Abstract
Nylon 6 is one of the most widely used polymers in the world. For some nylon products, hydrophobic coatings are used for impregnation. However, it has been suggested in the literature that coating could accelerate aging. Therefore, in this paper, we focused on the degradation process of dyed nylon yarns with and without perfluorinated coating under accelerated weathering conditions. To monitor the degradation process, we used methods such as tensile test, molecular weight analysis, ultraviolet–visible spectroscopy, Fourier-transform infrared spectroscopy, differential scanning calorimetry, energy dispersive X-ray analysis, and scanning electron microscopy. We found that the hydrophobic coating is unlikely to have a negative effect on the degradation process. However, the coating decomposes during weathering, and its concentration on the fiber's surface decreases. The type of dye used was identified as the most significant factor influencing the degradation rate. This was explained by the screening effect of dyes in the UVA region of the light spectrum. Manufacturers of nylon products, which require a pleasant appearance and safety, should, therefore, consider a careful selection of dyes.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
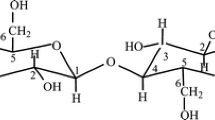
Aliphatic polyamides (nylons) are among the most widely used engineering polymers. Many nylon products must withstand harsh outdoor conditions involving UV radiation, water, and atmospheric oxygen. The effect of different environmental conditions on nylon degradation was summarized by Shamey and Sinha [1]. The main degradation pathways caused by weathering include autoxidation [2], photooxidation [3], and hydrolysis [4]. These degradation processes lead to the deterioration of mechanical properties through the scission of the molecular backbone or crosslinking (Scheme 1).
Combined effect of temperature, UV light, water, dyes, and hydrophobic coating on nylon degradation. The number m in the molecular structure of the hydrophobic coating is according to the technology—old 8, new 6, or 4 [6]
In our previous work [5], we found that the tensile strength of hydrophobically coated nylon 6 yarns decreased by 10% compared to 4% for uncoated yarns after four months of outdoor weathering. However, nylon products can be weathered for much longer than four months. The more than twice as fast degradation of hydrophobically coated nylon would significantly affect safety in applications where such coatings are desirable, such as static ropes for canyoning and caving, mooring ropes, and others.
Hydrophobic coatings based on fluorocarbons [6] are used by manufacturers of sportswear and sports equipment, but also in many other areas [7], to improve water repellency or other properties such as resistance to dirt and abrasion [8, 9]. The molecular structure is usually based on acrylate polymers with perfluoroalkyl side chains (see Scheme 1) and initially utilized C8 fluorocarbons. Recently, in response to environmental and health concerns, the industry has shifted towards using C6 and C4 fluorocarbons [10]. The effect of a hydrophobic coating based on fluorocarbons on nylon's photodegradation has not been studied to the author's knowledge. Only the biodegradation of nylon fabric coated with fluoropolymer was studied by Setua et al. [11], but no comparison with uncoated nylon was presented.
Furthermore, nylon fibers are often dyed to achieve an attractive appearance for end consumers. However, dyes and coatings may affect the degradation of nylon — they can either initiate or hinder it [12]. The oxidation process of nylon initiated by coatings and dyes starts with the absorption of photons by the dye or coating molecules, which leads to their conversion into excited states or radicals. These photoactivated species can then initiate the oxidation process of nylon by abstracting a hydrogen atom from its molecular backbone. This photo-induced degradation generally starts at the surface and progresses into the material [12]. The dye-sensitized photodegradation of polymers, also known as phototendering, was studied or reviewed by several authors [13,14,15,16,17,18]. However, no definitive guideline was established due to the number of dyes on the market [17] and their structural complexity [19]. Coatings and dyes can hinder the photooxidation reaction in nylon through two processes. The first process involves the absorption of harmful UV radiation, while the second process involves the deactivation of photoexcited species [14]. Furthermore, because of its hydrophobic nature, the coating reduces the contact of water with the nylon, which could prevent its hydrolysis [20] and plasticization. The latter occurs when nylon absorbs water into its structure, significantly lowering the glass transition temperature (plasticization) from around 66 °C in dry conditions to -9 °C when fully saturated with 9% wt. water. The increased mobility of the polymer chains above the glass transition temperature accelerates polymer degradation through the Norrish II reaction [3, pp. 17–18]. The combination of dyes and hydrophobic coating effects on nylon degradation is shown in Scheme 1.
The finding of a higher tensile strength decrease of hydrophobically coated nylon 6 yarns compared to uncoated yarns as a result of outdoor weathering found in our previous study has far-reaching implications for the safety, design, and manufacture of nylon products. Therefore, we decided to study the same yarns in more detail. The goal was to confirm or reject the hypothesis that a commercially used hydrophobic coating based on fluorocarbons accelerates the degradation of nylon yarns. A secondary objective was to monitor the degree of influence of dyes on degradation. To achieve these goals, we aged dyed nylon yarns in an accelerated weathering machine and used methods such as tensile test, molecular weight analysis (MWA), ultraviolet–visible spectroscopy (UV–Vis), Fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), energy dispersive x-ray analysis (EDX), and scanning electron microscopy (SEM) to study the degradation process.
