pistillate flowers of selected species of Clusia sect. Retinostemon. A C. reginae; B C. decussata; C C. rosiflora; D C. pratensis; E – G C. minor; H C. odorata; J C. cupulata (Maguire) Maguire; K C. uvitana Pittier; L C. croatii D'Arcy; M C. mocoensis Cuatrec.; N C. pallida Engl.; P C. haughtii Cuatrec.; Q C. guabalensis Luján & J.Aranda; R C. petiolaris Planch. & Triana; S C. lineata (Benth.) Planch. & Triana; T C. diguensis Cuatrec.; U C. hirsuta Hammel; V C. radicans Pav. ex Planch. & Triana. Arrows in E – G indicate anthers embedded in the resiniferous ring. photos: m. luján; except e j. paolini-ruiz.
distribution. Clusia reginae occurs mostly in secondary vegetation and montane forest edges, between 900 – 1625 m elev., its distribution is the foothills and valleys of the Venezuelan Andes (Map 1). One collection (R. Liesner & M. Guariglia 11830) comes from a locality at a lower elevation (450 m), in the Táchira depression. The species is likely to occur in neighbouring Colombia, although specimens from that country were not located for this study.
specimens examined. venezuela. Mérida: Municipio Libertador, vía Mérida-Jají, frente al Mirador cerca del Hotel Las Lomas, borde de carretera, 08°34.365'N, 71°12.265'W, [8.5727061°N, -71.206756°W], 1541 m, 10 Aug. 2014, Luján M. & G. Ghiselli 464 (holotype MERC!; isotype MER!); Barinas: Distrito Bolívar, San Isidro, 27 km de Barinitas, 900 – 1200 m, 11 Oct. 1983, G. Aymard 2189 (VEN! [203600]); Lara: between Paso de Angostura and Parque Nacional Yacambú, 7.5 km from Paso de Angostura, 900 m, 29 Dec. 1973, J. A. Steyermark & V. Carreño Espinoza 108814 (VEN! [99018]); Lara: Distrito Jiménez: La Gran Parada, 15 km ESE of Sanare, Parque Nacional Yacambú, 69°32'W, 9°42'N, 1200 m, 28 Oct. 1982, G. Davidse & A. C. González 21296 (PORT! [22071]; VEN! [376147]); Mérida: río Chama, al borde E de Mérida, 1600 m, 10 May 1953, E. L. Little Jr. 15165 (VEN! [296067]); Mérida: 2 km SE of Ejido, 1100 m, 19 Oct. 1953, B. Maguire 39435 (VEN! [66722], NY!); Mérida: camino Mérida-El Morro, en la vertiente hacia Mérida, 1360 m, 28 Sept. 1970, Castellano 134 (VEN! [252430]); Mérida: Municipio Arzobispo Chacón, taludes a orilla del camino entre San Isidro Bajo y San Isidro Alto, arriba de Santa Cruz de Mora, 30 Aug. 1989, R. Bonaci, S. Gainze & B. Stergios 9 (VEN! [273859]); Mérida: Municipio Campo Elías, Vía Mérida-Jají, cerca de la intersección hacia El Salado, frente al restaurant Las Brisas, borde de carretera, frente al Mirador cerca del Hotel Las Lomas, borde de carretera, 8.5839103°N, -71.2261979°W, 1625 m, 10 Aug. 2014, Luján M. & G. Ghiselli 463 (MERC!); Táchira: nearly level slopes, E and in the vicinity of Michelena, 1200 m, 27 – 30 Aug. 1966, J. A. Steyermark & M. Rabe 96728 (VEN! [251512]); Táchira: 7 km W of Rubio, 7°42'N, 72°25'W, 900 – 1000 m, 18 March 1981, R. Liesner & A. González 10704 (VEN! [156366]). Táchira: Río Negro, 0.5 – 2 km from main highway, road adjacent to river, 7°35'N 72°10'W, 450 m, 5 May 1981, R. Liesner & M. Guariglia 11830 (VEN! [156360]); Táchira: Distrito Uribante, ± 3 km outside Siberia on road to Pregonero, 8°55'N, 71°40'W, 1300 m, 11 July 1983, H. van der Werff & A. González 5306 (VEN! [368404], MO!).
conservation status. The extent of occurrence (EOO) for Clusia reginae, is approximately 10,500 km2 and its area of occupancy (AOO) is 60 km2. There are 6 identified locations, some of which are geographically relatively remote which partially mitigates the risk from a single threatening event. All the specimens studied occur outside protected areas and the plants grow as part of the secondary vegetation in disturbed and ruderal habitats. Some individuals are cultivated in private gardens and others seem to be maintained in public spaces and roadsides, although the ornamental use of C. reginae does not seem to be an extended practice in the region. Given the number of known locations, the likely decline in quality of habitat due to its proximity to urban centres and that some locations are outliers, we provisionally assess the conservation status for C. reginae as Vulnerable [VU B1ab(iii) + 2ab(iii)] based on the IUCN (2012, 2019) guidelines and criteria.
