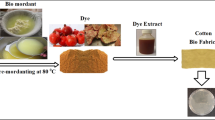
Abstract
The research reported in this paper concerns the examination of the ability to dye cotton textiles with plant-derived colorants in the presence of various natural additives. For this purpose, cotton textile samples were dyed with commercially available plant-derived dyes, which are usually used for food application, using a cold dyeing process in acidic conditions. The natural origin additives which were applied during the dyeing process were cosmetic grade and low molecular weight chitosan, nettle extract and shellac in an ethanol solution. The dyed fabrics were analyzed using FTIR spectroscopy, and the mechanical properties were tested to study the influence of colorants and additives on cotton textile properties or the dyeing process. Furthermore, the color stability under the influence of UVC irradiation was studied, using a colorimeter. The obtained results indicated that applied plant-derived colorants may effectively dye natural fabrics, such as cotton. The application of natural additives had a beneficial influence on cotton textile properties and the dyeing process. The pretreatment of cotton with chitosan, nettle extract or shellac improves the color stability following UVC irradiation of the material. Moreover, those additives can influence the mechanical properties of cotton textiles. Further research, however, is required to develop the most favorable dyeing conditions in each case.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Color is essential in textile production, thus leading to one of the largest consumptions of dyes across all industries. Since the second half of the nineteenth century, textile coloration has been dominated by synthetic dyes, which consistently replaced plant-derived ones that had been in use for thousands of years. Apart from the economic benefits, these synthetic dyes unfortunately have an enormously negative impact on the natural environment and human health. According to Berradi et al. the residue of synthetic dyes has an adverse impact on the environment, particularly the aquatic ecosystems, due to their toxic and carcinogenic properties [1]. Synthetic dye effluents containing organic components contribute to the formation of undesirable, harmful byproducts, which flow into the fields and thus influence soil productivity [2]. In addition to their advantages, such as their resistance to UV irradiation and color stability, they are becoming increasingly abandoned by textile manufacturers [3], as much research has confirmed their unfavorable impact on every form of life, from agriculture to aquaculture to public health [4, 5]. More ecological, eco-friendly alternatives might be the plant-derived colorants that were successfully applied in the past and have now become the subject of numerous scientific studies, which may help to increase their importance with regard to not only local, craft or artistic fabric dyeing but also the global textile industry [6,7,8,9,10,11,12,13,14,15,16,17,18].
Among fabrics, those of animal origin, like silk and wool, are far easier to dye. Cotton, linen, and other cellulose textiles absorb dyes less efficiently because of the negative charges on their surface when placed in water, so the dyeing of such fibers requires the use of mordants, which allow the dye to bond with the fibers. For many years, chemical compounds, such as alum, potash (potassium carbonate obtained from wood ash), calcium salts, sodium chloride, vinegar, tartaric acid, and citric acid, were used as mordants during natural textile dyeing, although aluminum, chromium, copper, and iron salts can also be used for this purpose. As some of them contain heavy metals, however, they can also contribute to environmental pollution [19, 20]. One sustainable alternative might be, for example, tannins [21]. Tannins and tannin-based biomacromolecules have been widely investigated with regard to improving the flame retardancy of cotton, as discussed in a recent review paper by Basak et al. [22].
Nowadays, many researchers focus not only on plant-derived colorants or environmentally friendly mordants but also on other substances which can modify and improve textile properties, increase dye uptake, or facilitate the dyeing process. During the pretreatment process, biopolymers can be successfully applied to influence wettability or ensure a uniform dyeing and finishing process. As an illustration, starch is commonly used but, because of its enormous demand in the food industry, other biodegradable biopolymers are more often considered, such as chitosan, whereas sericin, alginate or cyclodextrins [23] are also of interest to scientists for use in the textile finishing process. One of these textile substances with added value is chitosan- nontoxic amino polysaccharide, which is one of the most abundant biopolymers that can cross-link with various fibers, like cotton, creating a cationic charge on textiles’ surfaces. The application of chitosan to textiles has been studied by many researchers; for instance, Silva et al. showed that cotton fabric that was pretreated with chitosan (instead of mordants) possessed improved fastness properties and increased color strength.
