Abstract
Aerobic methane-oxidizing bacteria (MOB) are an environmentally significant group of microorganisms due to their role in the global carbon cycle. Research conducted over the past few decades has increased the interest in discovering novel genera of methane-degrading bacteria, which efficiently utilize methane and decrease the global warming effect. Moreover, methanotrophs have more promising applications in environmental bioengineering, biotechnology, and pharmacy. The investigations were undertaken to recognize the variety of endophytic methanotrophic bacteria associated with Carex nigra, Vaccinium oxycoccus, and Eriophorum vaginatum originating from Moszne peatland (East Poland). Methanotrophic bacteria were isolated from plants by adding sterile fragments of different parts of plants (roots and stems) to agar mineral medium (nitrate mineral salts (NMS)) and incubated at different methane values (1–20% CH4). Single colonies were streaked on new NMS agar media and, after incubation, transferred to liquid NMS medium. Bacterial growth dynamics in the culture solution was studied by optical density—OD600 and methane consumption. Changes in the methane concentration during incubation were controlled by the gas chromatography technique. Characterization of methanotrophs was made by fluorescence in situ hybridization (FISH) with Mg705 and Mg84 for type I methanotrophs and Ma450 for type II methanotrophs. Identification of endophytes was performed after 16S ribosomal RNA (rRNA) and mmoX gene amplification. Our study confirmed the presence of both types of methanotrophic bacteria (types I and II) with the predominance of type I methanotrophs. Among cultivable methanotrophs, there were different strains of the genus Methylomonas and Methylosinus. Furthermore, we determined the potential of the examined bacteria for methane oxidation, which ranged from 0.463 ± 0.067 to 5.928 ± 0.169 μmol/L CH4/mL/day.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivation of methanotrophs was started in 1906 by Söhngen, who isolated Bacillus methanicus (now known as Methylomonas methanica) (Söhngen 1906). Since that time, the number and diversity of cultured methanotrophs have significantly increased. The crucial studies on cultivation of methanotrophs were performed by Whittenbury et al. (1970), who isolated more than 100 strains of these bacteria from various environments. The two mineral media containing nitrate or ammonium mineral salts (NMS and AMS) optimized by Whittenbury et al. are still widely used. So far, methanotrophic bacteria have been detected in different types of ecosystems both natural: soils, deserts, landfills, tundra, wetlands, rice paddies, sediments, lakes, marine (Hanson and Hanson 1996), as well as in the atmosphere (Santl-Temkiv et al. 2013), coal mines (Stępniewska et al. 2006), and mercury mines (Baesman et al. 2015) as well as engineered ecosystems, e.g., waste water treatment plant (Ho et al. 2013).
Wetlands are the main natural source of methane. This emission is the result of the balance between methanogenesis and methanotrophic processes and is actively affected by the composition of wetland plants, which can influence CH4 production, consumption, and transport in the soil. Studies on this phenomenon indicated a significant role of methanotrophic bacteria in CH4 emissions, both the free-living microorganisms in the rhizosphere attached to the root surface in the form of a biofilm (rhizoplane) and those living inside host tissues (endophytes) and colonizing parts of plants (Raghoebarsing et al. 2005; Liebner et al. 2011; Stępniewska et al. 2013; Stępniewska and Kuźniar 2013; Putkinen et al. 2014). The mechanism of the association of methanotrophs with peat vascular plants is poorly recognized and is currently the subject of studies undertaken by many researchers throughout the world (Fig. 1).
During the first step in methane oxidation to CO2, an important role is played by methane monooxygenase (MMO), which catalyzes the conversion of methane to methanol (Hanson and Hanson 1996). Methane monooxygenase occurs in two forms: membrane-bound, particulate (pMMO) and cytoplasmic soluble (sMMO). sMMO is responsible for the catalysis of oxidation of some hydrocarbons (saturated, unsaturated, linear, branched, and cyclic hydrocarbons, single- and double-ring aromatics, heterocycles, halogenated alkenes, and ethers (Dunfield et al. 1999)). All aerobic methanotrophs contain one or both MMOs. pMMO is coded by a pmoCAB operon and sMMO by genes including mmoXYBZDC. The pmoA and mmoX genes are usually used as functional markers for methanotrophic bacteria. Detection of pmoA or mmoX gene sequences from new isolates combined with determination of the 16S ribosomal RNA (rRNA) gene sequence is a necessary requirement for identification of methanotrophs (Dedysh and Dunfield 2014). mmoX is characteristic of most type II methanotrophs as well as in members of the genus Methylococcus and some Methylomonas strains (Shigematsu et al. 1999), Methylovulum miyakonense HT12 (Iguchi et al. 2011), but not in most of the other type I methanotrophs (Hanson and Hanson 1996).
Methanotrophs are multifunctional, harmless bacteria with promising applications in environmental bioengineering of methane removal and biodegradation of toxic compounds. The biodiversity of the methanotroph habitat especially with an established presence of sMMO gives many opportunities for application of these unique bacteria (Sullivan et al. 1998; Pandey et al. 2014).
