Summary
We describe six new species in the neotropical fern genus Danaea Sm. (Marattiaceae). These are D. alansmithii Tuomisto & Keskiniva in subgenus Arthrodanaea and D. gracilis Tuomisto & Keskiniva, D. lanceolata Tuomisto & Keskiniva, D. stricta Tuomisto & Keskiniva, D. tenuicaulis Tuomisto & Keskiniva, and D. vanderwerffii Tuomisto & Keskiniva in subgenus Holodanaea. Recent synonymisations in the genus are discussed, a former synonymisation of D. lucens A.Rojas is reverted and D. quebradensis Christenh. is synonymised under D. lucens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fern genus Danaea Sm. (Marattiaceae) has a distribution spanning most of the Neotropics, from Veracruz in southern Mexico to Bolivia and southern Atlantic Brazil (Tryon & Tryon 1982). Danaea can be found from sea level up to 2600 m but the highest species richness seems to be between 100 and 1000 m (Christenhusz 2006). Danaea species occur in rain forests and cloud forests, often on steep slopes and well drained soils, but some species occur along creeks or in swamps. The genus has been in taxonomic flux, as authors have disagreed about species delimitations. Tuomisto & Moran (2001) recognised 18 species for Ecuador alone, and described eight species as new. Rolleri (2004) preferred extremely broad species circumscriptions and recognised only 18 species for the entire genus. She lumped many species under D. elliptica Sm., D. moritziana C.Presl and D. nodosa (Sm.) L., including seven of the eight species that had been described by Tuomisto & Moran (2001). However, field trips and herbarium work continued to uncover new diversity in the genus in the next few years, resulting in the description of another 19 new species by Christenhusz (Christenhusz 2006; Christenhusz & Tuomisto 2006; Christenhusz 2010; Christenhusz et al. 2018) and four new species by Rojas-Alvarado (2006, 2009). The overview provided by Christenhusz (2010) recognised 48 species. He considered all the species that had been described by Tuomisto & Moran (2001) as valid, thereby reverting their synonymisation that had been proposed by Rolleri (2004). However, at the same time Christenhusz (2010) synonymised all four species that had been described by Rojas-Alvarado (2006, 2009). A few years later, Rojas-Alvarado (2013) described another three new species and reinstated the four he had described previously. Here we contribute to the discussion on species delimitations and some of the recent synonymisations. In addition, we describe six new species, one in subgen. Arthrodanaea C.Presl and five in subgen. Holodanaea C.Presl. The species descriptions are primarily based on morphology, but a new phylogeny (to be published elsewhere when finished) provides additional support for all those for which material has been available for DNA sequencing.
New taxa
Danaea alansmithii Tuomisto & Keskiniva sp. nov. (subgen. Arthrodanaea C.Presl). Type: Colombia, Amazonas, H. Tuomisto 12340 (holotype COAH!; isotypes TUR!, UC!).
http://www.ipni.org/urn:lsid:ipni.org:names:77295613-1
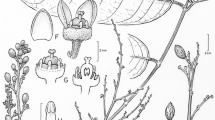
Rhizomes erect, slender, 0.9 – 1.7 cm in diam. when dry, 30 – 80 cm long, leaves arranged spirally. Sterile leaves 32 – 60 cm long; petioles 11 – 30 cm long, with 0 – 2 nodes, winged adaxially from the node upwards, wings to 1 mm wide, petiole bases sparsely scaly, scales dark brown; laminae 19 – 33 × 13 – 27 cm, 3 – 5 pinna pairs, imparipinnate, terminal pinna usually of equal size with lateral pinnae, proximal pinnae sometimes smaller than the others, lamina rather thin in texture, uniformly reddish dark brown when dry, costae and rachises very dark, rachises winged adaxially, wings to 1 mm wide, rachises not scaly; terminal pinna 10.4 – 17.5 × 2.1 – 4.7 cm, elliptic to slightly obovate, bases acute, apex acuminate, apical margins entire to slightly sinuate; largest lateral pinna 9.7 – 17 × 2.1 – 4.1 cm, pinnae elliptic to slightly obovate, ascending, petiolulate proximally, sessile distally, bases concave distally, slightly auriculate proximally but often symmetrical in the lowest pinna pairs, pinna apex 1.5 – 3.5 cm long, acuminate-caudate, apical margins entire to slightly sinuate, veins 9 – 11 per cm, mostly simple or paired at the costa. Fertile leaves 49 – 60 cm long; petioles 24 – 38 cm long, 1 node, winged adaxially from the node upwards, wings to 1 mm wide, petiole bases sparsely scaly adaxially; laminae 13 – 23 × 13 – 18 cm, 3 – 4 pinna pairs, imparipinnate, terminal pinna of equal size with lateral pinnae, costae and rachises very dark brown, sterile zone of 1 – 4 mm on each side of the costa, rachises winged adaxially, wings to 1 mm wide, rachises not scaly; terminal pinna 10.4 – 17.5 × 2.1 – 4.7 cm, narrowly elliptic to lanceolate, bases acute, apex acuminate, margins entire; largest lateral pinna 8.5 – 14.5 × 1.4 – 2.6 cm, pinnae narrowly elliptic to lanceolate, bases concave distally, auriculate proximally but sometimes symmetrical in the proximal pinna pair, apex acuminate, margins entire. Juveniles often more greenish in colour, longest simple juvenile 21 cm long, smallest pinnate juvenile 6 cm long, terminal pinna largest, oblong to elliptic, lateral pinnae elliptic. Figs 1A – F, 2, 3.
A – F Danaea alansmithii: A plant with fertile leaf in black; B medial fertile pinna; C medial pinna apex; D juvenile; E juvenile; F rhizome. G Danaea lingua-cervina Christenh. & Tuomisto from Loreto, Peru: medial fertile pinna. A – C H. Tuomisto 12340, TUR; D H. Tuomisto 12511, COAH; E H. Tuomisto 12511, TUR; F H. Tuomisto 12539, TUR; G H. Tuomisto 15127, TUR. drawn by janina keskiniva.
recognition. Danaea alansmithii is unique in that the synangia do not reach the costa but leave an irregular sterile zone of 2 – 7 mm width in the middle of the fertile pinnae. The synangia are also unusually slender and widely spaced. The leaf dimorphism in D. alansmithii is not as pronounced as in other species of Danaea, with fertile pinnae almost the same size as the sterile pinnae. The sterile leaves of D. alansmithii resemble those of D. lingua-cervina Christenh. & Tuomisto, D. arbuscula Christenh. & Tuomisto and D. bipinnata Tuomisto but are more parallel-sided (vs clearly broadest at or above the middle) and obtain a reddish-brown colour with almost black rachises and costae when dried. The other three species have paler rachises and generally remain greener, except D. arbuscula, which often dries very dark brown. Danaea arbuscula differs further in having a thicker lamina texture, and D. lingua-cervina in that the terminal pinna is generally clearly larger than the lateral ones.
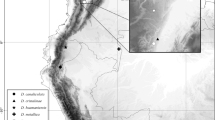
distribution. Only known from Colombian Amazonia (Amazonas). Map 1.
specimens examined. colombia. Amazonas: Near mouth of Río Popeyacá, 0°20'S 70°30'W, 200 m, 22 – 26 Feb. 1952, R. E. Schultes 15583 (COL! (2), U!, US!); Pto. Santander, Quebrada La Manchurria, 15 Aug. 1998, R. Alfonso 117 (COL!); Río Caquetá, Villa Azul, 3 Sept. 1998, R. Alfonso 449 (COL!); Río Caquetá, E of río Metá and 6 km N of its confluence with Caquetá, 0°53'S 71°36'W, 150 – 250 m, 22 April 1998, H. Tuomisto 12048 (AAU!, COAH!, TUR!, U!, UC!); Río Caquetá, 3 km NW of the mouth of río Oso, 1°7'S 71°35'W, 150 – 250 m, 26 April 1998, H. Tuomisto 12206 (AAU!, COAH! (3), MO!, TUR! (2), UC!, Z!); Río Caquetá, 0.5 – 6 km SE of Estrecho, 0°59'S 71°31'W, 150 – 250 m, 30 April 1998, H. Tuomisto 12259 (COAH!, TUR!); Río Caquetá, 0.5 – 6 km SE of Estrecho, 0°59'S 71°31'W, 150 – 250 m, 30 April 1998, H. Tuomisto 12261 (COAH!, TUR!); Río Caquetá, 6 – 7 km SE of Estrecho, 1°0'S 71°30'W, 150 – 250 m, 1 May 1998, H. Tuomisto 12268 (COAH!, TUR!); Río Caquetá, 6 – 7 km SE of Estrecho, 1°0'S 71°30'W, 150 – 250 m, 1 May 1998, H. Tuomisto 12269 (COAH!, TUR!, UC!); Río Caquetá, 2 km ESE of the confluence of río Culebra and Caquetá, 0°59'S 71°44'W, 150 – 250 m, 18 May 1998, H. Tuomisto 12340 (holotype COAH!, isotypes TUR!, UC!); 0 – 6 km SW of the confluence of Quebradón Oso with Río Caquetá, 1°9'S 71°36'W, 150 – 250 m, 25 May 1998, H. Tuomisto 12511 (COAH!, TUR!); 8 – 13 km SW of the confluence of Quebradón Oso with Río Caquetá, 1°12'S 71°39'W, 150 – 250 m, 30 May 1998, H. Tuomisto 12539 (COAH!, TUR!).