Thanks to the elimination of electrolytes, the dyeing process was also more ecological. Additionally, it may also enhance antimicrobial activity and anti-UV protection [24]. Other studies also focused on whether the addition of chitosan improved the properties or dyeing process of textile green finishing [25,26,27,28,29,30,31]. Moreover, chitosan and lignosulfonate were selected to treat cotton fabric [32], while chitosan and sodium alginate were employed to treat another cellulose fiber-jute [33] to create green, flame-retardant materials. Furthermore, proteins rich in phosphorus and sulfur, such as caseins and hydrophobins, were investigated as eco-friendly flame retardants for cotton [34]. Not only these natural compounds but also wastage plant biomolecules, proteins (whey protein or casein), chitosan, and starch, among others, were discussed, in terms of sustainable fire retardancy for textiles [35]. Another additive that may favorably affect dyed textiles is nettle extract. Its ability to dye cotton textile was studied by Eser and Onal [36] but, due to its UV protection properties and antioxidant potential, the addition of nettle extract in the dyeing process may affect cotton’s color stability under the influence of UV irradiation. In addition, a natural origin polymer that may improve textile properties in terms of green fabric finishing is shellac-resin. It is obtained from lac insect secretion, which is usually used as a source of lac dye. Shellac can enhance strength and stiffness and make fabric resistant to moisture. Shellac was also used to create a new biocomposite, based on ramie fiber, by embedding this cellulose material into a biopolymeric matrix to obtain fiber-reinforced material [37].
The aim of the present research was to study the ability to dye plant origin textiles with plant-derived colorants through the application of various natural additives. This work also entailed examining the influence of colorants and additives on the mechanical properties of textiles and their color fastness under the influence of UVC irradiation. For this purpose, cotton textile samples were dyed using commercially available, plant-derived dyes for food application during a cold dyeing process under acidic conditions. Various additives of natural origin were applied during the dyeing process, such as nettle extract, shellac solution and two grades of chitosan: one of cosmetic grade and the second with a low molecular weight chitosan to examine whether or not its quality (and therefore the cost of the process) had an impact on the results obtained. The color fastness after dyeing and UVC irradiation was examined using a colorimeter. The structure of the dyed cotton samples was analyzed using FTIR spectroscopy. Finally, the mechanical properties of the fibers were measured.
2 Experimental
2.1 Materials
2.1.1 Fabric
Two types of non-dyed textiles of plant origin (cotton 225 gsm and cotton 240 gsm) were used for the experiment. Both textiles were made of 100% cotton and were unbleached and undyed, with width 159 cm and weave 3/1. The cotton textile samples were obtained from a Textile Agency Aris (Poland).
2.1.2 Colorants
To dye cotton, plant-derived food colorants from EXBERRY® by GNT were applied in two forms: liquid and powder. Both food colorants consist of fruit, vegetables, and other edible plant concentrates obtained only through a physical manufacturing process involving water.
2.1.3 Additives in the Dyeing Process
During the dyeing studies, two different grades of chitosan were used. Low molecular weight chitosan powder was purchased from Sigma Aldrich (CAS number 9012-76-4), while cosmetic-grade chitosan powder was purchased from Calaya-natural cosmetic raw materials. Citric acid monohydrate pure P.A. was purchased from Avantor Performance Materials Poland SA (CAS number 5949-29-1). Liquid nettle extract containing water, Urtica dioica L. folium and citric acid (concentration 30 g/l) was purchased from EkaMedica. Dewaxed blonde shellac flakes were purchased from a local art conservation and restoration shop. Food grade rectified spirit (95%) was purchased from Polmos SA.
