Summary
A new species of Salvia (S. celendina J.R.I.Wood & Uría) is described from the Marañón valley hotspot in northern Peru. The new species is illustrated with photographs and its distribution is mapped. Notes on its cultivation and distinctive characteristics are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Salvia L. is the largest genus in the Lamiaceae with around 1000 species (Mabberley 2017) distributed throughout most of the World apart from the polar regions, tropical forests and Australia. In the Americas, Mexico is an outstanding hotspot with some 294 recorded species, of which 242 are endemic, but the genus is also very diverse throughout the Andes of South America, Peru having the greatest diversity with 77 species of which 49 are endemic (González-Gallegos et al. 2020). New species of Salvia are being described quite regularly from the neotropics with 59 in the last decade, according to IPNI, nearly all from Mexico but two, Salvia hunzikeri A.Granda and S. vargas-llosae Sagást. & E.Rodr. from Peru. The discovery of another new species from Peru is not, therefore, entirely unexpected.
The American salvias were last revised by Carl Epling (1939) who divided the genus into numerous sections. Although Epling subsequently published regular papers updating his infrageneric classification until his death, it has long been known that this classification was out of date (Wood & Harley 1989; Fragoso-Martínez et al. 2018; González-Gallegos et al. 2020). However, in the absence of any alternative it has been followed by almost all national and regional revisions to this day. In recent years, molecular studies (Walker et al. 2004) have shown that Salvia as traditionally circumscribed is not monophyletic. There has been tension between those who wish to split the genus into segregate genera (Will & Claßen-Bockhoff 2017) and those who advocate an expanded Salvia to encompass Rosmarinus L. and a few other small genera (Drew et al. 2017). This latter approach seems to be gaining acceptance and must surely please all who want nomenclatural stability, including most gardeners.
Salvia is an important genus horticulturally and different American species are cultivated in temperate, Mediterranean and subtropical gardens in many parts of the world. Indeed, the number of entries in the internet related to the cultivation of salvias or sages, as well as attendance at International Salvia meetings, such as the Salvia Summit held at Huntington Botanical Gardens in Pasadena in 2013, suggests that the popularity of salvias amongst gardeners is steadily increasing.
This paper is a collaboration between two salvia enthusiasts who represent different but overlapping traditions. The first author became an academic botanist later in life and developed an interest in salvias while living in Colombia and Bolivia. The second author is a professional horticulturalist and plant hunter from Argentina who has cultivated salvias for many years. The new species described below results from field collections in Peru combined with the study of herbarium specimens at Kew and a review of the relevant salvia literature. The description is based on herbarium specimens supplemented by habit and colour details derived from photographs of wild populations and field observations
Salvia celendina J.R.I.Wood & Uría, sp. nov. Type: Peru, Celendín, D. N. Smith 6194 (holotype USM-104439, isotypes K, MO).
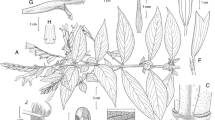
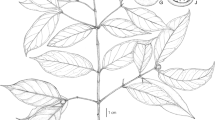
Much-branched aromatic shrub 80 – 150 cm high; stems square, floccose with short, branched white hairs, somewhat glabrescent when old. Leaves petiolate; petioles 4 – 10 mm, densely white-floccose; lamina 1 – 6.4 × 0.6 – 2.5 cm, ovate, apex acute, base shallowly cordate, margin crenate, adaxially green, glabrous, somewhat bullate, the depressions often filled with white hair bases, gland-dotted, abaxially white-tomentose with branched hairs, gland-dotted. Inflorescence of solitary raceme-like thyrses terminal on the branches, occasionally with two lateral thyrses arising at base of the central thyrse, mostly 4 – 7 cm long; rachis 4 – 9 cm, floccose, verticillasters c. 1 cm apart below, becoming confluent above, 3 – 8-flowered; bracteoles 6 – 8 × 2 – 3 mm, ovate, acuminate, floccose, caducous; pedicels c. 3 mm long; calyx 2-lipped, gaping in fruit, 5 mm long at anthesis, teeth acute and mucronate; upper lip 5-veined, veins darker but mostly hidden by floccose indumentum, internally bright green, net-veined, covered in short, sparse appressed hairs; corolla 11 mm long, blue or white, exterior lanate, interior glabrous, tube cylindrical, c. 5 mm long, upper lip c. 5 mm long, enclosing the style, lower lip 6 – 7 mm long, 4 mm wide, shallowly 3-lobed; filaments glabrous; anthers c. 1.5 mm, style shortly exserted from upper lip, pilose except for the two arms, the upper arm recurved or even coiled, 2 – 3 times longer than lower arm. Nutlets 1.5 × 1 mm, brown, ellipsoid, apically rounded, smooth. Fig. 1.