habitat. Grows in tall terra firme rainforest at 150 – 250 m elevation on well-drained sites such as slopes in dissected and undulating terrain.
conservation status. We estimate that Danaea alansmithii belongs in the Least concern category (IUCN 2012). Danaea alansmithii has a known Area of Occupancy of 28 km2 and an Extent of Occurrence of 478 km2, which correspond to the EN category (IUCN 2012). However, the species is locally very abundant and similar forest apparently extends far beyond the observed area.
etymology. Named in honour of Alan R. Smith, who has made huge contributions to fern systematics in general and has generously provided personal advice and help with species identification and other taxonomic problems.
notes. Danaea alansmithii is unique in having a 2 – 7 mm wide sterile zone around the costae of fertile pinnae. Sometimes a sterile zone can appear in incompletely fertile pinnae of other species, but in D. alansmithii this trait is consistently present. The synangia are also rather thin and widely spaced, which contributes to the characteristic appearance of the fertile leaves. The leaf dimorphism in D. alansmithii is also less pronounced than in other species of Danaea, with fertile pinnae almost the same size as the sterile ones. Although most of the known specimens of D. alansmithii come from a relatively small area along the Caquetá river in Colombian Amazonia, in that area the species was found to be locally extremely abundant (Fig. 3). It was mostly found on slopes and other well-drained sites, in contrast to D. lingua-cervina, which occurred in the same area but was restricted to wetter sites. Morphologically D. lingua-cervina differs in having a larger terminal pinna (larger than the lateral pinnae vs all equal in size in D. alansmithii) and remaining greener when dried (vs becoming dark reddish-brown with blackish costae and rachis). In addition, D. lingua-cervina grows larger than D. alansmithii before producing pinnate leaves, with simple juveniles of up to 40 cm long (vs up to 21 cm long) and lateral pinnae not produced in leaves under 22 cm long (vs smallest pinnate leaves 6 cm long). Danaea arbuscula shares a dark brown lamina colour in dried samples, but lacks the reddish tint of D. alansmithii and also has a thicker lamina texture and more broadly elliptic pinna shape. Danaea bipinnata dries to a dark green colour and has a rachis without wings or thinly winged in the distal part of the lamina (vs rachis with wings to 1 mm wide) and more broadly elliptic to oblanceolate pinnae. Bipinnate leaves are often present in D. bipinnata, but were found on only one individual in a very large population of D. alansmithii (H. Tuomisto 12269). Danaea leprieurii Kunze is a smaller plant (leaves 18 – 38 cm vs 32 – 60 cm long) with smaller pinnae (6 – 10 × 1.4 – 2.8 cm vs 10 – 17 × 2.1 – 4.1 cm), and it usually has more nodes on the petiole (2 – 3 vs 0 – 2), and can be readily separated in the field by its yellow-green (vs dark green) colour. Juveniles of D. bipinnata and D. leprieurii usually start to produce pinnate leaves at a smaller size than D. alansmithii, as their simple leaves do not exceed 13 cm in length (vs up to 21 cm long).
Danaea gracilis Tuomisto & Keskiniva sp. nov. (subgen. Holodanaea C.Presl). Type: Colombia: Chocó, O. L. Haught 5452 (holotype COL!; isotypes G!, NY!, US!).
http://www.ipni.org/urn:lsid:ipni.org:names:77295614-1
Small plants with sterile leaves arranged in a rosette and erect fertile leaves up to twice the length of the sterile leaves. Rhizomes creeping, 0.5 – 1 cm in diam. when dry, to 6 cm long, with leaf bases arranged radially. Sterile leaves 19 – 32 cm long; petioles 4 – 12 cm long, with 0 – 1 (– 2) nodes, winged adaxially, wings to 1 mm wide but indistinct in the proximal part of larger plants, petioles very scaly proximally, scales atrocastaneous to brown; laminae 14 – 24 × 6 – 12 cm, 19 – 25 pinna pairs, medial pinnae 0.6 – 1.3 cm apart, paripinnate with the terminal pinnae replaced by buds that may grow into plantlets while the leaf is still vigorous, laminae lanceolate to parallel-sided, thin and slightly translucent, uniformly dark green in colour; rachises winged adaxially, wings to 1 mm wide, narrowest at nodes, rachises and costae scaly abaxially; largest lateral pinna 3 – 5.9 × 0.7 – 1.2 cm, pinnae linear-oblong to slightly oblanceolate, perpendicular to the rachis, not or only very slightly falcate distally, petiolulate proximally, sessile distally, bases truncate to obtuse, symmetrical in basal pinnae and increasingly concave in more distal pinnae, apices obtuse to acute, apical margins slightly crenulate to serrulate, veins 12 – 20 per cm, mostly paired at the costa. Fertile leaves to 40 cm long, linear-lanceolate; petiole without nodes, to 26 cm long; laminae paripinnate, to 15 cm long; largest pinna 1.6 – 2.7 × 0.2 – 0.4 cm, linear-oblong. Juveniles with pinnate leaves produced at a very small size (2.5 cm). Figs 4A – D & 5.
A – D Danaea gracilis: A sterile leaf (J. A. Duke 15321, US); B medial pinnae (L. O. Haught 5452, G); C rhizome (C. Whitefoord 203, BM); D whole plant with fertile pinnae in black (B. Øllgaard 105343, TUR). E Danaea wendlandii Rchb.f. from Limón, Costa Rica (L. D. Gómez 23363, BM): medial pinnae. drawn by janina keskiniva.
recognition. Danaea gracilis is most similar to D. wendlandii Rchb.f., D. lanceolata and D. chococola Christenh. but can be distinguished from all of these by its lamina being very thin in texture and almost translucent (vs clearly opaque) and uniformly dark (vs usually darker adaxially and paler abaxially). Danaea gracilis also differs from the three other species in having symmetrical medial pinna bases and the pinnae being perpendicular to the rachis (vs asymmetrical medial pinna bases and ascending pinnae). Danaea gracilis further differs from D. wendlandii in having more pinnae (19 – 25 vs 10 – 15 pairs) that are generally narrower in shape (4 – 5 times vs 2 – 4 times as long as wide). Danaea gracilis differs from Danaea lanceolata in having more pinnae (19 – 25 vs 13 – 19 pairs) that are generally smaller (3 – 5.9 × 0.7 – 1.2 cm vs 4.2 – 14 × 1 – 2.4 cm), more crowded (medial pinnae 0.7 – 1.3 cm vs 1.0 – 2.3 cm apart), always linear (vs usually falcate) and have obtuse to acute (vs acute-acuminate) pinna apices. Danaea gracilis differs from D. chococola in having terminal pinnae replaced by a bud (vs usually present) and shorter petioles (4 – 12 cm vs 12 – 17 cm) with fewer nodes (0 – 2 vs 3 – 4).