2.2 Methods
2.2.1 Dyeing
Cotton textile 225 gsm was dyed yellow and cotton textile 240 gsm was dyed purple. The dyes were dissolved in distilled water in concentrations of 1% (w/w). Samples of cotton textiles were cold dyed for 24 h in a liquor ratio of 1:25 under acidic conditions. In addition to colorants, the selected additives were added to the samples of cotton textiles. One of them was 1% (w/w) cosmetic grade chitosan solution in 3% (w/w) citric acid (CGCS/CA). Moreover, chitosan was also used in the pretreatment process prior to the dyeing: for instance, the samples were pretreated with two grades of chitosan: a 1% (w/w) low molecular weight chitosan solution in 3% (w/w) citric acid (LMWCS/CA) and a 1% (w/w) cosmetic grade chitosan solution in 3% (w/w) citric acid (CGCS/CA) with a liquor ratio of 1:10 for one hour at room temperature, and then dyed. To part of the samples, nettle extract with a concentration of 30 g/L was added instead of water. Moreover, once dyed, some of the samples were covered with a thin layer of 20% (w/w) dewaxed blond shellac solution in ethanol (SH). To clarify the process of dyeing the cotton textiles, all of the parameters are listed in Table 1.
2.2.2 UV Irradiation of Fabric
All of the sample fabrics were irradiated using a UV lamp (ULTRAVIOL NBV 15) mainly emitting UVC (with a wavelength equal to 254 nm and radiation intensity of 21.5 W/m2). Samples of non-dyed and dyed textiles were irradiated at a distance of 5 cm from the UV lamp for 30 min, 2 h, and 4 h.
2.2.3 Colorimetric Measurements
The color of the dyed fabrics was evaluated using a colorimeter (Colorimeter CL 400, Courage, Khazaka, Köln, Germany). The mean values of the three measurements of the color parameter L*a*b* were used to calculate the ΔE value to examine the color change of all of the dyed fabrics following UVC irradiation (30 min, 2 h, and 4 h). The ΔE values were calculated using equation ΔE = (ΔL2 + Δa2 + Δb2)0.5, where ΔL = L − L0, Δa = a − a0, Δb = b − b0, with L0, a0, b0 being the values of the reference samples corresponding to the non-irradiated dyed textiles.
2.2.4 ATR-FTIR (Attenuated Total Reflection-Fourier Transform Infrared) Spectroscopy
The ATR-FTIR spectra were evaluated for all of the samples of both non-dyed and dyed textiles before and after UVC irradiation in the range of 400–4000 cm−1 (with the absorption mode at 4 cm−1 intervals and 64-times scanning) with a Nicolet iS10 spectrophotometer using an ATR accessory equipped with a diamond crystal (Thermo Fisher Scientific, Waltham, MA, USA). Before the measurements, background scanning was performed. OMNIC software was used to process the data.
2.2.5 Mechanical Properties
The mechanical properties were measured for the sample fabrics using a mechanical testing machine (Z.05, Zwick and Roell, Germany). The sample fabrics were cut into rectangular shapes, 5 cm long and 1 cm wide (at least four to six measurements were performed for each tested sample, and the samples were tested along the weft direction). The testing program parameters were as follows: the speed starting position was 50 mm/min; the speed of the initial force was 5 mm/min; and the initial force was 0.1 MPa. The TestXpert II 2017 program was used to collect the data. The obtained results were listed as average values with standard deviation (SD), for which a statistical analysis using the Q-Dixon test was performed.
3 Results
3.1 Colorimetric Measurements
The ΔE values for all of the dyed cotton textile samples are presented in Tables 2, 3, 4 and 5. According to the ISO 11664-4:2019 standard, if the ΔE value is greater than 5, it is considered a color change.
For the cotton 225 gsm, the color change after 2 h of UVC irradiation was observed for all dyed samples except for the yellow dyed one which was pretreated with CGCS and the yellow dyed sample with the addition of nettle extract covered with a shellac solution; however, after 4 h of UVC irradiation, the color of all of the samples tested in this study had changed. In the case of cotton 240 gsm, after 30 min of UVC irradiation, the color remain unchanged for the purple dyed sample with nettle extract and with nettle extract covered with shellac solution and pretreated with LMWCS before dyeing. After 2 and 4 h of UVC irradiation, however, all of the samples suffered color change.
3.2 ATR FTIR Spectroscopy
Infrared spectra were registered for all of the non-dyed and dyed textile samples with various additives and for the dyed textiles after 30 min, 2 h, and 4 h of UVC irradiation. The ATR FTIR spectra of the yellow dyed cotton textile samples are shown in Figs. 1 and 2 and those of the purple dyed cotton in Figs. 3 and 4. The wavenumbers for the IR characteristic bands’ position for the non-dyed and dyed cotton (225 and 240 gsm) textiles before and after 30 min, 2 h, and 4 h of UVC irradiation are listed in Table 6 (yellow dyed cotton) and Table 7 (purple dyed cotton) and discussed.
