Abstract
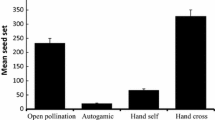
We studied the extrinsic factors behind the low number of offspring in two populations of Anagyris foetida, a bird-pollinated leguminous shrub of the Mediterranean region. Fruit initiation was pollen limited, but fruit maturation was not. This limiting effect varied between flowering seasons and between populations, and also within a given phenological stage. In the first season, the early flowers had the lowest fruit set, while in the second season fruit set was highest in these early flowers. This was possibly related to the pollination environment. Seed initiation (fertilized ovules) increased following pollen supplementation, but this was not translated into a significant increase in either the number of seeds per fruit, or seed mass. This indicates that seed initiation is pollen limited but that other factors (e.g., resource availability) are involved in seed maturation. Abiotic factors such as excess humidity during flowering were responsible for the loss of inflorescences, especially in one of the two populations. In this population, the prevailing wind in autumn-winter was less effective in drying the flowers when there was excess humidity. Also, significantly fewer inflorescences were lost from solitary plants than from clustered plants, probably reflecting the beneficial action of the wind and the greater light levels during flowering. Of the biotic factors analyzed, sheep predation was the most important, being worse in drought years. This predation, by affecting population density, could modify the plant-pollinator interaction and severely reduce the plant’s breeding success because of its pollen limitation.



Similar content being viewed by others
References
Amarasekare P (2004) Spatial dynamics of mutualistic interactions. J Anim Ecol 73:128−142
Bawa KS, Buckley DP (1989) Seed: ovule ratios, selective seed abortion, and mating systems in Leguminosae. In Stirton CH, Zarucchi JL (eds) Advances in legume biology. Monogr Syst Bot Missouri Bot Gard 29:243−262
Bawa KS, Webb CJ (1984) Flower, fruit and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. Amer J Bot 71:736−751
Bishop JG, Schemske DG (1998) Variation in flowering phenology and its consequences for lupines colonizing Mount St. Helens. Ecology 79:534−546
Bradford DF, Smith CC (1977) Seed predation and seed number in Scheelea palm fruits. Ecology 58:667−673
Brink RA, Cooper DC (1936) The mechanism of pollination in alfalfa (Medicago sativa). Amer J Bot 23:678−683
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83−139
Campbell DR, Halama KJ (1993) Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology 74:1043−1051
Casper BB (1988) Evidence for selective embryo abortion in Cryptantha flava. Amer Naturalist 132:318−326
Charlesworth D (1989) Evolution of low female fertility in plants: pollen limitation, resource allocation and genetic load. Trends Ecol Evol 4:289−292
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annual Rev Ecol Syst 18:237−268
Devesa, JA, Ortega-Olivencia A (2004) Especies vegetales protegidas en España: plantas vasculares. Ministerio de Medio Ambiente, Madrid
Ehrlén J (1991) Why do plants produce surplus flowers? A reserve-ovary model. Amer Naturalist 138:918−933
Ehrlén J (1992) Proximate limits to seed production in a herbaceous perennial legume, Lathyrus vernus. Ecology 73:1820−1831
Fenster CB (1991) Effect of male pollen donor and female seed parent on allocation of resources to developing seeds and fruit in Chamaecrista fasciculata (Leguminosae). Amer J Bot 78:13−23
Horvitz CC, Schemske DW (1988) A test of the pollinator limitation hypothesis for a neotropical herb. Ecology 69:200−206
Janzen DH (1969) Seed-eaters versus seed size, number, toxicity and dispersal. Evolution 23:1−27
Karoly K (1992) Pollinator limitation in the facultatively autogamous annual, Lupinus nanus (Leguminosae). Amer J Bot 79:49−56
Kaye TN (1999) From flowering to dispersal: reproductive ecology of an endemic plant, Astragalus australis var. olympicus (Fabaceae). Amer J Bot 86:1248−1256
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman T-L (2005) Pollen limitation of plant reproduction: pattern and process. Annual Rev Ecol Evol Syst 36:467−497
Mikesell J (1988) Comparative development of viable and aborted ovules in Phytolacca americana L. (Phytolaccaceae). Bot Gaz 149:196−202
Mitchell R (1977) Bruchid beetles and seed packaging by Palo Verde. Ecology 58:644−651
Ortega-Olivencia A, Rodríguez-Riaño T, Valtueña FJ, López J, Devesa JA (2005) First confirmation of a native bird-pollinated plant in Europe. Oikos 110:578−590