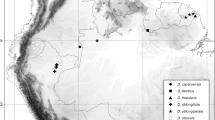
distribution. Known from moist lowland forests along the Pacific coast of northern South America from Ecuador (Esmeraldas, Pichincha) through Colombia (Chocó, Antioquia) to adjacent Panama (Darién). Map 2.
specimens examined. colombia. Antioquia: Río Guapá, 7°36'N 76°40'W, 100 m, 13 May 1945, O. L. Haught 4662 (COL!, NY!, S); Between Río Guapá and León, 100 m, March 1948, E. Ruiz Landa 18C362 (US!). 20 July 1880, W. Kalbreyer 1822 (K!). Chocó: Cabita Bay, Cape Corrientes, 13 Feb. 1934, W. R. Taylor 1269 (US!); Port Utria, 0 – 150 m, 14 Feb. 1934, W. R. Taylor 1294 (UC!); Río Catripe, 100 m, 17 Jan. 1947, O. L. Haught 5452 (holotype COL!, isotypes G!, NY!, US!); Bahía Solano; near Ciudad Mutis, 0 – 100 m, 21 – 23 Feb. 1939, E. P. Killip 33625 (US!); Río Nuquí, 6°4'N 77°11'W, 200 m, 23 Jan. 1947, O. L. Haught 5471 (COL!, F!, S, US!); Río San Juan, 3.5 km SW Andagoya, 5°4'N 76°43'W, 50 m, 24 Feb. 1971, D. B. Lellinger 497 (COL!, US!); Bahía Solano, W Pto. Mutis, road to Miniquia, 6°7'N 77°21'W, 0 – 100 m, 26 Jan. 1971, D. B. Lellinger 29 (COL!, US!); Nuquí, Alto del Buey, 1000 m, May 1940, K. von Sneidern A 20 (C!, GH!, S, WAG!); Río Truando, 2 km SSW Río Mercua confluence, 7°1'N 77°29'W, 100 m, 4 March 1971, D. B. Lellinger 577 (COL!, US!); Río Mutatá c. 3 km above its junction with Río El Valle, NW of Alto del Buey, 7°14'N 76°26'W, 100 m, 7 Feb. 1971, D. B. Lellinger 183 (COL!, US!). Peak over Río Curundú, 550 m, March 1968, J. A. Duke 15321 (MO, US!); Loma del Cuchillo, c. 15 km WSW Chigorodó, 7°27'N 76°50'W, 150 – 400 m, 9 March 1971, D. B. Lellinger 651 (COL!, US!). ecuador. Esmeraldas: Río Cayapa, Zapallo Grande, 0°48'N 78°55'W, 100 m, 1 – 2 Aug. 1982, L. P. Kvist 40815 (AAU!, QCA!); Cerro de Río Bravo de Cayapas, 0°41'N 78°56'W, 250 m, 1 Sept. 1980, L. B. Holm-Nielsen 25529 (AAU!, QCA!); Río San Miguel, one hour upstream from San Miguel de Cayapas, 0°43'N 78°52'W, 200 m, 1 Sept. 1980, L. B. Holm-Nielsen 25459 (AAU!, QCA!); Río Cayapa, Zapallo Grande, 0°47'N 78°59'W, 200 m, 11 – 15 Oct. 1983, A. Barfod 48062 (AAU!, QCA!); Lita – San Lorenzo road, 1°4'N 78°39'W, 150 m, 3 Nov. 1994, B. Øllgaard 105343 (AAU, QCA!, TUR! (2)); Pueblo San Miguel, 0°45'N 78°54'W, 200 m, 31 Aug. 1980, L. B. Holm-Nielsen 25377 (AAU! GH!, NY!, QCA!, UC!). Pichincha: Quito – Puerto Quito road, km 113, 10 km N of the main road, 0°5'N 79°2'W, 800 m, 28 April 1984, A. Arguello 287 (QCA!); NE of Pedro Vicente Maldonado, km 113 on Quito – Puerto Quito road, 0°4'N 79°1'W, 600 m, July 1994, H. Navarrete 624 (AAU, QCA!). panama. Darién: Cana – Cuasi Trail, 600 – 650 m, 12 March 1940, R. G. Terry 1519 (F!); Río Cocalito, 250 m, 15 Feb. 1982, C. Whitefoord 203 (BM!, PMA!); Cap Corrientes, Feb. 1848, B. C. Seemann 996 (BM!, K! (2)).
habitat. Grows mostly in lowland forests below 600 (– 1000) m elevation. Has often been found on clay soil in shade, can also be found in disturbed forests.
conservation status. We estimate that Danaea gracilis belongs in the Least concern category (LC) (IUCN 2012). Danaea gracilis has an Area of Occupancy of 68 km2, which corresponds to the EN category, and an Extent of Occurrence of 103,364 km2, which corresponds to the LC category, with some collection locations inside protected areas (Darién National Park in Panama and Utría National Natural Park in Colombia). The large number of collections suggests that D. gracilis is relatively abundant throughout its distribution, and there is no plausible threat to all of the subpopulations.
etymology. Named for the slender thin-textured pinnae and the generally graceful appearance of the leaves.
notes. Danaea gracilis is a small fern that has generally been identified as D. wendlandii Rchb.f. in herbaria. This was also done by Tuomisto & Moran (2001): we identify all the specimens they cited as examples of D. wendlandii in Ecuador as D. gracilis. Another similar species is Danaea lanceolata, but both of these have fewer pinnae (at most 19 vs 19 – 25 in D. gracilis) and medial pinnae that are ascending and asymmetrical at the base (vs perpendicular to the rachis and symmetrical at the base in D. gracilis). The three species differ in geographical range: Danaea gracilis occurs along the Pacific coast of South America from Ecuador to Colombia and into southeastern Panama, D. wendlandii occurs in Central America from western Panama to Costa Rica and D. lanceolata is confined to northwestern Amazonia (Map 2). Danaea chococola Christenh. occurs in the same geographical area as D. gracilis, but differs in having terminal pinnae usually present (vs replaced by a bud in D. gracilis), strongly asymmetrical pinna bases (vs symmetrical in D. gracilis), and longer petioles (12 – 17 cm vs 4 – 12 cm) with more nodes (3 – 4 vs 0 – 2). One of the paratypes of D. gracilis was cited as a syntype of Danaea humilis T.Moore (B. C. Seemann 996), which differs in having strongly bicolorous laminae (dark adaxially, whitish abaxially vs concolorous), the terminal pinna usually being present, lateral pinnae having clearly asymmetrical bases, and petioles being generally longer (8 – 14 vs 4 – 12 cm) and having more nodes (1 – 3 vs 0 – 2). Danaea imbricata Tuomisto & R.C.Moran and Danaea trichomanoides Spruce ex T.Moore share the thin lamina texture but differ clearly in pinna shape, these being much shorter (2 – 3 times as long as wide vs 4 – 5 times in D. gracilis). In addition, D. trichomanoides has ovate to obovate pinnae with sinuate to widely denticulate apical margins and simple venation (vs mostly forked in D. gracilis), and D. imbricata has ovate, overlapping pinnae which are coarsely serrulate to crenulate at apex, and veins forked well above their base (vs at the costa in D. gracilis).
Danaea lanceolata Tuomisto & Keskiniva sp. nov. (subgen. Holodanaea C.Presl). Type: Ecuador: Napo, H. Tuomisto 10686 (holotype QCA!; isotypes AAU!, QCNE, TUR!).
http://www.ipni.org/urn:lsid:ipni.org:names:77295615-1
Small to intermediate plants with sterile leaves arranged in a rosette and erect fertile leaves up to twice the length of the sterile leaves. Rhizomes creeping to ascending, 0.5 – 1.5 cm in diam. when dry, to 7 cm long, with leaf bases arranged spirally. Sterile leaves 19 – 50 cm long; petioles 3 – 14 cm long with 0 – 2 nodes, petioles winged adaxially, in distal part wings 1 mm wide, indistinct in proximal part, petioles very scaly abaxially, scales atrocastaneous to brown; laminae 15 – 36 × 6 – 18 cm, 13 – 19 pinna pairs, medial pinnae 1.0 – 2.3 cm apart, usually paripinnate with the terminal pinna replaced by a bud, lamina lanceolate, thin in texture but opaque, bicolorous, dark green adaxially, paler abaxially; rachises winged adaxially, wings to 1 mm wide, narrowest at nodes, rachises and costae very scaly abaxially; terminal pinna 0.5 – 5.5 × 0.6 – 1.6 cm, lanceolate, bases cuneate to acute, apices gradually tapering, acute to acuminate, apical margins crenulate; largest lateral pinna 4.2 – 14 × 1 – 2.4 cm, pinnae lanceolate to parallel-sided, ascending, falcate (sometimes only slightly so), petiolulate proximally, sessile distally, bases asymmetrical, truncate, increasingly concave in more distal pinnae, pinna apices obtuse in proximal pinnae, acute-acuminate in distal pinnae, tip 0.2 – 2 cm long, apical margins slightly crenulate to serrulate; veins 10 – 15 per cm, veins bifurcated or simple with proportions variable both within and among individual plants. Fertile leaves 16 – 52 cm long, linear-lanceolate; petiole without nodes, 6 – 29 cm long; laminae 8.5 – 33 × 3 – 8.6 cm, 7 – 18 pinna pairs, usually paripinnate with terminal pinna replaced by a bud; terminal pinna c. 2.1 × 0.5 cm; largest lateral pinna 1.5 – 4.3 × 0.3 – 0.7 cm, pinnae linear-oblong. Juvenile laminae parallel sided in shape, pinnae rounder, often with terminal pinna present but interrupted by a bud, longest simple juvenile 2.5 cm. Figs 6A – F & 7.