The characteristic vibrations for cellulose-based fibers according to the literature are H-bonded OH stretching at 3570–3200 cm−1, C–H stretching at 3000–2800 cm−1, asymmetric bridge C–O–C at 1130 cm−1, asymmetric in-plane ring stretching at 1092 cm−1, and C–O stretching at 1042 cm−1. Peaks at 2918 and 2849 cm−1 are characteristic of long alkyl chains (asymmetric and symmetric methylene groups stretching) and indicate that, in the case of cellulose-based textiles, like cotton, there exist impurities, such as wax residue.
As a result of dyeing for all yellow dyed cotton textiles (225 gsm), the H-bonded OH stretching band and CH stretching band were shifted to a higher wavenumber, only in the case of yellow dyed cotton with addition of nettle extract, the position of the CH stretching band remained the same. The other positions of the characteristic band after dyeing remained unaltered.
Following exposure to UVC irradiation, both non-dyed cotton textiles and yellow dyed cotton with the addition of nettle extract showed a shift of CH stretching band to higher wavenumber, while for the yellow dyed cotton, either nontreated or pretreated with both grades of chitosan, the CH stretching band position was altered to a lower wavenumber. For the other samples, the yellow dyed cotton with the addition of chitosan and the yellow dyed cotton with the addition of nettle extract covered with shellac solution, the CH stretching bands’ positions remained unchanged. For non-dyed cotton, yellow dyed cotton pretreated before dyeing with both types of chitosan and yellow dyed cotton with the addition of nettle extract covered with shellac solution, once exposed to UVC irradiation, their OH stretching bands were shifted to higher wavenumbers; whereas, for the yellow dyed cotton, yellow dyed cotton with the addition of chitosan and yellow dyed cotton with the addition of nettle extract, the OH stretching bands remained unaltered. Finally, one can observe that all of the other characteristic band positions were unaltered by UVC irradiation. As a result of dyeing, for the purple dyed cotton (240 gsm) with the addition of nettle extract covered with shellac solution and pretreated with both grades of chitosan, the CH stretching band was shifted to a higher wavenumber. For the purple dyed cotton, the CH stretching band’s position remained unaltered, whereas for the purple dyed cotton with the addition of CGCS and purple dyed cotton with the addition of nettle extract, these bands were shifted to a lower wavenumber. In most cases, the OH stretching band positions were altered (shifted to higher wavenumbers), with only both the purple dyed cotton and purple dyed cotton with the addition of CGCS’s band position of the OH group remaining the same. The rest of the characteristic bands remained unchanged.
As a result of the purple dyed cotton and purple dyed cotton with the addition of CGCS’s exposure to UVC irradiation, the position of the CH stretching band was altered (it was shifted to higher wavenumbers), whereas for cotton pretreated before dyeing with LMWCS, it was shifted to lower wavenumbers. For all of the remaining samples, once they had undergone UVC irradiation, their CH stretching band position was unaltered. Following the UVC exposure of the non-dyed cotton and purple dyed cotton samples, the OH stretching band position was shifted to higher wavenumbers; conversely, for purple dyed cotton with the addition of nettle extract, for both the purple dyed cotton with the addition of nettle extract covered with shellac solution and also the sample dyed with the addition of CGCS, the OH stretching band position was shifted to lower wavenumbers. For both of the chitosan pretreated samples, the OH band position was virtually unchanged. The rest of the characteristic bands were unaltered.
3.3 Mechanical Properties
The following parameters were measured for all of the specimens: the Young modulus, breaking force, elongation at break, and tensile strength. The corresponding mechanical properties for the yellow dyed cotton textiles are presented in Figs. 5, 6, 7 and 8, and those for the purple dyed cotton are presented in Figs. 9, 10, 11 and 12.
As one can see in Fig. 5, the Young modulus for the cotton textile samples (225 gsm) was the highest for the non-dyed sample (0.18 GPa) and the lowest was for the yellow dyed cotton pretreated with LMWCS (0.09 GPa). In addition, all of the dyed samples showed similar values of Young modulus parameter, which appeared to be almost halved compared to the non-dyed cotton textile.