Material and methods
Plant samples
Plant materials were collected from the Moszne Lake area located in the northwestern part of the Poleski National Park (51° 23′ N, 23° 63′ E), situated in the province of Lublin, in the Polish part of Polesie, where vegetation was dominated by Sphagnum magellanicum, Sphagnum fuscum, Sphagnum fallax, Aulacomium palustre, Polytrichum strictum, and Drosera rotundifolia with dense colonies of Vaccinium species, Eriophorum vaginatum, Eriophorum angustifolium, Carex rostrate, Carex nigra, and Carex gracilis.
Methanotrophic bacteria isolation and culture
Methanotrophic bacteria were isolated from plants C. nigra (C), Vaccinium oxycoccus (V), and E. vaginatum (E) by adding sterile fragments of each part of plant: roots (R) and stems (S) to agar mineral medium (NMS) (Whittenbury et al. 1970) and incubated in methane atmosphere 10% (v/v) CH4. Fragments of plants were sterilized by sequential immersion in 70% (v/v) ethanol for 5 min, and 0.9% (w/v) sodium hypochlorite solution for 20 min, and then surface-sterilized samples were washed in sterile water for three times to remove surface sterilization agents. Single colonies were streaked on new NMS agar media and, after incubation, transferred to liquid NMS medium. The cultures were incubated at 30 °C and 180 rpm (Innova 42R, New Brunswick Scientific, USA) under atmospheric conditions supplemented with 1, 5, 10, and 20% (v/v) CH4.
Bacterial growth dynamics was studied by absorbance and methane consumption. Changes in the methane concentrations were controlled during incubation by the gas chromatography technique. The headspace concentrations of gases (CH4, CO2, O2, N2) were analyzed by a gas chromatograph Shimadzu GC 2010 (Japan) equipped with a flame ionization detector and a thermal conductivity detector, after CH4, CO2, O2, and N2 calibrations. Bacterial growth dynamics was assessed by measuring absorbance at 600 nm (A 600) (UV1800, Shimadzu, Japan).
Calculation and statistical analysis
The methanotrophic activity of cultures was calculated from the slope of the regression line of the measured CH4 concentration vs. time. Adjustments were made with r 2 ≤ 0.95 with Sigma Plot 10.0 software (USA) and written as micromole per liter CH4 per milliliter of culture per day. MMO enzyme activity was characterized by a curve of initial velocity vs. substrate concentration, referred to as a Michaelis-Menten plot, with an inset Lineweaver-Burk plot. The maximum specific methane oxidation rate (Vmax) and saturation constant (Km) were determined using GraphPad Prism 6 software (USA).
DNA isolation and amplification
Bacterial DNA was isolated from the cultures with the method of Sambrook with own modifications. (Sambrook 1989). Cultured cells were harvested by centrifugation at 17500g for 5 min and subjected to lysis using 5 mol/L guanidine thiocyanate, 100 mmol/L EDTA, and 0.5% sarcosyl (pH 8.0). DNA was purified by extraction with ice-cold 7.5 mol/L ammonium acetate (10 min) and, subsequently, using a chloroform/3-methyl-1-butanol (24:1, v/v) mixture (1 min). The two-phase mixture was centrifuged at 17500g for 10 min. The upper layer was collected into a new tube. DNA was precipitated at −20 °C with 0.8 volumes of 2-propanol for 1 h. The pellet was separated by centrifugation at 17,500g for 30 min, rinsed five times with 70% (v/v) ethanol, dried under vacuum (RVC 2_18, Christ, Germany), and resuspended in 30 mL of ultrapure, DNAse-free water. The purity and concentration of the isolates were evaluated spectrophotometrically (UV_1800, Shimadzu, Japan). 16S rRNA genes were amplified from enrichment culture DNA using primers 27f (AGAGTTTGATCMTGGCTCAG) and 1492r (TACGGYTACCTTGTTACGACTT), (DeLong 1992). The presence of the mmoX gene in the isolates encoding sMMO, which are key enzymes in the bacterial methane metabolism pathway, was used for authentication of the isolates. mmoX gene was detected due to the oxidation of methane in sMMO is critical for understanding the microbial applications of C-H activation in one-carbon substrates and limited diversity of sMMO-containing methanotrophs (Lee 2016). The presence of genes in the isolates was detected by partial amplification of the genes using specific primers. The following primer pairs were used for mmoX (mmoX1 CGGTCCGC TGTGGAAGGGCATGAAGCGCGT and mmoX2 - GGCTCGACCTTGAACTTGGAGCCA TACTCG) (Miguez et al. 1997). The PCR reaction mixture (50 μL) contained PCR Master Mix (2×): 0.05 U/μL TaqDNA polymerase, reaction buffer, 4 mmol/L MgCl2 and 0.4 mmol/L of each dNTP, (Thermo Scientific); forward and reverse primers at 0.1 mmol/L and 3 μL of template DNA. PCR amplification was performed in a TProfessional gradient system thermocycler (Biometria, Germany) using the following conditions: initial denaturation at 95 °C (1 min); 30 cycles consisting of denaturation 95 °C (0.30 min), annealing 55 °C (1 min) for 16S rRNA, 62.5 °C (0.55 min) for mmoX primers; extension 72 °C (1 min); and final extension 72 °C (5 min). Isolated DNA and PCR products were analyzed by gel electrophoresis on MiniSubR Cell GT (Bio_Rad Laboratories Ltd., UK), stained with ethidium bromide, and visualized in UV light in the Red™ Imaging System (Alpha Innotech, USA).