recognition. The new species clearly belongs to the Tomentellae clade II sensu Jenks et al. (2013) and Fragoso-Martínez et al. (2018) of subgen. Calosphace, one of whose distinct characteristics is branched hairs. It has sometimes been identified as and most closely resembles Salvia cuspidata Ruiz & Pav. in sect. tomentellae (Epling) Epling but differs from the various subspecies of S. cuspidata most obviously by the abundant, dense white-floccose indumentum. The floccose indumentum recalls the Argentinian species S. calolophos Epling, which itself may prove to belong to S. cuspidata, but S. celendina is more lanate and differs in the clearly ovate, rather than lanceolate leaves, which are less strongly crenate and, basally, have rounded, rather than subhastate auricles. It also resembles some species placed in sect. flocculosae (Epling) Epling, especially Salvia flocculosa Benth. from Ecuador and S. griseifolia Benth. from Huánuco in Peru, sharing with these two species similar ovate leaves that are floccose beneath. Both these, however, are readily distinguished by the longer inflorescence (> 10 cm), shorter subcanescent indumentum and smaller leaves (< 2.5 cm long); S. flocculosa is distinguished additionally by the distinct persistent lanceolate bracteoles and subentire leaves, and S. griseifolia by the distant verticillasters, usually > 1 cm apart.
habitat & distribution. Endemic to the Río Marañón basin in northern Peru where it is found in a few scattered populations between 1600 and 2000 m altitude approximately. It is a plant of dry bushland on steep rocky slopes, often in areas adjoining cultivated land. Fig. 2, Map 1.
specimens examined. peru. Cajamarca: Prov. Celendín, ladera frente el puente sobre el Río Marañón, subiendo hasta Limón, abundante en matorral bajo con Acacia, Bombax y Bougainvillea, 1600 m, 16 April 1982, I. Sanchez Vega 2805 (MO - http://legacy.tropicos.org/Name/17601180?tab=images); ibid. with white flowers; I. Sanchez Vega 2806 (MO); Los Blancos, 4 km al norte de Celendín, siguiendo la ruta a Llanguat, “ladera de arbustos y árboles: Escallonia pendula,” 6°47'6.5"S 78°10'41"W, 19 Feb. 2010, I. Sanchez Vega et al. 14 118 (CPUN); Balsas-Celendín road, 16 – 23 km from Balsas 6°50'S 78°48'W, Rio Marañon Valley, 1800 – 2100 m, “brushfields” in area of permanent agriculture, 24 Feb. 1984, D. N. Smith 6194 (K, MO, USM); north of Celendín on way to Rio Grande, rocky slopes, 6°48'16.7"S 78°10' 17"W, 1982 m, 23 July 2016, R. Uría PE16-005 (K, CIIDIR); on road from Celendín to Balsas, rocky slopes, 6°51'35.7"S 78°04'19"W, 23 July 2016, 1763 m, R. Uría PE16-009 (K, CIIDIR).
notes. Salvia celendina is restricted to the Marañon River area whereas S. cuspidata is apparently restricted to coastal Peru in Lima and Ancash (Wood 2007).
conservation status. The populations of Salvia celendina seem to be highly localised and, although the populations where R. Uría PE16-005 and I. Sanchez Vega 2805 were collected were recorded as “quite big” or “abundant”, the species was not seen elsewhere in northern Peru, apart from the small population where R. Uría PE16-009 was collected. Our information at the present time is so limited that this species can only be classified as Data Deficient (DD) under IUCN guidelines. The Marañón River Valley, where this species grows, is a major biodiversity hotspot with high levels of endemism in fauna and flora. Over 140 species of flowering plants are endemic to the area and new species are constantly being described (Marcelo-Peña et al. 2016). The landscape and habitat face a generalised threat from fragmentation due to agriculture, mining and population growth, and a specific threat from the proposed construction of a series of dams to produce hydroelectricity. However, none of these directly threaten the known populations of S. celendina. Information about threats to the Marañon River valley and efforts at conservation can be found at https://www.actualidadambiental.pe/amazonas-busca-proteger-especies-unicas-en-bosques-del-canon-del-maranon/
etymology. The specific epithet “celendina” is derived from Celendín Province, to which this species is nearly endemic.