A – F Danaea lanceolata: A rhizome; B juvenile; C sterile leaf; D medial pinna; E whole young plant; F fertile leaf. G Danaea oblanceolata Stolze from Pasco, Peru: medial pinna. A J. A. Ewan 16771, UC; B, E H. Tuomisto 10624, TUR; C R. C. Moran 6035, TUR; D R. C. Moran 6124, TUR; F B. Øllgaard 1384, AAU; G E. P. Killip 26777, US. drawn by janina keskiniva.
recognition. Danaea lanceolata is most similar to D. oblanceolata Stolze, D. wendlandii Rchb.f. and D. gracilis. Danaea lanceolata differs from D. oblanceolata in having generally more pinnae (13 – 19 vs 11 – 14 pairs) that are ascending (vs perpendicular to the rachis) in addition to being lanceolate to parallel-sided in shape (vs oblanceolate) and generally narrower (1.0 – 2.4 cm vs 1.9 – 2.7 cm wide) with asymmetrical bases (vs symmetrical) and acute-acuminate apices (vs short-caudate) that are usually clearly falcate (vs only slightly falcate). Danaea lanceolata differs from D. gracilis in having opaque bicolorous laminae (vs translucent and uniformly green), fewer pinnae (10 – 19 vs 19 – 25 pairs) that are less crowded (medial pinnae 1.0 – 2.3 cm vs 0.6 – 1.3 cm apart) and ascending (vs perpendicular to the rachis), pinna apices usually being gradually tapering (vs obtuse to acute), clearly falcate (vs only slightly falcate), and medial pinna bases being asymmetrical (vs symmetrical). Danaea lanceolata differs from D. wendlandii in being generally larger (leaves 19 – 59 × 6 – 18 cm vs 13 – 27 × 5 – 10 cm) with more pinnae (13 – 19 vs 10 – 15 pairs) that are longer (4.2 – 14 cm vs 2.4 – 5 cm) and lanceolate to parallel-sided (vs parallel-sided), with acute-acuminate apices (vs obtuse to acute).
distribution. Found in northwestern Amazonia from Ecuador to Colombia (Putumayo) and northern Peru (Loreto). Map 2.
specimens examined. colombia. Putumayo: Oreto on Río Oretopungo, 450 m, 14 Jan. 1945, J. A. Ewan 16771 (BM!, GH!, UC!, US!). ecuador. Morona-Santiago: Taisha, Río Panguientza about 5 km NW of the military camp, 2°23'S 77°30'W, 250 – 300 m, 21 June 1980, J. Brandbyge 32190 (AAU!, QCA!, UC!); Patuca – Santiago road, km 44, 3°1'S 78°12'W, 800 – 950 m, 12 – 14 March 1998, B. Øllgaard 2907 (AAU!, QCA!). Napo: Yasuní National Park, 1°4'S 76°13'W, 200 – 300 m, 26 Feb. 1998, H. Tuomisto 11615 (QCA, QCNE, TUR!); Yasuní National Park, 1 km E of Estacion Cientifica Yasuni, 0°40'S 76°28'W, 10 April 1996, R. C. Moran 6035 (AAU! (2), TUR! (4), QCA!, but not NY! which is Danaea acuminata Tuomisto & R.C.Moran); Cerro Antisana, Talag, 15 km SSW from Tena, 11 July 1960, P. J. Grubb 130 (BM!, NY!, US!); Yasuní National Park, 1 km N of Río Tivacuno, 0°40'S 76°26'W, 200 m, 13 April 1996, R. C. Moran 6124 (AAU, NY! (2), QCA, QCNE, TUR! (4), UC); Yasuní National Park, 0°40'S 76°26'W, 200 – 300 m, 15 April 1997, H. Tuomisto 10624 (NY!, QCA (2), QCNE (2), TUR! (3), U!, UC!); Yasuní National Park, 0°39'S 76°28'W, 15 April 1996, R. C. Moran 6196 (AAU!, NY!, QCA!, QCNE, TUR!); Yasuní National Park, 0°33'S 76°31'W, 250 m, 16 April 1996, R. C. Moran 6210 (AAU!, NY!, QCA!, QCNE, TUR! (3)); Río Napo, 8 km E of Misahuallí, 1°4'S 77°36'W, 450 m, 19 – 28 March 1987, C. E. Cerón 1095 (QCA!, UC!); Yasuní National Park, 0°40'S 76°28'W, 19 April 1997, H. Tuomisto 10686 (holotype QCA!, isotypes AAU!, QCNE, TUR!); Río Payamino, 0°29'S 77°12'W, 19 June 1968, L. B. Holm-Nielsen 802 (AAU!, S); Yasuní National Park, 0°59'S 76°12'W, 200 – 300 m, 19 March 1998, H. Tuomisto 11915 (QCA, QCNE, TUR!, UC!); Yasuní National Park, 0°40'S 76°28'W, 20 April 1997, H. Tuomisto 10807 (QCA, QCNE, TUR!, U!, US!); Upper Río Tiputini, 0°43'S 76°57'W, 300 m, 21 – 23 July 1991, B. Øllgaard 99039 (AAU!); Upper Río Tiputini, 0°43'S 76°57'W, 300 m, 21 – 23 July 1991, B. Øllgaard 99071 (AAU!, QCA!); Yasuní National Park, 0°40'S 76°28'W, 22 April 1997, H. Tuomisto 10814 (QCA!, QCNE, TUR!); Yasuní National Park, 0°40'S 76°28'W, 200 – 300 m, 23 April 1997, H. Tuomisto 10844 (AAU!, NY!, QCA (3), QCNE (2), TUR! (3)); Yasuní National Park, 0°55'S 76°11'W, 200 m, 26 May – 8 June 1988, C. E. Cerón 3963 (MO, UC!); Yasuní National Park, Añangu, 0°31.5'S 76°23'W, 250 – 350 m, 30 May – 21 June 1982, B. Øllgaard 38927 (AAU!, F!, GH, NY!, QCA, UC!, US!); Parque Nacional Yasuní, 2 km S of Estación Científica Río Yasuní, 0°40'S 76°24'W, 350 m, 4 Sept. 1994, H. Navarrete 637 (AAU!, QCA!). Pastaza: ARCO Moretecocha, 1°30'S 78°30'W, 500 m, 20 – 27 Feb. 1991, E. Gudiño 1325 (MO!); Chapeton on Río Bobonaza, trail E of village to Río Aulapi, 1°38'S 77°41'W, 450 m, 3 Nov. 1995, B. Øllgaard 1353 (AAU!, QCA!, TUR!); Comunidad Canelos, c. 4.5 km S of Yanapuma, 1°25'S 77°41'W, 750 m, 5 March 1997, B. Øllgaard 2350 (QCA!, TUR!); Comunidad Canelos, c. 0.3 km E of Yanapuma, 1°25'S 77°42'W, 900 m, 3 June 1997, B. Øllgaard 2378 (QCA!); Chapeton on Río Bobonaza, Shiuna, N of the river, 1°38'S 77°42'W, 450 m, 5 Nov. 1995, B. Øllgaard 1384 (AAU!, QCA!). Sucumbios: Sinangue, upstream from Lumbaqui on Río Aguarico, c. 2.3 km NW of village near Río Candué, 0°6'S 77°26'W, 600 m, 23 Oct. 1996, B. Øllgaard 1855 (AAU!); Sinangue, upstream from Lumbaqui on Río Aguarico, c. 2.3 km NW of village near Río Candué, 0°6'S 77°26'W, 600 m, 23 Oct. 1996, B. Øllgaard 1865 (AAU!, QCA); Trail Chuscuyacu – Río Candué, 0°5'N 77°24'W, 480 – 520 m, 27 Jan. 1992, B. Øllgaard 99662 (AAU, NY!, QCA!, TUR!). peru. Loreto: Río Corrientes, Cachuela, 23 Sept. 1968, S. T. McDaniel 11198 (F!, GH!, MO!); Río Tigre, 1 – 2 km SW of the river. 2°53'S 75°25'W, 100 – 200 m, 27 Jan. 2005, H. Tuomisto 14712 (AMAZ, TUR!, USM, UC); Río Tigre, 1 km SW of the river, 2°53'S 75°25'W, 100 – 200 m, 27 Jan. 2005, H. Tuomisto 14725 (AMAZ, TUR!, USM); 30 km NE of Shiviyacu, along road between Río Pastaza and Río Tigre, 2°26'S 75°53'W, 200 m, 28 Feb. 2006, M. Higgins 992 (AMAZ, TUR! (2)).