The values of the breaking force (Fig. 6) and elongation at break (Fig. 7) for all of the dyed cotton textile samples (225 gsm) were higher than those for the non-dyed samples (41.92 N and 17.96%, respectively); the highest value for a breaking force parameter was observed for the yellow dyed sample covered with shellac solution, while the highest value for elongation at break parameter was observed for the yellow dyed cotton pretreated before dyeing with CGCS. Of all of the dyed samples used in this experiment, the lowest values for breaking force were obtained for the yellow dyed sample with the addition of nettle extract while, in the case of elongation at break parameter, it was the yellow dyed cotton covered with shellac solution.
Finally, as Fig. 8 shows, all of the tensile strength parameter results for the dyed cotton samples were lower than those for the non-dyed samples. Among the dyed samples, the highest values were obtained for the yellow dyed cotton textiles pretreated with CGCS while the lowest were for the yellow dyed sample pretreated with LMWCS.
As Fig. 9 shows, the highest value for the Young modulus parameter for the cotton textile samples (240 gsm) was obtained for the non-dyed sample while the lowest was observed for the purple dyed cotton pretreated with CGCS. Values for the Young modulus parameter for all of the remaining dyed samples were similar and significantly lower when compared to non-dyed cotton.
Figure 10 shows that all of the values for the breaking force parameter obtained for the purple dyed cotton textiles were lower than those for the non-dyed cotton sample, with the lowest value obtained for the purple dyed sample pretreated with CGCS. Among the sample of dyed cotton textiles (240 gsm), the highest value for this parameter was obtained for purple dyed cotton with the addition of nettle extract (87.9 N).
As Fig. 11 shows, all of the values of the elongation at break parameter obtained for the purple dyed cotton textiles were higher when compared to the non-dyed cotton sample (28.03%), with the highest value obtained for the purple dyed sample pretreated with CGCS (57.80%). Finally, values for all of the remaining dyed samples were similar and ranged from 39.10 to 44.88%.
For all of the dyed samples, the tensile strength parameters (Fig. 12) were significantly lower than those for the non-dyed cotton textile samples. Nevertheless, among the dyed samples, the highest value was obtained for the purple dyed cotton with the addition of nettle extract, while the lowest was obtained for the purple dyed samples pretreated with CGCS.
4 Discussion
The results presented in this paper show that the plant-derived colorants examined, which are used for food application, possess the ability effectively to dye natural textiles, like cotton, in a concentration of 1%, and so can be considered as an ecological alternative to the use of synthetic colorants. The process requires neither heating nor the use of inorganic mordants, thus limiting the negative impact on the environment. The application of additives to the colorants influenced the color fastness of the dyed textiles. For the yellow dyed cotton, despite the fact that the color of all of the samples, after 4 h of UVC irradiation, according to the colorimetric measurements, had changed, the addition of nettle extract, the addition of nettle extract with shellac solution and pretreatment with both types of chitosan imparted color stability. Only in the case of the addition of CGCS did the color quality deteriorate after UVC irradiation was observed. A similar situation applied to the purple dyed cotton, where the addition of nettle extract, the addition of nettle extract with shellac solution and pretreatment with chitosan influence beneficially the color fastness. In this latter case, the addition of CGCS only slightly improved the color stability, which was insignificant compared with the other samples.
The FTIR spectra recorded for the dyed cotton (240 gsm) samples suggest that dyeing with purple colorant does not influence the chemical structure of this cellulose textile. The obtained results indicate that shifts occurred in the characteristic bands wavenumbers in the FTIR spectra for the samples dyed with additives, thus suggesting that newly created interactions occurred between the components. For cotton (225 gsm), after dyeing with yellow colorant, wavenumber shifts of characteristic bands were observed, thus again resulting from these newly created interactions. Compared to the yellow dyed sample, those with chitosan application did not show any wavenumber shift of characteristic bands, whereas those with the addition of nettle extract, and nettle extract with shellac, did. For the yellow and purple dyed cotton, following UVC irradiation, wavenumber shifts of the characteristic bands may indicate photodegradation of the cellulose-based textiles.