DNA sequence analysis
The identification of the cultured plant endophytes was performed after PCR product sequencing (Genomed S.A., Poland) and compared with sequences stored in NCBI (USA) using the BLASTN algorithm.
Nucleotide sequence
The 16S rRNA gene sequences of the methanotrophic communities examined in this study were deposited in GenBank databases under accession numbers KT860052, KT860053, KT860054, KT860055, and KT860056 numbers. Moreover, as a result of our investigation, we added mmoX gene sequences as KT962955, KT962956, KT962957, KT962958, and KT962959 to the GenBank databases (Table 1).
FISH from endophyte cultures
FISH was performed according to Eller et al. (2001) with minor modifications using cells growing in the logarithmic phase. Cells were harvested by centrifugation and resuspended in 0.5 mL of phosphate-buffered saline (PBS). The suspensions were then mixed with 1 mL of 4% (w/v) paraformaldehyde and fixed for 3 h. The fixed cells were collected by centrifugation (6000g for 1 min) and washed twice with PBS. The resulting pellet was resuspended in 50% ethanol in PBS and stored at −20 °C until use. The hybridization was carried out on slides where 15 μL of fixed cells suspensions were transferred and left to dry. Dehydration was performed by washing the slides in 50, 80, and 96% (v/v) ethanol for 3 min each. A 50-mL polypropylene falcon tube containing a slip of filter paper soaked in hybridization buffer was used as a hybridization chamber. Ten-microliter hybridization buffer (1 mol/L Tris-HCl (pH 8.0), 5 mol/L NaCl, 10% sodium dodecyl sulfate and 20% formamide), and 1 μL of a fluorescent probe solution was placed on each spot of the fixed cells. The specificity of the probes applied is presented in Table 2. The chamber was incubated for 90 min at the hybridization temperature (47 °C). Then, the slides were washed at the hybridization temperature for 15 min in washing buffer (1 mol/L Tris-HCl, 0.5 mol/L EDTA, and 5 mol/L NaCl) and rinsed twice with distilled water. The slides were air-dried and stained with Vectashield Mounting medium containing DNA-staining DAPI (Vector Laboratories, USA). The slides were analyzed using fluorescence with a Nikon Eclipse 80i microscope. The pictures were taken with a Digital Sight camera (Nikon) and processed with software provided by the manufacturer.
Results
Endophyte enrichment culture growth
Endophytes of C. nigra, V. oxycoccus, and E. vaginatum were grown on 5, 10, and 20% of CH4 at 30 °C. Cultivations of all methane-oxidizing bacteria (MOB) consortia were characterized by the dynamics of the gaseous phase. A steady decrease in the methane and oxygen concentration was shown, as well as accumulation of carbon dioxide (Fig. 2). The CH4 consumption in the all investigated enrichment culture showed a decrease in the CH4 concentration during 4–6 days of incubation.
Rapid growth of all investigated MOB consortia was observed; it was reflected by an increase absorbance in the range from about 0.1 to 0.8–1.8 after 3 to 6 days of cultivation, which was accompanied by a decline in the CH4 mixing ratio in the headspace, while no growth was observed in the same medium in the absence of methane. The complete consumption of CH4 by methanotrophic consortium cultures after 3 days under 1–10% CH4 was demonstrated. At the highest substrate concentration (20% CH4), the presence of 6% of methane was shown after 6 days of cultivation (Fig. 3).
Methanotrophic activity of the endophytes
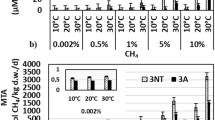
On the basis of the consumption of methane during culture, the methanotrophic activity of the endophytes was determined. A clear decrease in the CH4 concentration tested in the headspace was shown after 4–6 days of incubation. Methane consumption by the consortium isolated from the roots of wetland plants ranged from 0.463 ± 0.067 to 5.928 ± 0.169 μmol/L CH4/mL/day and from the stems of these plants from 0.499 ± 0.119 to 5.305 ± 0.022 μmol/L CH4/mL/day, depending on the initial CH4 concentration (Fig. 4). The dependence of the methanotrophic activity of isolated methanotrophs was significantly related to the substrate concentration (p < 0.0001) and did not differ significantly between the plant species.