etymology. The specific epithet of this new species honours Jorge Paolini-Ruiz’s wife Reyna, in recognition of her invaluable lifetime companionship.
notes. Discriminant analyses of morphological characters demonstrate that Clusia reginae can be recognised as a distinct species (Fig. 4, Appendix Table 1.). Canonical plots show that there is minimal overlap in leaf dimensions between C. reginae and the closely related and sympatric species C. minor and C. rosiflora, and no overlap in fruit dimensions between these species. This highlights that statistical analyses of morphological data provide valuable evidence for species delimitation even within closely related taxa.
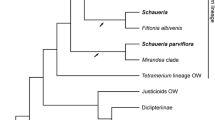
The phylogenetic analyses resulted in an alignment 739 bp long containing 310 (41.9 %) variable sites. Maximum likelihood reconstructions indicate that Clusia reginae is closely related to C. decussata, C. pratensis, C. rosiflora and C. minor, although their evolutionary relationships are unresolved. These species form a strongly supported clade within Clusia section Retinostemon Planch. & Triana (Fig. 5), which is the most species-rich section with flower resins in the genus. Species in Clusia sect. Retinostemon display an extraordinary diversity in flower morphology, with their pistillate flowers having carpels surrounded by a ring of resin-producing staminodes (Fig. 3). Clusia minor is one of the most widespread species of Clusia in the Neotropics and may represent a species complex that includes dioecious, apomictic, and apparently hermaphroditic forms (Hammel 1986). It is unknown whether anthers embedded in the resiniferous ring of Clusia minor’s flowers produce viable pollen (Fig. 3E – G). Our field observations suggest C. reginae is apomictic, like C. pratensis, in which no staminate individuals have been identified. Detailed anatomical examination of flowers within the C. minor complex is needed to assess the variation in pollen viability across closely related species.
Phylogenetic relationships among Clusia species, based on maximum likelihood analysis of nrITS. Species names are followed by NCBI accession numbers. Clusia reginae is marked in red, the Clusia minor species complex is highlighted in orange and Clusia sect. Retinostemon in grey. Bootstrap values >70% are indicated above the branches, lower values are not shown. Insert in the lower left corner shows a cladogram with untransformed branches, with the scale bar indicating substitutions per site.
The production of flower resins as a reward for pollinators is an unusual trait, present in a reduced number of flowering plant groups (Armbruster 1984). Flower resins, as well as resins from vegetative parts, are collected by social stingless bees (Meliponini) for nest construction and defence against predators and pathogens (Shanahan & Spivak 2021). The main components of Clusia flower resins are polyisoprenylated benzophenones, although this has only been determined from a limited number of species included in Clusia sections Chlamydoclusia Engl., Cordylandra Planch. & Triana, and Phloianthera Planch. & Triana, including C. grandiflora Splitg., C. nemorosa G.Mey and C. rosea Jacq. (de Oliveira et al. 1996; Porto et al. 2000). A comparative analysis of the flower resin’s biochemical composition across Clusia species, including taxa from Clusia sect. Retinostemon, such as C. reginae, is warranted to assess the variation of this trait across species in the genus and to better understand its ecological and evolutionary relevance.
References
Akaike, H. (1974). Automatic control: A new look at the statistical model identification. IEEE. Transactions on Biomedical Engineering 19: 716 – 723. https://doi.org/10.1109/TAC.1974.1100705.
Armbruster, W. S. (1984). The role of resin in angiosperm pollination: ecological and chemical considerations. Amer. J. Bot. 71: 1149 – 1160. https://doi.org/10.1002/j.1537-2197.1984.tb11968.x.
Bachman, S., Moat, J., Hill, A. W., de la Torre, J. & Scott, B. (2011). Supporting Red List threat assessments with GeoCAT: geospatial conservation assessment tool. In: V. Smith & L. Penev (eds), e-Infrastructures for data publishing in biodiversity science. ZooKeys 150: 117 – 126. https://doi.org/10.3897/zookeys.150.2109.
Beentje, H. (2020). The Kew Plant Glossary: an illustrated dictionary of plant terms (Second Edition). Royal Botanic Gardens, Kew.
Dayrat, B. (2005). Towards integrative taxonomy. Biol. J. Linn. Soc. 85: 407 – 417. https://doi.org/10.1111/j.1095-8312.2005.00503.x.
de Oliveira, C. M., Porto, A., Bittrich, V., Vencato, I. & Marsaioli, A. J. (1996). Floral resins of Clusia spp.: chemical composition and biological function. Tetrahedron Lett. 37: 6427 – 6430. https://doi.org/10.1016/0040-4039(96)00656-9.