cultivation. After a four-year trial period in the second author´s garden we can conclude that Salvia celendina has ornamental potential and can be grown for decorative purposes in gardens and for landscape in mild climates. The grey-green foliage and the attractive blue flowers are its main aesthetic attributes. It thrives in full sun on a well-drained soil and withstands temperatures of -5°C for short periods; it is also drought-tolerant once established. Propagation is by seed or softwood cuttings and it takes about two years to progress from seed to a blooming plant. This low maintenance shrub is perfect along fence lines and as a hedge and can be used as a background plant. Potted specimens offer year-round interest and structure on patios and terraces; it is suitable for rockeries among other xeric shrubs and perennials. It has a relatively long blooming period during the Southern Hemisphere autumn and winter, from May to August.
References
Drew, B. T., González-Gallegos, J. G., Xiang, C, Kriebel, R., Drummond, C. P., Walker, J. B. & Sytsma, K. J. (2017). Salvia united: the greatest good for the greatest number. Taxon 66: 133 – 145. doi:https://doi.org/10.12705/661.7
Epling, C. (1939). A revision of Salvia subgenus Calosphace. Repert. Spec. Nov. Regni Veg. Beih. 110: 1 – 380.
Fragoso-Martínez, I., Martínez-Gordillo, M., Salazar, G. A., Sazatornil, F., Jenks, A. A., García Peña, M. del R., Barrera-Aveleida, G., Benitez-Vieyra, S., Magallón, S., Cornejo-Tenorio, G. & Granados Mendoza, C. (2018). Phylogeny of the Neotropical sages (Salvia subg. Calosphace; Lamiaceae) and insights into pollinator and area shifts. Pl. Syst. Evol. 304: 43 – 55. doi.org/10.1007/s00606-017-1445-4
González-Gallegos, J. G., Bedolla-García, B. Y., Cornejo-Tenorio, G., Fernández-Alonso, J. L., Fragoso-Martínez, I., García-Peña, M. R., Harley, R. M., Klitgaard, B., Martínez-Gordillo, M. J., Wood, J. R. I., Zamudio, S., Zona, S. & Xifreda, C. C. (2020). Richness and Distribution of Salvia subgenus Calosphace (Lamiaceae). Int. J. Pl. Sci. 181(8): 831 – 856. 2020. DOI: https://doi.org/10.1086/709133
Jenks, A. A., Walker, J. B. & Kim, S.-C. (2013). Phylogeny of New World Salvia subgenus Calosphace (Lamiaceae) based on cpDNA (psbA-trnH) and nrDNA (ITS) sequence data. J. Pl. Res. 126: 483 – 496. DOI: https://doi.org/10.1007/s10265-012-0543-1
Mabberley, D. J. (2017). Mabberley’s Plant Book. Cambridge University Press, Cambridge.
Marcelo-Peña, J. L., Huamantupa, I., Särkinen, T. & Tomazello, M. (2016). Identifying Conservation Priority Areas on the Marañón Valley (Peru) based on Floristic Inventories. Edinburgh J. Bot. 73 (1): 95 – 123. DOI: https://doi.org/10.1017/S0960428615000281
Walker, J. B., Sytsma, K. J., Treutlein, J. & Wink, M. (2004). Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Amer. J. Bot. 91: 1115 – 1125.
Will, M. & Claßen-Bockhoff, R. (2017). Time to split Salvia s.l. (Lamiaceae) — New insights from Old World Salvia phylogeny. Molec. Phylogenet. Evol. 109: 33 – 58. doi:https://doi.org/10.1016/j.ympev.2016.12.041
Wood, J. R. I. (2007). The Salvias (Lamiaceae) of Bolivia. Kew Bull. 62: 177 – 222.
Wood, J. R. I & Harley, R. M. (1989). The genus Salvia (Labiatae) in Colombia. Kew Bull. 44: 211 – 278.
Acknowledgements
Rolando Uría thanks Juan Montoya Quino (Universidad Nacional de Cajamarca) for his kind assistance during a visit to the herbarium CPUN in 2016 and also Francisco Javier Lozando for his support and assistance during the field trips. Both authors are grateful to Hamilton Beltrán and Enoc Jara for locating the type at USM and sending us photographs of the specimen. They are also grateful to Lynsey and John Pink for putting them in touch with each other and for sharing their enthusiasm for salvias.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wood, J.R.I., Uría, R. Salvia celendina (Lamiaceae), a new species from Peru. Kew Bull 76, 421–425 (2021). https://doi.org/10.1007/s12225-021-09963-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-021-09963-2