habitat. Grows in primary Amazonian rainforests at elevations from 100 – 900 m. This species has been found in non-inundated areas and on river terraces, and on the banks and floodplains of creeks and small rivers.
conservation status. We estimate that Danaea lanceolata belongs in the Least concern (LC) category (IUCN 2012). Danaea lanceolata has an Area of Occupancy of 108 km2, which corresponds to the EN category, and an Extent of Occurrence of 154,464 km2, which corresponds to the LC category. Danaea lanceolata seems to be rather abundant especially in Ecuadorian Amazonia, and there is no plausible threat to all of its subpopulations.
etymology. The name acknowledges the fact that this species has earlier been confused with Danaea oblanceolata, but rather than having oblanceolate pinnae it has (narrow-) lanceolate pinnae and (broad-)lanceolate laminae.
notes. In Flora of Ecuador, Tuomisto & Moran (2001) noted that the Ecuadorian material of Danaea oblanceolata Stolze differs from typical material from Peru, and indeed all the Ecuadorian specimens they cited as D. oblanceolata are here referred to D. lanceolata. This species differs from D. oblanceolata by having lanceolate rather than oblanceolate pinnae that are ascending (vs perpendicular to the rachis) and also generally narrower (1 – 2.4 cm vs 1.9 – 2.7 cm) and more asymmetrical, and have acute-acuminate (vs short-caudate) and typically strongly falcate (vs symmetric or only slightly falcate) apices. Danaea oblanceolata has only been found in southern to central Peru (Pasco and Ucayali), whereas D. lanceolata is common in northern Peru (Loreto) and especially Ecuador (Map 2). The Central American Danaea wendlandii Rchb.f. is generally smaller (leaves 13 – 27 cm vs 19 – 50 cm long in D. lanceolata) and has generally fewer pinnae (10 – 15 vs 13 – 19 pairs) that are shorter (2 – 4 vs 3 – 7 times as long as wide), oblong rather than lanceolate, and have obtuse rather than acute-acuminate apices. Danaea gracilis from the Pacific side of the Andes has more pinnae (19 – 25 pairs) that are very thin, almost translucent and uniformly dark (vs opaque and usually darker adaxially and paler abaxially in D. lanceolata), symmetrical at the base, perpendicular to the rachis and straight or with only the apices slightly bending. Danaea acuminata Tuomisto & R.C.Moran differs in having almost always a terminal pinna (vs terminal pinna usually replaced by a bud), lateral pinnae with more sharply falcate and more coarsely serrate pinna apices (vs slightly crenulate to serrulate), and concolorous darker brownish lamina colour in dried specimens (vs bicolorous and green in D. lanceolata). Danaea humilis T.Moore differs in the parallel-sided rather than lanceolate lamina, presence of terminal pinnae, shorter lateral pinnae (2.2 – 3.4 cm vs 4.2 – 14 cm) and petioles with generally more nodes (1 – 3 vs 0 – 2). Danaea imbricata Tuomisto & R.C.Moran and D. trichomanoides Spruce ex T.Moore differ especially in shorter pinnae (2 – 3 vs 3 – 7 times as long as wide) and the laminae being so thin in texture that they are translucent.
Danaea stricta Tuomisto & Keskiniva sp. nov. (subgen. Holodanaea C.Presl). Type: Panama: Panamá, H. Tuomisto 15166 (holotype PMA!; isotypes AAU!, BM!, TUR!, UC!, US!).
http://www.ipni.org/urn:lsid:ipni.org:names:77295616-1
Rhizomes erect, 2.2 – 4.0 cm in diam. when dry, 30 – 45 cm tall, leaves arranged spirally and roots produced on all sides. Sterile leaves 68 – 85 cm long; petiole 20 – 32 cm long, 0 – 2 nodes, if no nodes, then the lowermost pinna is solitary and very small, petioles not winged, dark brown with many dark brown scales; laminae 37 – 58 × 21 – 33 cm, 16 – 22 pinna pairs, paripinnate with the terminal pinnae replaced by prolific buds, these sometimes forming terminal plantlets, pinnae widely spaced proximally, lamina parallel-sided, concolorous to somewhat lighter green (but not whitish) abaxially, rachis winged adaxially, wings up to 1 mm wide in the upper part of internodes in the apical part of the lamina, less elsewhere, rachises and costae moderately scaly abaxially; largest lateral pinna 11.4 – 16.8 × 1.5 – 2 cm, pinnae petiolulate, perpendicular to the rachis, straight and linear, pinna bases symmetrical and truncate, pinna apices tapering gradually to an acuminate-caudate tip 1.5 – 2 cm long, apical margins serrate to serrulate, 17 – 20 veins per cm, mostly simple but sometimes forked at the costa. Fertile leaves 79 – 95 cm long; petioles 22 – 53 cm long, 0 – 1 nodes; laminae 41 – 62 × 16 – 40 cm, 18 – 21 pinna pairs, laminae parallel-sided, paripinnate, terminal pinna replaced by a bud; largest fertile pinna 9.8 – 12.7 × 1 – 1.5 cm, pinnae perpendicular to the rachis, linear, symmetrical, bases truncate, apices acuminate to caudate, with serrulate margins. Lowermost pinna very small, solitary. Figs 8A – D & 9.
recognition. Danaea stricta is most similar to D. inaequilatera A.Rojas, but has a sturdier trunk (2.2 – 4 cm vs 0.5 – 2.3 cm in diam.), longer pinnae (11.4 – 16.8 cm vs 6.5 – 11.3 cm) with typically longer, more sharply serrate apices (1.5 – 2.7 vs 0.3 – 1.8 cm long), and has its terminal pinna always replaced by a bud (vs terminal pinna usually present in D. inaequilatera). Danaea stricta also resembles species in the D. cuspidata – D. moritziana complex, but can be identified by the thicker lamina texture, the strictly linear (vs falcate) sterile lateral pinnae that have truncate, symmetrical bases (vs obtuse to cuneate, asymmetrical pinna bases), almost uniform colour (vs whitish abaxially), and the pinnae being organised parallel to each other and perpendicular to the rachis (vs ascending). In addition, the fertile pinnae of D. stricta are wider (1 – 1.5 cm vs 0.3 – 1 cm) but thinner than in the D. cuspidata – D. moritziana complex, the terminal pinna is always replaced by a proliferous bud in both sterile and fertile leaves (vs terminal pinna usually present), and the rhizome is generally larger (up to 40 cm tall and 2.2 – 4 cm in diam. vs up to 30 cm tall and 0.8 – 2.5 cm in diam.).
distribution. Known from Panama (Darien, Panamá, San Blas) and the Pacific side of the Andes in Colombia (Chocó). Map 1.
specimens examined. colombia. Chocó: Hills above junction of Río Capá and Río Mumbú, upriver from Lloró, 5°37'N 76°25'W, 100 m, 2 Dec. 1983, A. Juncosa 1481 (MO!). panama. Darien: Darién National Park, top of Cerro Sapo, 7°58'N 78°21'W, 1100 m, 14 April 2014, O. O. Ortiz 2339 (PMA!). Panamá: Panama to San Blas, trail from end of road past Los Altos de Pacora region of Cerro Jefé. On to Cerro Brewster, 9°17'N 79°17'W, 600 – 800 m, 20 – 25 April 1985, B. E. Hammel 13550 (PMA!, UC!); El Jefe in Cerro Azul, 9°14'N 79°23'W, 950 – 1000 m, 26 Oct. 2005, H. Tuomisto 15166 (holotype PMA! (mounted on 3 sheets), isotype AAU!, BM! (2), TUR! (3), UC!, US!). San Blas: El Llano – Carti road, 17.5 km from Interamerican Hwy, 9°19'N 78°55'W, 350 m, 13 Oct. 1984, G. C. de Nevers 4003 (BM!, NY!, PMA!, US!).
habitat. Grows in moist forests from lowlands to 1000 m. One site described as elfin forest with signs of waterlogging.
conservation status. Danaea stricta has a known Area of Occupancy of 20 km2 and has been collected at only 5 locations, which corresponds to the EN category, and an Extent of Occurrence of 12,133 km2, which corresponds to the VU category. This suggests that it is rare and its range is small. However, two of the locations are inside protected areas (Chagres National Park and Darien National Park in Panama). There is no plausible threat to all of the subpopulations, and thus we assess D. stricta as belonging in the Least Concern (LC) category (IUCN 2012).
etymology. Named for the strict organisation of the pinnae, which are stiff, straight, parallel to each other and perpendicular to the rachis.
notes. The fertile pinnae of Danaea stricta are unusually broad and thin for Holodanaea but its sterile pinnae have a thicker texture than the species of the D. cuspidata – D. moritziana complex. Danaea stricta has a sturdier trunk than D. inaequilatera A.Rojas (2.2 – 4 cm vs 0.5 – 1.3 cm in diam.), and the latter has clearly shorter (6.7 – 11 cm vs 13.5 – 16.8 cm) pinnae that are also more densely packed along the rachis. All other species of the D. cuspidata – D. moritziana complex (especially D. cuspidata Liebm., D. moritziana C.Presl., D. mazeana Underw., D. jamaicensis Underw., D. betancurii A.Rojas., D. lucens A.Rojas) usually have a terminal pinna present (vs bud in D. stricta) and their lateral pinnae are more ascending and falcate (vs perpendicular to the rachis and straight in D. stricta). Furthermore, the laminae of D. betancurii, D. cuspidata and D. moritziana are generally clearly whitish underneath (vs almost concolorous in D. stricta) and laminae of D. lucens have a reddish colour.