Kinetic parameters of CH4 oxidation
The CH4 oxidation rates were written correctly in the substrate saturation curves as a function of initial methane concentrations (Fig. 5) and showed typical Michaelis-Menten characteristics. Lineweaver-Burk plots based on regression of 1/V vs. 1/S are presented as insets in Fig. 6. The apparent Km and Vmax values obtained for the tested enrichment culture are summarized in Table 3. The methane oxidation by the methanotrophic consortium enrichment cultures followed the Michaelis-Menten mechanism. In a majority of the analyzed cases, the specific methane oxidation rates increased in parallel with the initial methane concentration, but only the CS consortium enrichment culture achieved the maximum rate at 10% methane 5.033 ± 0.823 μmol/L CH4/mL/day. Based on the Lineweaver-Burk plot (Fig. 5), Vmax, and Km were calculated in the range from 9.521 ± 1.280 to 7.539 ± 1.115 μmol/L CH4/mL/day and from 142.3 ± 40.69 to 95.42 ± 34.96 μmol/L CH4/mL/day, respectively (Table 3).
Fluorescent in situ hybridization of the consortium from C. nigra roots (a) and stems (b), V. oxycoccus stems (c) and E. vaginatum roots after hybridization with Mg84 and Mg705 probes (type I) green colored, Ma450 probe (type II) red colored, and DAPI staining probes (non-methanotrophic) blue colored
Fluorescence in situ hybridization
Characterization of MOB was performed by fluorescence in situ hybridization (FISH) with Mg705 and Mg84 for type I methanotrophs and Ma450 for type II methanotrophs. Microscopic observations confirmed the presence of methanotrophic microorganisms isolated from the plants and grown on NMS medium. The combination of oligonucleotide probes Mg84 and Mg705 with Ma450 allowed parallel detection of type I and type II methanotrophs with the predominance of type I. Type I cells (shown as green) were short 1.5–3-μm-long rods and were all similar in size and shape. Type II methanotrophs were less numerous but more diverse. These bacterial cells represented at least two distinct morphologies: straight (1–2 μm long) and irregular, curved bacilli (0.5–1 μm). Simultaneously, the staining with DAPI revealed that methanotrophic bacteria were present in the community with non-methanotrophic cells which are almost as numerous as the methanotrophic cells (Fig. 6).
Identification of cultured endophytic methanotrophs
Analysis of the partial 16S rRNA gene sequences of the isolated MOB showed that they clustered closely with homologous sequences from different strains of the genus Methylomonas and numerous uncultured bacteria (Table 4).
It was found that sequences obtained from the endophyte enrichment culture with C. nigra stream (PPN CS1) were related with 99–98% identity to Methylomonas sp. [KJ081955.1, KT799650.1, NR_113033.1]. Isolate of C. nigra root (PPN CR2) demonstrated affiliation to bacteria but only 93% identity to Methylomonas sp. [KT799650.1, FR798966.1, FR798963.1]. The sequences of the methanotrophic microbial consortium isolated from V. oxycoccus (PPN VS1) showed affiliation to bacteria with 8–9% divergence from Methylomonas sp. [KJ081955.1, FR798968.1] and uncultured bacterium clone DS-10 [KC424661.1]. The population isolated from E. vaginatum (PPN E1) was related with 97–96% identity to Methylomonas sp. [KJ081955.1, AB683103.1, AB636300.1]. In the PPN ER2 isolate, we identified 95% similarity mainly to the uncultured bacterium (JN698029.1, AM086104.1, KR095256.1). Molecular identification on the basis of mmoX revealed high similarity of the methanotrophs cultured on the NMS medium to the genera Methylosinus and Methylomonas (Table 5).
Discussion
It was confirmed that methanotrophic bacteria have great potential as microbial sinks for the greenhouse gas methane as well as for industry in different biotechnological solutions (Semrau et al. 2010). However, their application in biotechnology is limited by the number of suitable strains readily accessible, which were not specifically isolated for this purpose, and the lack of the necessary properties for efficient use on an industrial scale (Jiang et al. 2010). Among the disadvantages, the relatively slow growth rate of the available strains may be mentioned (Hoefman et al. 2010). In this context, searching for new methanotroph strains and investigation of methanotrophic bacterial communities takes on a new meaning.
The endophytic bacterial community isolated in our study from vascular peatland plants C. nigra (PPN CS1) and E. vaginatum (PPN ER1) are closely related to Methylomonas sp. belonging to type Ia methanotrophs, known as fast-growing MOB with a short generation time of 3.5 h (Whittenbury et al. 1970). Type II MOB is generally known to grow more slowly, with generation times ranging from 5 h up to several days (Whittenbury et al. 1970; Dedysh et al. 2000, 2002; Vorobev et al. 2010). The investigated methanotrophic consortium cultures were characterized by rather rapid growth, reaching absorbance 0.8–1.8 after 3–6 days of cultivation, depending on the initial CH4 concentration. As reported by literature, methanotrophs possessing pMMO show good growth with A 600 reaching 0.8–1.5 within 3–6 days. By contrast, methanotrophs with only sMMO may require up to 2–3 weeks until the cultures reaches A 600 of 0.2–0.3 (Dedysh and Dunfield 2014). The fast growth rate and lower initial cell numbers in combination shows typical R-type life strategy of the methanotrophs, investing in reproduction (Steenbergh et al. 2010). Methanotrophic enrichment culture isolated from C. nigra (PPN CS2), V. oxycoccus (PPN VS1), and E. vaginatum (PPN ER2) revealed 16S rRNA gene sequence similarities of less than 98% which does not allow to determination of the genus.