Two of the most important mechanical properties of textiles are elongation at break and tensile strength. Both types of cotton after dyeing indicated increased elongation at break parameter and a decreased tensile strength parameter. For yellow dyed cotton with additives, the results did not reveal any significant differences: on one hand, cotton fibers were more flexible than in the non-dyed sample, while on the other hand only the sample covered with shellac solution appeared stiffer, with a value for elongation at break closer to the non-dyed cotton sample. For purple dyed cotton with various additives, the elongation at break values were similar, and close to the value obtained for the purple dyed sample; among dyed samples no significant differences were observed except for the chitosan pretreated samples, which displayed greater flexibility. Finally, for purple dyed cotton, CGCS pretreatment significantly improved the cotton textile flexibility in the most visible manner. As the Young modulus describes the stiffness of the measured material, the results for the yellow dyed cotton (225 gsm) and purple dyed cotton (240 gsm) suggest that the natural dyeing process, conducted with and without natural additives, caused a decrease in the Young modulus parameter values, thus indicating a decrease in the textile stiffness and thus an increasing flexibility and softness. Chitosan particles, instead, form a film on the cotton fiber surface and may be symmetrically distributed, so the chitosan particles may not reach the internal space between the fibers [38]; however, the purple dyed sample pretreated with cosmetic grade chitosan was characterized by significantly greater flexibility (the Young modulus values were lower) compared to the purple dyed samples without additives and those treated with low molecular weight chitosan, which may be due to the use of different types of chitosan and their properties. A decrease in the tensile strength of the dyed cotton samples may be associated with the fact that, under acidic conditions, the hydrolysis of cellulose causes damage to cotton fibers [38].
5 Conclusion
The applied food colorants have the potential to dye natural cotton fabrics effectively. Both the dyeing process and the properties of the dyed cotton can be modified by the addition of natural substances. It is striking that different types of treatment, namely the pretreatment of cotton with chitosan prior to dyeing, and the addition of nettle extract or shellac, significantly modify the cotton’s properties and efficiently improve the color stability following UVC irradiation. Furthermore, those additives are likely to influence the mechanical properties of cotton textiles, although further research is required to refine the dyeing method by, for example, using higher concentrations of dyes and different natural additives or plant mordants.
References
M. Berradi, R. Hsissou, M. Khudhair, M. Assouag, O. Cherkaoui, A. El Bachiri, A. El Harfi, Heliyon 5(11), e02711 (2019)
K.A. Wani, in Impact of textile dyes on public health and the environment, 1st edn., ed. by K.A. Wani, N.K. Jangid, A.R. Bhat (IGI Global, Hershey, 2020), pp.162–170
M.S. Beldean-Galea, F.M. Copaciu, M.V. Coman, J. AOAC Int. 101, 1353 (2018)
D.A. Yaseen, M. Scholz, Int. J. Environ. Sci. Technol. 16, 1193 (2019)
M. Islam, M. Mostafa, J. Environ. Sci. Nat. Resour. 11(1–2), 131 (2019)
A.K. Samanta, P. Agarwal, Indian J. Fibre Text. Res. 34, 384 (2009)
E.-K. Hwang, Y.-H. Lee, H.-D. Kim, Fibers Polym. 9, 334 (2008)