Danaea tenuicaulis Tuomisto & Keskiniva sp. nov. (subgen. Holodanaea C.Presl). Type: Colombia: Valle de Cauca, M. Kessler 14866 (holotype TUR!, isotype HUA).
http://www.ipni.org/urn:lsid:ipni.org:names:77295617-1
Rhizomes erect to decumbent, 0.4 – 1.2 cm in diam. when dry, to 37 cm long, leaves and roots widely spaced, bases 2 – 4 cm apart, leaves arranged spirally, roots produced on all sides. Sterile leaves 48 – 66 cm long; petioles 18 – 33 cm long, 1 – 3 nodes, not winged, green except brownish-violet towards base, scales brown; laminae 23 – 39.5 × 10 – 16 cm, 8 – 12 pinna pairs, pinnae 2.3 – 4.0 cm apart, rather widely spaced, lamina lanceolate, imparipinnate or sometimes paripinnate with terminal pinna replaced by a proliferous bud, thin, bicolorous, dark green above and lighter green below, rachises winged adaxially, wings up to 0.5 mm wide in the distal part of internodes in the apical part of the lamina; terminal pinna 6.5 – 12.5 × 1.3 – 2.2 cm, lanceolate to oblong, bases acute, pinna apex 1.5 – 2.6 cm, long-acuminate to abruptly long-caudate, (deeply) crenate to serrate; largest lateral pinna 7.2 – 12.6 × 1.5 – 2.5 cm, oblong, lanceolate or oblanceolate, very slightly falcate distally, bases asymmetrical, acute to truncate or obtuse, increasingly concave in more distal pinnae, apices abruptly long-caudate (to acuminate), 1.5 – 2.6 cm long, apical margins (deeply) crenate to serrate, veins 13 – 20 per cm, usually forked at the costa. Fertile leaves with lamina c. 31 × 9.4 cm, linear-lanceolate, pinnae widely spaced proximally, rather crowded distally; fertile terminal pinna c. 5.1 × 0.4 cm, linear, base acute, apex acuminate, long-caudate, apical margins slightly sinuate, lateral pinnae 4.7 × 0.5 cm, linear, base acute, apex acuminate, apical margins slightly sinuate. Juveniles with creeping to ascending rhizome, terminal pinna abruptly long-caudate to long acuminate, apical margins crenate to serrulate, lateral pinnae rounder, bases asymmetrical, apices short-caudate to cuspidate. Figs. 10A – D & 11.
recognition. Danaea tenuicaulis is most similar to species of the D. cuspidata – D. moritziana complex, but differs in having a long and slender rhizome (0.4 – 1.2 cm vs 0.8 – 2.5 cm in diam.) which has leaf bases spaced wide apart (internodes 2 – 4 cm vs 0.5 – 1.5 cm), and long abruptly tapering pinna apices.
distribution. Known from the Pacific coast of Colombia (Cauca, Nariño and Valle de Cauca). Map 1.
specimens examined. colombia. Cauca: La Gallera, Micay Valley, 3°0'N 77°30'W, 1400 – 1500 m, 29 – 30 June 1922, E. P. Killip 7682 (GH!, PH!, US! (2)); W Slope of Cordillera Occidental, W of Tambo, 2°24'N 77°0'W, 2200 m, 6 Nov. 1946, O. L. Haught 5206 (COL!, S, US!); SW slope of Cerro Munchique, 40 km W of Popayan, 2°31'N 76°56'W, 2400 – 2500 m, 6 Oct. 1961, R. M. Tryon 6005 (GH!). Nariño: above Paramo, 1100 m, 8 May 1939, A. H. G. Alston 8539 (BM!); Reserva Natural La Planada, Sendero “Al Hondon” SW of the science center, 1°10'N 77°59'W, 1850 – 1950 m, 7 June 1996, J. Bittner 2471 (UC!); Ricaurte, Reserva La Planada, 2°38.457'N 77°58.942'W, 1700 m, 14 Feb. 2016, M. Kessler 14880 (HUA, TUR!). Valle del Cauca: near Queremal, 3°31'N 76°43'W, 1300 m, 5 April 1939, A. H. G. Alston 7921 (BM! (2)); Parque Nacional Munchique, road W towards Pacific slope, 2°42.268'N 76°53.145'W, 2050 m, 12 Feb. 2015, M. Kessler 14866 (holotype TUR!, isotype HUA).
habitat. Grows at mid to high elevations, from 1100 – 2500 m, in wet to very wet montane forests.
conservation status. We estimate that Danaea tenuicaulis belongs in the Vulnerable (VU B1+2ab(iii)) category (IUCN 2012). It has an Area of Occupancy of 28 km2, which corresponds to the EN category. It has an Extent of Occurrence of 16,712 km2 and has been found in only 7 locations, which corresponds to the VU category. None of the locations are in protected areas. The area, extent, and quality of suitable habitats were inferred to be suffering continuing decline from deforestation and urbanisation.
etymology. The name refers to the unusually long and thin rhizomes and is a compilation of Latin words for slender (tenuis) and stem (caulis).
notes. Danaea tenuicaulis is recognised by the combination of a long, unusually thin rhizome with widely spaced leaf bases and roots, and short pinnae with a strikingly long-caudate, serrate to serrulate apices. The characteristically abrupt apices are already visible in the terminal pinnae of juveniles. Danaea inaequilatera A.Rojas also grows on the Pacific coast of Colombia, but at lower altitudes. It differs in having concolorous laminae (vs clearly bicolorous) and more pinnae (13 – 19 vs 9 – 12) with acute to acuminate apices (vs usually abruptly long-caudate). Danaea lucens A.Rojas can be found in the same area and shares the long-caudate, serrate pinna apices, but it is a larger plant (sterile leaves 57 – 117 cm vs 48 – 66 cm long) with a sturdier rhizome (1 – 2 cm vs 0.3 – 1.2 in diam.) that is creeping to ascending (vs erect to decumbent) and with concolorous laminae, a thicker lamina texture, and longer pinnae (11 – 22 cm vs 7.2 – 12.6 cm). Danaea ypori Christenh. differs in having terminal pinnae usually replaced by a bud (vs terminal pinna usually present), more pinna pairs (13 – 16 vs 9 – 12), pinnae with acute to acuminate apices (vs abruptly long-caudate), and a creeping-ascending rhizome that is thicker (1.3 – 1.9 cm vs 0.4 – 1.2 cm in diam.) and shorter (up to 6 cm long vs up to 37 cm long). Another species with a long, relatively slender erect rhizome is D. arbuscula, but this species belongs to subgen. Arthrodanaea and has elliptic concolorous pinnae with entire, acuminate apices (vs parallel-sided bicolorous pinnae with abruptly tapering, serrate apices).