Methanotrophs associated with C. nigra stream were closely related to the strains found previously in waterlogged conditions such as wastewater (KJ081955.1), waste sludge (KT799650.1) and floodwater of paddy fields (NR_113033.1). Bacteria similar to these inhabiting C. nigra roots were found in wetlands (FR798966.1, FR798963.1). V. oxycoccus stream endophytes were similar to bacteria isolated from waste water (KJ081955.1) and wetland (FR798968.1) and to uncultured bacteria from lake sediments. The endophytes of E. vaginatum roots were similar to bacteria isolated from wastewater (KJ081955.1), root of Acorus calamus var. angustatus (AB683103.1), rice root (AB636300.1), and numerous uncultured bacteria e.g. isolated from the rice root endosphere (JN698029.1), profundal lake sediment (AM086104.1), and Typha rhizosphere in wetlands of the Yongding River (KR095256.1) (Table 3). PPN CR2, PPN VS1, and PPN ER2 isolates may be particularly important because the nearest phylogenetic neighbor indicates the presence of a novel species. The full characterization and description of novel species which will be made in the future will complement these studies.
Stępniewska and Kuźniar (2014)) cultured endophytic methanotrophs isolated from different Sphagnum species originating from Moszne peatbog. The growth of this endophytic population was characterized by an increase in absorbance in the range from 0.3 to 2.0 after 12–13 days of incubation. Among cultivable type I methanotrophs, Stepniewska and Kuzniar (2014) identified different strains of the genus Methylomonas, whereas type II methanotrophs were represented by cultured strains belonging to the genera Methylocystis and Methylosinus. Methane consumption by the isolated consortiums ranged from 0.463 ± 0.067 to 5.928 ± 0.169 μmol/L CH4/mL/day. Methanotrophs isolated from different Sphagnum species and cultured at the same conditions were characterized by a lower MA, reaching maximum of 4.7 μmol/L CH4/mL/day in the isolated populations from S. magellanicum (Stepniewska and Kuzniar 2014).
sMMO is present in most type II methanotrophs and in members of the genus Methylococcus and Methylomonas (Shigematsu et al. 1999). In this study, based on the 16S rRNA, gene Methylomonas sp. affiliation was found, but amplification of the mmoX gene and DNA sequencing indicates the presence of the genus Methylosinus and Methylomonas. This data suggest that analysis of sequence of mmoX allowed noting a closer affinity to the GenBank database sequences and classification of isolates to Methylosinus sp. The limited diversity of sMMO may be related to constraints on the genetic diversity of this enzyme due to the conservation of the enzyme function, or may simply be found because strategies used to isolate methanotrophs by culturing or detection of mmoX by PCR are yet to reveal the true diversity of sMMO-containing methanotrophs.
Methanotrophic bacteria of the genus Methylosinus and Methylomonas are widespread in different environments such as: peatland soil (Szafranek-Nakonieczna et al. 2012; Danilova et al. 2013; Esson et al. 2016), peatland plants (Raghoebarsing et al. 2005; Iguchi et al. 2012; Stępniewska and Kuźniar 2014), lake sediment (Nercessian et al. 2005), paddy field (Ogiso et al. 2012), seawater (Boden et al. 2011), and coal mine (Wolińska et al. 2013). On the basis of these studies and the other data (e.g., Iguchi et al. 2012; Stępniewska and Kuźniar 2014), which indicate the presence of Methylomonas sp. and Methylosinus sp. associated with plants (mosses, herbaceous plants, woody plants), we can assume that these microorganisms may be particularly preferred by plants. The mechanism of this specific association requires further study. However, the coexistence of these bacteria with vascular plants can be justified by the ability to utilize methanol by Methylomonas sp. and Methylosinus sp. (Gayazov et al. 1990; Xin et al. 2010; Danilova et al. 2013). It is known that based on plant structure and metabolic properties, particularly the demethylation of pectin in the primary cell walls, plants produce methanol (Galbally and Kirstine 2002) and plant-associated methylobacteria was confirmed (Kutschera 2007).