De Queiroz, K. (2007). Species concepts and species delimitation. Syst. Biol. 56: 879 – 886. https://doi.org/10.1080/10635150701701083.
Efron, B. (1979). Bootstrap methods: another look at the jackknife. Annals of Statistics 7: 1 – 26. https://doi.org/10.1214/aos/1176344552. https://www.jstor.org/stable/2958830
Goulding, T. C. & Dayrat, B. (2016). Integrative taxonomy: ten years of practice and looking into the future. Archives of the Zoological Museum Lomonosov Moscow State Univ. 54: 116 – 133.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59: 307 – 321. https://doi.org/10.1093/sysbio/syq010.
Gustafsson, M. H. G., Winter, K. & Bittrich, V. (2007). Diversity, phylogeny and classification of Clusia. Pp. 95 – 116. In: U. Luttge (ed.), Clusia: a woody Neotropical genus of remarkable plasticity and diversity. Springer, Berlin / Heidelberg. https://doi.org/10.1007/978-3-540-37243-1_7.
Hammel, B. E. (1986). New species of Clusiaceae from Central America with notes on Clusia and synonymy in the tribe Clusieae. Selbyana 9: 112 – 120. https://www.jstor.org/stable/41888793.
IUCN (2012). IUCN Red List Categories and Criteria, version 3.1. IUCN Species Survival Commission. International Union for Conservation of Nature, Gland and Cambridge. Available from: http://cmsdocs.s3.amazonaws.com/keydocuments/Categories_and_Criteria_en_web%2Bcover%2Bbckcover.pdf
IUCN (2019). Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prepared by the IUCN Standards and Petitions Subcommittee. International Union for Conservation of Nature, Gland and Cambridge. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf. [Accessed 30 April 2023].
Luján, M. (2016). Clusia scariosepala (Clusiaceae), a distinct species of Clusia sect. Anandrogyne endemic to the Venezuelan Andes. Harvard Pap. Bot. 21: 137 – 140. https://www.jstor.org/stable/90000318.
____ (2019). Playing the taxonomic cupid: matching pistillate and staminate conspecifics in dioecious Clusia (Clusiaceae). Syst. Bot. 44: 548 – 559. https://doi.org/10.1600/036364419x15620113920590.
Nascimento Jr., J. E. do, Bittrich, V. & Amaral, M. (2019). Taxonomic novelties in Clusia (Clusiaceae) from Venezuela. Phytotaxa 400: 191 – 202. https://doi.org/10.11646/phytotaxa.400.3.6.
Padial, J. M., Miralles, A., De la Riva, I. & Vences, M. (2010). The integrative future of taxonomy. Frontiers in Zoology 7: 1 – 14. https://doi.org/10.1186/1742-9994-7-16.
Pipoly, J. & Cuello, N. (2008). Clusiaceae. Pp. 330 – 335. In: O. Hokche, P. E. Berry & O. Huber (eds), Nuevo catálogo de la flora vascular de Venezuela. Fundación Instituto Botánico de Venezuela, Dr. Tobías Lasser, Caracas.
Porto, A. L., Machado, S. M., de Oliveira, C. M., Bittrich, V., Amaral, M. do C. E. & Marsaioli, A. J. (2000). Polyisoprenylated benzophenones from Clusia floral resins. Phytochemistry 55: 755 – 768. https://doi.org/10.1016/s0031-9422(00)00292-2.
QGIS Development Team (2023). QGIS Geographic Information System. Version 3.32. Lima. Open Source Geospatial Foundation Project. Available from: http://qgis.osgeo.org. [Accessed 1 Oct. 2023].
Schliep, K. (2011). Phangorn: phylogenetic analysis in R. Bioinformatics 27: 592 – 593. https://doi.org/10.1093/bioinformatics/btq706.
Shanahan, M. & Spivak, M. (2021). Resin use by stingless bees: a review. Insects 12: 719. https://doi.org/10.3390/insects12080719.
Thiers, B. (2022, continuously updated). Index Herbariorum: a global directory of public herbaria and associated staff. Available from: http://sweetgum.nybg.org/science/ih/. [Accessed 9 May 2023].
Acknowledgements
The authors are thankful to Ana Escalona (MERC), Ana M. Torres and Adela Ortega (MER) for their generous assistance during herbarium work in Mérida, and to Gabriella Ghiselli for her kind assistance during fieldwork in Venezuela. Fieldwork in Venezuela was generously supported by a grant given to ML by the California Botanic Garden’s graduate research fund. Two anonymous reviewers provided helpful suggestions for manuscript improvement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luján, M., Paolini-Ruiz, J., Sanoja, E. et al. Integrative taxonomy led to recognising Clusia reginae (Clusiaceae), a new tree species from the Venezuelan Andes. Kew Bull 79, 191–200 (2024). https://doi.org/10.1007/s12225-023-10150-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-023-10150-8