Danaea vanderwerffii Tuomisto & Keskiniva sp. nov. (subgen. Holodanaea C.Presl). Type: Panama: Colón, H. van der Werff 22275 (holotype PMA!; isotypes MO!, TUR!).
http://www.ipni.org/urn:lsid:ipni.org:names:77295618-1
Rhizomes erect, forming a short trunk, 1.4 – 3.5 cm in diam. when dry. Sterile leaves 48 – 107 cm long; petioles 8 – 43 cm long, 0 – 1 nodes, not winged, atrocastaneous to brown, moderately scaly, scales dark brown; laminae 38 – 76 × 12 – 27 cm, 8 – 13 pinna pairs, lamina bicolorous, whitish abaxially, dark green to castaneous adaxially when dry, long-lanceolate, imparipinnate, pinnae ascending, widely spaced especially proximally, the most proximal pinnae solitary, elliptic to round and very small; rachises winged adaxially, wings to 1 mm wide, rachises sparsely scaly abaxially, costae very scaly abaxially; terminal pinna 10.5 – 16 × 2.0 – 4.1 cm, lanceolate-oblong, base acute, apex acuminate-caudate, apical margins sinuate; largest lateral pinna 10.3 – 16.7 × 1.7 – 3.3 cm, pinnae elliptic to oblong, petiolulate proximally, sessile distally, bases acute, asymmetrical in distal pinnae, which are concave distally towards apex and auriculate proximally, in proximal pinnae bases symmetrical, truncate to obtuse, apices acuminate to abruptly caudate, 1.5 – 4.0 cm long, generally bending towards leaf apex, tip with crenate (to serrulate) margins, base of apex serrulate to serrate, veins 12 – 17 per cm, mostly simple, sometimes bifurcated. Fertile leaves 64 – 109 cm long; petioles 17 – 44 cm long, 0 – 1 nodes; laminae 40 – 75 × 14 – 15 cm, 11 – 13 pinna pairs, imparipinnate, pinnae ascending, widely spaced especially proximally, the most proximal pinna solitary, small; largest lateral pinnae 9.2 – 12.8 × 0.8 – 1.5 cm, long-lanceolate, bases asymmetrical, auriculate in distal pinnae, symmetrical in proximal pinnae, apices acuminate, crenulate (to serrulate). Fig. 12.
recognition. Danaea vanderwerffii resembles D. bicolor Tuomisto & R.C.Moran, but can be recognised by the strongly reduced proximal pinnae, short erect rhizome (probably decumbent in D. bicolor), 8 – 13 pinna pairs (vs 3 – 6 in D. bicolor), fewer nodes on petioles (0 – 1 vs 2 – 3 nodes in D. bicolor), and the winged rachis (terete or only narrowly winged in the uppermost internode in D. bicolor).
distribution. Panama (Cocle, Colón, Veraguas). Map 1.
specimens examined. panama. Cocle: Distrito de La Pintada. 8°43'N 80°37'W, 200 m, 17 Oct. 2012, J. Aranda 4324 (PMA!); La Pintada, El Harino, El Copé, Parque Nacional G.D. Omar Torrijos Herrera, 8°40'N 80°35'W, 750 m, 20 Oct. 2012, L. Martínez 1092 (PMA!). Colón: Concession of Minera Panamá, 2 June 2012, J. F. Carrión 782 (PMA!); Donoso, Coclé del Norte, 8°48'N 80°43'W, 100 m, 26 Aug. 2012, H. Espinosa 167 (PMA!); Dibisim Coclé del Norte, Río Escribano, 8°51'N 80°50'W, 50 m, 24 Aug. 2012, L. Martínez 976 (PMA!); Teck Cominco Petanquilla mining concession, 8°49.6'N 80°39.7'W, 250 – 350 m, 30 Nov. 2007, H. van der Werff 22226 (MO!, PMA!, TUR!); Teck Cominco Petanquilla mining concession, 8°50.2'N, 80°41.6'W, 100 m, 5 Dec. 2007, H. van der Werff 22262 (MO!, PMA!, TUR!); Teck Cominco Petanquilla mining concession, 8°50.2'N 80°41.3'W, 100 m, 6 Dec. 2007, H. van der Werff 22275 (holotype PMA!, isotypes MO!, TUR!). Veraguas: Parque Nacional Santa Fe, Río Mulabá, 8°32'N 81°9'W, 750 m, 17 Nov. 2012, A. Espinosa 6085 (PMA!); Parque Nacional Santa Fe, Mulabá, 8°32'N 81°8'W, 650 m, 16 Nov. 2012, L. Martínez 1121 (PMA!). Parque Nacional Santa Fe,Río Mulabá, 8°31'N 81°8'W, 650 m, 19 Nov. 2012, L. Martínez 1161 (PMA!). Parque Nacional Santa Fe,Río Calovébora, 8°33'N 81°10'W, 18 Nov. 2012, O. O. Ortiz 1021 (PMA!).
habitat. Grows in lowland forests from 55 – 850 m elevation. Often found on banks of rivers and creeks. Has been found in locations disturbed by cultivation and animal husbandry.
conservation status. Danaea vanderwerffii has a known Area of Occupancy of 48 km2 and Extent of Occurrence of 1,061 km2, which correspond to the EN category (IUCN 2012). It has been collected at 12 locations, many of which are inside protected areas (Santa Fé National Park and Omar Torrijos National Park in Panama). There is no plausible threat to all of the subpopulations, and thus we assess D. vanderwerffii as belonging to the Least Concern category (IUCN 2012).
etymology. Named after Henk van der Werff, who has made extensive collections of tropical American ferns, and collected many of the known specimens of this species.
notes. Danaea vanderwerffii is a species of intermediate size that is most similar to D. bicolor and some species of the D. cuspidata – D. moritziana complex. Its most striking characters are the extreme degree of reduction in the pinnae formed at the most proximal pinna-bearing node, and the strongly bicolorous laminae with abaxial side almost white. The abaxial colour is similar to that of D. bicolor, but D. vanderwerffii has fewer nodes in the petiole (0 – 1 vs 2 – 3), clearly more pinnae (8 – 13 vs 3 – 6 pinna pairs) and a winged rachis (unwinged to only very narrowly winged in the uppermost internode in D. bicolor). Species of the D. cuspidata – D. moritziana complex can also have bicolorous laminae, but the contrast is less striking and their pinnae are usually more falcate, with the tip of the apex being usually serrate (vs crenulate in D. vanderwerffii). In addition, D. vanderwerffii has generally broader pinnae than species of the D. cuspidata – D. moritziana complex, especially in the terminal pinnae of sterile leaves (2.0 – 4.1 cm vs 1.0 – 2.6 cm wide) and the lateral pinnae of the fertile leaves (0.8 – 1.5 cm vs 0.3 – 1.0 cm wide). Pinnae are also spaced more widely apart (length of lamina divided by the number of pinnae is 4.4 – 6.4 in D. vanderwerffii and 1.5 – 4.6 in the D. cuspidata – D. moritziana complex). Furthermore, D. vanderwerffii has only 0 – 1 nodes on the petiole, whereas species of the D. cuspidata – D. moritziana complex usually have more than one node.
Notes on previously synonymised Danaea species and synonymisation of Danaea quebradensis Christenh. under Danaea lucens A.Rojas
As mentioned in the Introduction, there has been considerable disagreement about species delimitation within Danaea. Tuomisto & Moran (2001) described eight species as new, but Rolleri (2004) synonymised seven of them. We disagree with these synonymisations and thereby agree with Christenhusz (2010), who considered all of them as valid, distinct species. Indeed, all of them are distinguishable on the basis of morphological characteristics, some of which are very obvious. One of these characters is rhizome habit. The structural difference between a creeping rhizome (especially a dorsiventral one) and an erect radially arranged trunk is so fundamental that we consider it impossible for both rhizome habits to coexist within a single species. Unfortunately, many herbarium specimens of the larger species contain no rhizomes or even descriptions or photographs of them, which makes their identification difficult at best and potentially impossible, especially if the specimen is also otherwise fragmentary and DNA sequences are not available.
The following list gives the most obvious morpological characters that distinguish each species from the one it was synonymised under by Rolleri (2004). All leaf characters refer to sterile leaves.
-
Danaea acuminata differs from D. moritziana C.Presl in being a smaller plant (leaves less than 40 cm vs up to 1 m long) with a creeping rhizome (vs erect trunk) and laminae with a uniformly dark brown colour when dried (vs rather pale and greenish but clearly bicolorous with abaxial side whitish).
-
Danaea bicolor differs from D. nodosa in having 2 – 3 nodes on the petiole (vs none), fewer pinna pairs (3 – 6 vs 8 – 16) and clearly bicolorous laminae with abaxial side almost white (vs concolorous).
-
Danaea bipinnata was synonymised under D. elliptica Sm., which has since been synonymised under D. nodosa because its type is a juvenile of D. nodosa (Christenhusz & Tuomisto 2006). Danaea bipinnata differs from D. nodosa in being a much smaller plant (leaves less than 70 cm vs to over 2 m long) with an erect trunk (vs creeping dorsiventral rhizome) and smaller pinnae (8.5 – 16.5 cm vs 21 – 34 cm long, 2.3 – 3.7 cm vs 3.6 – 6.0 cm wide).
-
Danaea erecta differs from D. nodosa in having an erect sturdy trunk with leaves arranged spirally (vs creeping dorsiventral rhizome with leaves in two rows) and proliferous buds often replacing the terminal pinna (vs terminal pinna always present).