Endophytes isolated from Sphagnum sp. and vascular peatland plants investigated in this study were characterized as communities. Many colonies that appear on solid mineral media after incubation in the presence of the methane belong to non-methanotrophs. Typical examples of such non-methanotrophic bacteria are members of the genera Burkholderia and Bradyrhizobium (Dedysh and Dunfield 2014). Microorganisms from communities interacting at multi-trophic levels (Naeem and Li 1997; Naeem et al. 2000) are grouped into autotrophs (primary producers) and heterotrophs (decomposers). This kind of relationships may be observed in methane driven ecosystems, where the methanotroph can be considered as a primary producer and interacts with heterotrophs (Hutchens et al. 2004; Iguchi et al. 2011; van Duinen et al. 2013; Agasild et al. 2014). Ho et al. (2014) showed that methanotrophic activity was positively correlated with heterotroph richness. Methanotrophs require associated microorganisms which can be important for growth and functioning (Hoefman et al. 2010).
This fact seems to be very important especially in connection with ecological significance of association MOB consortia with plants. Studies about Sphagnum sp. and endophytic methanotrophs such as: Raghoebarsing et al. (2005), Kip et al. (2010), Liebner et al. (2011), Stępniewska et al. (2013) and Stępniewska and Kuźniar (2014) showed that methane, being oxidized by methanotrophs to carbon dioxide, becomes an additional source of C to the plants. On the other hand the mosses provide oxygen and ecological niche for the methanotrophs. Recent studies Ho and Bodelier (2015) and Kox et al. (2016), emphasize that CH4–N cycle interactions were found in the methanotrophs associated with submerged mosses. The presence of a methanotrophic diazotrophs and fixation of N2 (diazotrophic activity) at a level of from 2 to 3 up to 35% of the N assimilated by Sphagnum sp. was demonstrated (Berg et al. 2013; Larmola et al. 2014). A similar interaction was observed in methanotrophic bacteria association with rice root (Minamisawa et al. 2016). These arguments testify to the fact that methanotrophs are an important component of the microflora of peat land.
Literature describes a wide range of methanotoph applications and is constantly looking for new efficient strains for biotechnology applications. The richness of non-methanotrophic bacteria associated with the endophyte community was confirmed by FISH analysis (Fig. 6). However, further studies are needed to determine whether the effect of the heterotroph richness is species specific and can be extrapolated to other methanotrophs.
In conclusion, the results of this study demonstrate that cultivable bacteria existing as endophytes of vascular plants belong to type I methanotrophs and are characterized by fast growth and relatively high methanotrophic activity. Moreover, we showed that the community composed of methanotrophs and non-methanotrophs has potential applications in biotechnology systems.
References
Agasild H, Zingel P, Tuvikene L, Tuvikene A, Timm H, Feldmann T et al (2014) Biogenic methane contributes to the food web of a large, shallow lake. Freshwat Biol 59:272–285. doi:10.1111/fwb.12263
Baesman SM, Miller LG, Wei JH, Cho Y, Matys ED, Summons RE, Welander PV, Oremland RS (2015) Methane oxidation and molecular characterization of methanotrophs from a former mercury mine impoundment. Microorganisms 3:290–309. doi:10.3390/microorganisms3020290
Berg A, Danielsson Å, Svensson BH (2013) Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant Soil 362(1–2):271–278
Boden R, Cunliffe M, Scanlan J, Moussard H, Kits KD, Klotz MG, Mikhailova N (2011) Complete genome sequence of the aerobic marine methanotroph Methylomonas methanica MC09. J Bacteriol 193(24):7001–7002
Danilova OV, Kulichevskaya IS, Rozova ON, Detkova EN, Bodelier PL, Trotsenko YA, Dedysh SN (2013) Methylomonas paludis sp. nov., the first acid-tolerant member of the genus Methylomonas, from an acidic wetland. Int J Syst Evol Microbiol 63(6):2282–2289
Dedysh SN, Liesack W, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD et al (2000) Methylocella palustris gen. Nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bags, representing a novel subtype of serine-pathway methanotrophs. Int J Syst Evol Microbiol 50:955–969
Dedysh SN, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Liesack W, Tiedje JM (2002) Methylocapsa acidiphila gen. nov., sp. nov., a novel methane-oxidizing and dinitrogen-fixing acidophilic bacterium from Sphagnum bog. Int J Syst Evol Microbiol 52:251–261
DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci U S A 89:5685–5689