Paiva J (1999) Anagyris. In Talavera S, Aedo C, Castroviejo S, Romero Zarco C, Sáez L, Salgueiro FJ, Velayos M (eds) Flora Iberica 7/1. Leguminosae (partim). Real Jardín Botánico Madrid, CSIC, Madrid, pp 37−39
Palamarev E (1989) Paleobotanical evidences of the Tertiary history and origin of the Mediterranean sclerophyll dendroflora. Pl Syst Evol 162:93−107
Rodríguez-Riaño T, Ortega-Olivencia A, Devesa JA (1999) Reproductive biology in two Genisteae (Papilionoideae) endemic of the western Mediterranean region: Cytisus striatus and Retama sphaerocarpa. Canad J Bot 77:809−820
Rodríguez-Riaño T, Ortega-Olivencia A, Devesa JA (2004) Reproductive biology in Cytisus multiflorus (Fabaceae). Ann Bot Fenn 41:179−188
Santandreu M, Lloret F (1999) Effect of flowering phenology and habitat on pollen limitation in Erica multiflora. Canad J Bot 77:734−743
Siemens DH, Johnson CD (1995) Number of seeds per pod in three species of perennial legumes: a compromise between ecological and physiological constraints? Amer Midl Naturalist 133:197−205
Siemens DH, Johnson CD, Ribardo KJ (1992) Alternative seed defense mechanisms in congeneric plants. Ecology 73:2152−2166
Sokal RR, Rohlf FJ (1979) Biometría. Blume, Madrid
SPSS (2002) SPSS for windows, release 11.5.1. SPSS, Chicago
Stephenson AG (1981) Flower and fruit abortion: proximate causes and ultimate functions. Annual Rev Ecol Syst 12:253−279
Stephenson AG (1984) The regulation of maternal investment in an indeterminate flowering plant (Lotus corniculatus). Ecology 65:113−121
Stephenson AG, Winsor JA (1986) Lotus corniculatus regulates offspring quality through selective fruit abortion. Evolution 40:453−458
Sutherland S (1986a) Floral sex ratios, fruit-set, and resource allocation in plants. Ecology 67:991−1001
Sutherland S (1986b) Patterns of fruit-set: what controls fruit-flower ratios in plants? Evolution 40:117−128
Sutherland S (1987) Why hermaphroditic plants produce many more flowers than fruits: experimental tests with Agave mckelveyana. Evolution 41:750−759
Valtueña FJ, Ortega-Olivencia A, Rodríguez-Riaño T (2007) Nectar production in Anagyris foetida (Fabaceae): two types of concentration in flowers with hanging droplet. Int J Pl Sci 168:627−638
Valtueña FJ, Ortega-Olivencia A, Rodríguez-Riaño T, López J (2008a) Reproductive biology in Anagyris foetida L. (Leguminosae), an autumn-winter flowering and ornithophilous Mediterranean shrub. Bot J Linn Soc 157:519−532
Valtueña FJ, Ortega-Olivencia A, Rodríguez-Riaño T (2008b) Germination and seed bank biology in some Iberian populations of Anagyris foetida L. (Leguminosae). Pl Syst Evol 275:231–243
Vaughton G, Ramsey M (1997) Seed mass variation in the shrub Banksia spinulosa (Proteaceae): resources constraints and pollen source effects. Int J Pl Sci 158:424−431
Vázquez DP, Simberloff D (2004) Indirect effects of an introduced ungulate on pollination and plant reproduction. Ecol Monogr 74:281−308
Wesselingh RA (2007) Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytol 174:26−37
Wiens D (1984) Ovule survivorship, brood size, life history, breeding systems, and reproductive success in plants. Oecologia 64:47−53
Wiens D, Calvin CL, Wilson CA, Davern CI, Frank D, Seavey SR (1987) Reproductive success, spontaneous embryo abortion, and genetic load in flowering plants. Oecologia 71:501−509
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends Pl Sci 7:270−277
Willson MF (1979) Sexual selection in plants. Amer Naturalist 113:777−790
Willson MF, Schemske DW (1980) Pollinator limitation, fruit production, and floral display in Pawpaw (Asimina triloba). Bull Torrey Bot Club 107:401−408
Zimmerman M, Pyke GH (1988) Reproduction in Polemonium: assessing the factors limiting seed set. Amer Naturalist 131:723−738
Acknowledgements
This work was financed by the Ministry of Education and Science of Spain through projects BOS2002-00703 and CGL2005-00783/BOS, both co-financed by ERDF. A pre-doctoral grant of that Ministry to FJV (BES-2003-2187) is greatly appreciated. We thank Dr. R.A. Wesselingh and an anonymous referee for their comments and criticisms that notably improved the earlier draft and Dr. Miguel González for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valtueña, F.J., Ortega-Olivencia, A., Rodríguez-Riaño, T. et al. Causes of Low Fruit and Seed Set in Bird-Pollinated Anagyris foetida (Leguminosae): Pollen Limitation and Other Extrinsic Factors. Folia Geobot 45, 77–94 (2010). https://doi.org/10.1007/s12224-009-9054-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-009-9054-9