-
Danaea falcata differs from D. moritziana in having a creeping dorsiventral rhizome (vs erect trunk) and leaves with a uniformly dark brown colour when dried (vs rather pale leaves that remain greenish and are clearly bicolorous with abaxial side whitish).
-
Danaea latipinna differs from D. nodosa in having broader pinnae (5 – 9 cm vs 3.6 – 6.0 cm wide) of a rounder shape (2 – 4 times vs more than 4 times as long as wide), the terminal pinna usually being replaced by a bud (vs terminal pinna always present) and petioles with up to 2 nodes (vs none).
-
Danaea longicaudata differs from D. nodosa in being a smaller plant (leaves less than 1 m vs to over 2 m long) with an erect trunk (vs creeping dorsiventral rhizome), petioles with up to 2 nodes (vs none) and smaller pinnae (10 – 16 cm vs 21 – 34 cm long, 1.8 – 2.6 cm vs 3.6 – 6.0 cm wide).
Although Christenhusz (2010) reinstated the species that had been synonymised by Rolleri (2004), he at the same time synonymised all the species described by Rojas-Alvarado: D. inaequilatera under D. falcata and D. betancurii, D. lucens and D. tuomistoana under D. moritziana. Rojas-Alvarado (2013) reinstated all four species he had described before. We are still in the process of verifying the taxonomic status of some of these species, but for two of them we have already made a decision and hereby support reverting their synonymisations.
-
Danaea inaequilatera differs from D. falcata in having an erect trunk (vs creeping dorsiventral rhizome), lighter and greenish lamina colour in dried specimens (vs dark brown), more pinna pairs (13 – 19 vs 6 – 12) that are more densely arranged (pinnae < 2.6 cm apart vs >2.6 cm apart), parallel-sided (vs oblanceolate) and with shorter apices (0.3 – 1.8 cm vs 1.5 – 2.8 cm).
-
Danaea lucens differs from D. moritziana in having a creeping to ascending rhizome with roots formed mostly on the ventral side (vs erect with both leaves and roots arranged radially), more falcate pinna shape, longer pinna apices (2.5 – 4.0 cm vs 0.5 – 2.5 cm), a thicker lamina texture, and lamina colour in dried specimens being more uniformly dark (vs whitish abaxially) with often a reddish tint in and around rachises and costae (vs brown) and a characteristic sheen on the adaxial surface (vs dull). In addition, the fertile pinnae of D. lucens are wider (1.5 cm vs 0.3 – 1.0 cm).
As to the species described by Christenhusz (2010), we consider Danaea quebradensis to be conspecific with D. lucens. Since D. lucens was described first, it has priority and D. quebradensis becomes a synonym. The two species were described from the same area in Colombia, both have types from the Anorí area in Antioquia, and both descriptions cite as a paratype a specimen from Municipio San José del Palmar in Chocó (Franco 1240 for D. quebradensis and Franco et al. 1551 for D. lucens). We have seen the types and most paratypes of both species, and are confident that they are conspecific. All specimens share the same distinguishing characteristics: they are similar in size, pinna number and pinna shape, they have a distinctly thick lamina texture, most have a characteristic reddish colour in and around rachises and costae, and the adaxial lamina surface is dark green with a characteristic sheen.
specimens examined. Previously cited as Danaea lucens: colombia. Antioquia: Municipio Anorí, Vereda San Antonio, Finca El Cielo, 7°16'N 75°3'W, 730 m, 15 Nov. 2003, W. D. Rodríguez 4325 (holotype: COL!; isotype: HUA; not previously cited: COL! (2nd sheet), NY!). Chocó: Municipio San José del Palmar, vereda “El Corcovado”, Finca “La Esperanza”, 1900 m, 19 Jan. 1983, P. Franco 1551 (COL!). Risaralda: Municipio de Mistrató, entre los corregimientos de Gaguadas y Puerto de Oro, selva de Pisones, 1550 m, 30 March 1992, J. Fernandez 9620 (COL!).
Previously cited as Danaea quebradensis, but here referred to D. lucens: colombia. Antioquia: Vic. Planta Providencia, 28 km SW of Zaragoza, valley of Río Anorí in areas surrounding the confluence of Quebrada La Tirana and Río Anorí, c. 3 km upriver from Planta Providencia, c. 07°18′N 75°04′W, 400 – 700 m, 3 April 1977, W. S. Alverson 336 (holotype NY! (3 sheets), not previously cited: COL!, WIS! (2 sheets)); Municipio Tarazá, Corregimiento El 12, 210 km NE of Medellín, Barro Blanco, Arbeláez 225 (NY!); Peñas Blancas, Woronow & Juzepczuk 4525 (US!); Río Guapá, 6 km E of Guapá, 53 km S of Turbo, Haught 4663 (NY!). Chocó: Municipio San José del Palmar, Vereda La Holanda, Franco 1240 (COL! not previously cited, MO!). Norte de Santander: Bellavista on pipeline, Foster 1675 (A, COL! not previously cited).
Not previously cited, here identified as Danaea lucens: colombia. Antioquia: Río Anori valley near Planta Providencia, 7°30'N, 75°50'W, 350 – 600 m, 27 Aug. 1976, J. D. Shepherd 587 (WIS! (2 sheets)); Municipio El Triunfo, Paraje El Doradal, 100 – 200 m, J. I. Santa S. 379 (COL!). Vereda San Antonio, 07°15'58.6"N 75°04'27.0"W, 790 m, 12 Nov. 2003, W. D. Rodríguez 4300 (COL!); Municipio Nechi, Vereda Santa Maria, 8°81'60"N 74°45'13"W, 60 m, 1 March 2010, W. D. Rodríguez 6558 (COL!). Norte de Santander: Catatumbo, Puerto Barco, 12 May 1959, H. Bischler 2594 (COL!).
References
Christenhusz, M. J. M. (2006). Three new species of Danaea (Marattiaceae) from French Guiana and the Lesser Antilles. Ann. Bot. Fenn. 43 (3): 212 – 19. https://www.jstor.org/stable/23727210.
____ (2010). Danaea (Marattiaceae) revisited: biodiversity, a new classification and ten new species of a neotropical fern genus. Bot. J. Linn. Soc. 163 (3): 360 – 385.
____, Almeida, E. M. & Felix, L. P. (2018). A new species Danaea (Marattiaceae) from the Atlantic forests of Brazil. Phytotaxa 356: 226 – 232.
____ & Tuomisto, H. (2006). Five new species of Danaea (Marattiaceae) from Peru and a new status for D. elliptica. Kew Bull. 61: 17 – 30.
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1. Second edition. Gland and Cambridge.
Rojas-Alvarado, A. F. (2006). Una nueva especie de helecho del género Danaea (Marattiales: Marattiaceae) endémica de Costa Rica. Revista Biol. Trop. 54 (3): 1057 – 60.
____ (2009). Novelties in Danaea moritziana complex (Marattiaceae) from Colombia. Mét. Ecol. Sist. 4: 8 – 19.
____ (2013). Notas taxonómicas en Danaea Sm. (Marattiaceae) para Costa Rica, Panamá y Colombia. Actual. Biol. 35: 11 – 20.
Rolleri, C. H. (2004). Revisión Del Género Danaea (Marattiaceae – Pteridophyta). Darwiniana 42 (1/4): 217 – 301.
Tryon, R. M. & Tryon, A. F. (1982). Ferns and Allied Plants With Special Reference to Tropical America. Springer-Verlag New York Inc., U.S.A.
Tuomisto, H. & Moran, R. C. (2001). Marattiaceae. In: Flora of Ecuador 66: 21 – 68.
Acknowledgements
We thank Michael Kessler, Henk van der Werff and Benjamin Øllgaard for duplicates of their specimens; COAH, COL and PMA for scanning and sending images of specimens, QCA for access to their physical collections, and the following herbaria for loans: AAU, BM, C, F, G, GH, K, MO, NY, U, UC, and US. Additional material from several herbaria has been seen online through Pteridophyte Collections Consortium (https://pteridoportal.org/portal/) and the databases of individual herbaria. The TUR herbarium has provided working facilities and technical support. JK has been funded by the Graduate School of the University of Turku and Varsinais-Suomi Regional Fund. Herbarium specimens have been collected during several earlier projects, many of which were funded by the Academy of Finland (grants to HT).
Funding
Open Access funding provided by University of Turku (UTU) including Turku University Central Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keskiniva, J.S., Tuomisto, H. Six new species of Danaea (Marattiaceae) and the synonymisation of Danaea quebradensis. Kew Bull 77, 189–210 (2022). https://doi.org/10.1007/s12225-022-10011-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-022-10011-w