Dunfield PF, Dedysh SN (2014) Methylocella: a gourmand among methanotrophs. Trends Microbiol 22(7):368–369. doi:10.1016/j.tim.2014.05.004
Dunfield PF, Liesack W, Henckel T, Knowels R, Conrad R (1999) High-affinity methane oxidation by a soil enrichment culture containing a type II methanotroph. Appl Environ Microbiol 65:1009–1014
Eller G, Stubner S, Frenzel P (2001) Group-specific 16S rRNA targeted probes for the detection of type I and type II methanotrophs by fluorescence in situ hybridisation. FEMS Microbiol Lett 198(2):91–97
Esson KC, Lin X, Kumaresan D, Chanton JP, Murrell JC, Kostka JE (2016) Alpha-and gammaproteobacterial methanotrophs codominate the active methane-oxidizing communities in an acidic boreal peat bog. Appl Environ Microbiol 82(8):2363–2371
Galbally IE, Kirstine W (2002) The production of methanol by flowering plants and the global cycle of methanol. J Atmos Chem 43(3):195–229
Gayazov RR, Chetina EV, Mshenskii YN, Trotsenko YA (1990) Physiological and cytobiochemical characteristics of Methylomonas methanica grown on methane in the presence of methanol. Prikl Biokhim Mikrobiol 26(3):394–398
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Ho A, Bodelier PL (2015) Diazotrophic methanotrophs in peatlands: the missing link? Plant Soil 389(1–2):419–423
Ho A, Vlaeminck SE, Ettwig KF, Schneider B, Frenzel P, Boon N (2013) Revisiting methanotrophic communities in sewage treatment plants. Appl Environ Microbiol 79(8):2841–2846
Ho A, De Roy K, Thas O, De Neve J, Hoefman S, Vandamme P, Boon N (2014) The more, the merrier: heterotroph richness stimulates methanotrophic activity. The ISME journal 8(9):1945–1948. doi:10.1038/ismej.2014.74
Hoefman S, Boon N, de Vos P, Heylen K (2010) Protecting the fragile: preservation of methanotrophic bacteria. Cryobiology 61:362–408. doi:10.1016/j.cryobiol.2010.10.023. 0 0
Hutchens E, Radajewski S, Dumont MG, McDonald I, Murrell C (2004) Analysis of methanotrophic bacteria in Movile cave by stable isotope probing. Environ Microbiol 6:111–120
Iguchi H, Yurimoto H, Sakai Y (2011) Stimulation of methanotrophic growth in cocultures by cobalamin excreted by Rhizobia. Appl Environ Microbiol 77:8509–8515. doi:10.1128/AEM.05834-11
Iguchi H, Sato I, Sakakibara M, Yurimoto H, Sakai Y (2012) Distribution of methanotrophs in the phyllosphere. Bioscience biotechnology and biochemistry 76(8):1580–1583
Jiang H, Chen Y, Jiang P, Zhang C, Smith TJ, Murrell JC, Xing XH (2010) Methanotrophs: multifunctional bacteria with promising applications in environmental bioengineering. Biochem Eng J 49:277–288. doi:10.1016/j.bej.2010.01.003
Kiene RP (1991) Production and consumption of methane in aquatic systems. In Microbial production and consumption of greenhouse gases: methane, nitrogen oxides and halomethanes, pp. 11 1–146. Edited by J. E. Rogers & W. B. Whitman. Washington, DC: American Society for Microbiology
Kip N, van Winden JF, Pan Y, Bodrossy L, Reichart GJ, Smolders AJ, den Camp HJO (2010) Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat Geosci 3(9):617–621
Kox MA, Lüke C, Fritz C, van den Elzen E, van Alen T, den Camp HJO, Lamers LPM, Jetten MSM, Ettwig KF (2016) Effects of nitrogen fertilization on diazotrophic activity of microorganisms associated with Sphagnum magellanicum. Plant Soil 406(1–2):83–100
Kutschera U (2007) Plant-associated methylobacteria as co-evolved phytosymbionts: a hypothesis. Plant Signal Behav 2(2):74–78
Larmola T, Leppänen SM, Tuittila ES, Aarva M, Merilä P, Fritze H, Tiirola M (2014) Methanotrophy induces nitrogen fixation during peatland development. Proc Natl Acad Sci 111(2):734–739
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol 37:25–50
Lee SJ (2016) Hydroxylation of methane through component interactions in soluble methane monooxygenases. J Microbiol 54(4):277–282
Liebner S, Zeyer J, Wagner D, Schubert C, Pfeiffer EM, Knoblauch C (2011) Methane oxidation associated with submerged brown mosses reduces methane emissions from Siberian polygonal tundra. J Ecol 99(4):914–922
Miguez CB, Bourque D, Sealy JA, Greer CW, Groleau D (1997) Detection and isolation of methanotrophic bacteria possessing soluble methane monooxygenase (sMMO) genes using the polymerase chain reaction (PCR). Microb Ecol 33(1):21–31
Minamisawa K, Imaizumi-Anraku H, Bao Z, Shinoda R, Okubo T, Ikeda S (2016) are symbiotic methanotrophs key microbes for N acquisition in paddy rice root? Microbes Environ 31(1):4
Naeem S, Li S (1997) Consumer species richness and autotrophic biomass. Ecol 79:2603–2615
Naeem S, Hahn DR, Schuurman G (2000) Producer-decomposer co-dependency influences biodiversity effects. Nature 403:762–764
Nercessian O, Noyes E, Kalyuzhnaya MG, Lidstrom ME, Chistoserdova L (2005) Bacterial populations active in metabolism of C1 compounds in the sediment of Lake Washington, a freshwater lake. Appl Environ Microbiol 71(11):6885–6899