A. Haji, M. Naebe, J. Clean. Prod. 265(20), 121866 (2020)
Y.S. Jung, D.G. Bae, Fibers Polym. 15, 138 (2014)
J. Arora, P. Agarwal, G. Gupta, Green Sustain. Chem. 7(1), 35 (2017)
J. Sonja, K. Marija, Z. Silvana, G.-L. Sashka, M. Kiro, Tekst. Ind. 68(4), 12 (2020)
M. Chairat, J.B. Bremner, K. Chantrapromma, Fibers Polym. 8, 613 (2007)
A. Fröse, K. Schmidtke, T. Sukmann, I. Juhász Junger, A. Ehrmann, Optik 181, 215 (2019)
S. Lykidou, M. Pashou, E. Vouvoudi, N. Nikolaidis, Fibers Polym. 22, 3336 (2021)
A.K. Guha, Int. J. Text. Sci. 8, 38 (2019)
S.R. Maulik, L. Chakraborty, P. Pandit, Fibers Polym. 22, 711 (2021)
J. Sheikh, N. Singh, M. Srivastava, Fibers Polym. 20, 312 (2019)
I. Zerin, N. Farzana, A.S.M. Sayem, D.M. Anang, J. Haider, Potentials of natural dyes for textile applications, in Encyclopedia of renewable and sustainable materials, vol. 2, 1st edn., ed. by I. Choudhury, S. Hashmi (Elsevier Science, Amsterdam, 2020), pp.873–883
Ö.E. İşmal, Fibers Polym. 18, 773 (2017)
K. Karabulut, R. Atav, Fibers Polym. 21, 1773 (2020)
K. Elsahida, A.M. Fauzi, I. Sailah, I.Z. Siregar, I.O.P. Conf, Ser. Earth Environ. Sci. 399, 012065 (2019)
S. Basak, A.S.M. Raja, S. Saxena, P.G. Patil, Polym. Degrad. Stab. 189, 109603 (2021)
F.H.H. Abdellatif, M.M. Abdellatif, Utilization of sustainable biopolymers in textile processing, in Green chemistry for sustainable textiles: modern design and approaches, 1st edn., ed. by N. Ibrahim, C.M. Hussain (Elsevier Science, Amsterdam, 2021), p. 453–467
M. Silva, M.A. Barros, R. Almeida, E. Pilau, E. Pinto, G. Soares, J. Santos, J. Clean. Prod. 199, 807 (2018)
Shahid-ul-Islam, B.S. Butola, Int. J. Biol. Macromol. 158, 94 (2020)
S. Vílchez, P. Jovančić, P. Erra, Fibers Polym. 11, 28 (2010)
L. Ammayappan, J. Jeyakodi Moses, Fibers Polym. 10, 161 (2009)
L. Jajpura, A. Rangi, A. Khandual, Natural finishes, technologies and recent developments, in Sustainable technologies for fashion and textiles, 1st edn., ed. by R. Nayak (Woodhead Publishing, Cambridge, 2020), pp.209–229
D. Shukla, P.S. Vankar, Fibers Polym. 19, 1913 (2018)
B.C.-E. Zhou, C.-W. Kan, C. Sun, J. Du, C. Xu, AATCC J. Res. 6(1), 8 (2019)
A. Haji, S. Ashraf, M. Nasiriboroumand, C. Lievens, Fibers Polym. 21, 743 (2020)
P. Li, C. Liu, Y.-J. Xu, Z.-M. Jiang, Y. Liu, P. Zhu, Polym. Degrad. Stab. 181, 109302 (2020)
S.-Q. Li, R.-C. Tang, C.-B. Yu, Polym. Degrad. Stab. 196, 109826 (2022)
J. Alongi, R.A. Carletto, F. Bosco, F. Carosio, A. Di Blasio, F. Cuttica, V. Antonucci, M. Giordano, G. Malucelli, Polym. Degrad. Stab. 99, 111 (2014)
S. Basak, S. Wazed Ali, Polym. Degrad. Stab. 133, 47 (2016)
F. Eser, A. Onal, J. Nat. Fibers 12, 222 (2015)
A.S. Herrmann, J. Nickel, U. Riedel, Polym. Degrad. Stab. 59(1–3), 251 (1998)
M.A. Rahman Bhuiyan, M.A. Hossain, M. Zakaria, M.N. Islam, M.Z. Uddin, J. Polym. Environ. 25, 334 (2017)
Author information
Authors and Affiliations
Contributions
Conceptualization; PB and AS Data curation; PB Formal analysis; PB, MG and AS Funding acquisition; AS, PB Investigation; PB Methodology; PB and AS Project administration MG and AS Resources; AS, PB Software; PB, AS Supervision MG and AS Validation; PB, MG and AS Visualization; PB Roles/Writing—original draft; PB Writing—review and editing AS and MG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brudzyńska, P., Sionkowska, A. & Grisel, M. Cotton Textile Dyeing by Plant-Derived Colorants in the Presence of Natural Additives. Fibers Polym 24, 3641–3655 (2023). https://doi.org/10.1007/s12221-023-00332-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00332-3