Ogiso T, Ueno C, Dianou D, Van Huy T, Katayama A, Kimura M, Asakawa S (2012) Methylomonas koyamae sp. nov., a type I methane-oxidizing bacterium from floodwater of a rice paddy field. Int J Syst Evol Microbiol 62(8):1832–1837
Pandey VC, Singh JS, Singh DP, Singh RP (2014) Methanotrophs: promising bacteria for environmental remediation. Int J Environ Sci Technol 11(1):241–250
Putkinen A, Larmola T, Tuomivirta T, Siljanen HM, Bodrossy L, Tuittila ES, Fritze H (2014) Peatland succession induces a shift in the community composition of sphagnum-associated active methanotrophs. FEMS Microbiol Ecol 88(3):596–611. doi:10.1111/1574-6941.12327
Raghoebarsing AA, Smolders AJ, Schmid MC, Rijpstra WI, Wolters-Arts M, Derksen J et al (2005) Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 436:1153–1156. doi:10.1038/nature03802
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, Cold Spring Harbor Laboratory, New York
Santl-Temkiv T, Finster K, Hansen BM, Pasic L, Karlson UG (2013) Viable methanotrophic bacteria enriched from air and rain can oxidize methane at cloud-like conditions. Aerobiologia 29:373–384. doi:10.1007/s10453-013-9287-1
Semrau JD, DiSpirito AA, Yoon S (2010) Methanotrophs and copper. FEMS Microbiol Rev 34(4):496–531. doi:10.1111/j.1574-6976.2010.00212.x
Shigematsu T, Hanada S, Eguch M, Kamagata Y, Kanagawa T, Kurane R (1999) Soluble methane monooxygenase gene clusters from trichloroethylene-degrading Methylomonas sp. Strains and Detection of Methanotrophs during In Situ Bioremediation Appl Environ Microbiol 65:5198–5206
Söhngen NL (1906) Über Bakterien, welche Methan als Kohlenstoffnahrung and Energiequelle gebrauchen. Zentralbl Bakteriol Parasitik. Abt I 15:513–517
Steenbergh AK, Meima MM, Kamst M, Bodelier LEP (2010) Biphasic kinetics of a methanotrophic community is a combination of growth and increased activity per cell. FEMS Microbiol Ecol 71:12–22
Stępniewska Z, Kuźniar A (2014) Cultivation and detection of endophytic aerobic methanotrophs isolated from sphagnum species as a perspective for environmental biotechnology. AMB Express 4:58. doi:10.1186/s13568-014-0058-3
Stępniewska Z, Szmagara A, Niewiarowska M (2006) The environmental requirements of methanotrophic bacteria inhabiting coal mine dump rock. International Workshop “Pathways of pollutant from landfills to air and water-soil systems and mitigation strategies of their impact on the ecosystems”. The Conference Proceedings Kazimierz Dolny, 17–20
Stępniewska Z, Kuźniar A, Pytlak A, Szymczycha J (2013) Detection of methanotrophic endosymbionts in Sphagnum sp. originating from Moszne peat bog (East Poland). African Journal Microbiology Research 7:1319–1325
Sullivan JP, Dickinson D, Chase HA (1998) Methanotrophs, Methylosinus trichosporium OB3b, sMMO, and their application to bioremediation. Crit Rev Microbiol 24(4):335–373
Szafranek-Nakonieczna A, Stepniewska Z, Woloszyn A, Ciepielski J (2012) Methanotrophs responsible for methane oxidation in natural peats from Polesie Lubelskie region. Acta Agrophysica 19(1)
Van Duinen GA, Vermonden K, Bodelier PLE, Hendriks AJ, Leuven RSEW, Middelburg JJ et al (2013) Methane as a carbon source for the food web in raised bog pools. Freshwat Sci 32:1260–1272. doi:10.1899/12-121.1
Vorobev AV, Baani M, Doronina NV, Brady AL, Liesack W, Dunfield PF, Dedysh SN (2010) Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium possessing only a soluble methane monooxygenase. Int J Syst Evol Microbiol 61:2456–2463. doi:10.1099/ijs.0.028118-0
Whittenbury R, Phillips KC, Wilkinson JF (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218
Wolińska A, Pytlak A, Stępniewska Z, Kuźniar A, Piasecki C (2013) Identification of methanotrophic bacteria community in the Jastrzebie-Moszczenica coal mine by fluorescence in situ hybridization and PCR techniques TC 7: 0–001.
Xin JY, Zhang YX, Dong J, Zhou QQ, Wang Y, Zhang XD, Xia CG (2010) Epoxypropane biosynthesis by whole cell suspension of methanol-growth Methylosinus trichosporium IMV 3011. World J Microbiol Biotechnol 26(4):701–708
Acknowledgements
The research on methane emissions from peatlands is conducted by the Department of Biochemistry and Environmental Chemistry of the John Paul II Catholic University of Lublin under the National Science Centre project N305 29 94 40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stępniewska, Z., Goraj, W., Kuźniar, A. et al. Enrichment culture and identification of endophytic methanotrophs isolated from peatland plants. Folia Microbiol 62, 381–391 (2017). https://doi.org/10.1007/s12223-017-0508-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-017-0508-9