2 Materials and Methods
2.1 Yarns
Nylon 6 yarns were manually extracted from the sheath of two dynamic climbing ropes using tweezers and scissors. The ropes were Mammut Phoenix Classic (uncoated) and Mammut Phoenix Dry (coated) from Mammut Sports Group AG, Switzerland. The coated rope had yarns treated with a coating called COATINGfinish™, resulting in a thin layer of coating on each fiber. The manufacturer does not disclose the exact chemical composition. However, a fluorinated repellent called Oleophobol® (Huntsman International LLC, The Woodlands, TX, USA) based on C6 technology was already described in a study testing hydrophobic coatings on Mammut ropes [8]. The yarns consisted of 140 fibers, each with a diameter of 30 μm. The uncoated yarns were white, dark blue, light blue, and purple, while the coated yarns were yellow, light blue, and purple (Fig. 1).
2.2 Accelerated Weathering
Yarns were placed into the accelerated weathering machine Q-Lab QUV/spray and tested according to ISO 4892-3:2006, Type 1A (UVA 340) fluorescent UV lamps, method A, cycle No.1 [21]. The weathering cycle consisted of an 8 h dry period with irradiation of 0.76 W/m2/nm at 340 nm, and a temperature of 60 °C and a 4 h condensation period with 100% relative humidity, the lights off, and a temperature of 50 °C. UVA-340 lamps simulate sunlight from about 370 nm down to a solar cut-off of 295 nm with an emission peak at 340 nm [22]. The sample holders with yarns were moved to a different position in the test machine every 2 days to achieve the most homogeneous irradiation. Yarns were sampled for further examination at three different time points: before accelerated weathering, after one week of exposure (168 h), and after 2 weeks of exposure (336 h). The total light dose was 306 and 613 kJ/m2nm at 340 nm for 168 and 336 h of testing, respectively. For the spectrum between 295 and 400 nm, the total light dose was approximately 17,470 and 34,930 kJ/m2 for the same respective time periods.
It should be noted that accelerated weathering is not a direct simulation of the outdoor environment, as factors such as temperature, humidity, the presence of microorganisms, ozone, and chemical pollutants in the air are not identical.
2.3 Tensile Test
Quasi-static tensile test of yarns was performed using the universal test machine Shimadzu AG-X Plus (Shimadzu Corporation, Kyoto, Japan). The gauge length of 115 mm, the pretension of 1.5 N, and the displacement speed of 115 mm/min were used. Maximum breaking force and elongation at break were calculated from the force–displacement curves. Mean values and standard deviations of 10 measurements per color are presented.
2.4 Molecular Weight Analysis
The molecular weight analysis was performed by measuring the viscosity of the solution on an Ubbelohde viscometer. Nylon yarns were dissolved in 99% formic acid (Merck KGaA, Darmstadt, Germany) in a concentration of 5 mg/mL. Five measurements per yarn were conducted to determine the relative viscosity. The flow durations were between 60 and 110 s, with standard deviations of the flow durations between 0.1 and 0.4 s. The intrinsic viscosity \(\left[\eta \right]\) was calculated using Solomon and Ciuta’s equation:
where \(c\) is the concentration in g/dL, \({\eta }_{\mathrm{sp}}\) is the specific viscosity (\({\eta }_{\mathrm{sp}}\) = \({\eta }_{\mathrm{rel}}\) − 1), and \({\eta }_{\mathrm{rel}}\) is the relative viscosity. Finally, the viscosity-average molecular weight \({M}_{\mathrm{v}}\) was calculated using the Mark–Houwink–Sakurada equation:
where \(K\) = 2.26 × 10–4 and \(\alpha \) = 0.82 are Mark–Houwink constants for the nylon 6—formic acid solution [23].
2.5 Ultraviolet–Visible Spectroscopy
UV–Vis spectroscopy was used for two purposes. First, to characterize dyes in the visible region and second, to investigate the UV screening effect of dyes and their influence on the degradation of mechanical properties. To perform the measurements, solutions were prepared by dissolving 15 mg of nylon yarns in 5 mL of 99% formic acid (Merck KGaA, Darmstadt, Germany). The UV–Vis spectrometer Zeiss MCS 521 System (Carl Zeiss AG, Oberkochen, Germany) equipped with CLD500 and CLH500 modules was used for measurement in the ultraviolet (200–400 nm) and visible (400–750 nm) spectra, respectively. The path length of the cuvette was 10 mm.
2.6 Infrared Spectroscopy
Fourier-transform infrared spectrometer Bruker Alpha (Bruker Corporation, Billerica, MA, US) with attenuated total reflection attachment (ATR-FTIR) was used to monitor the degradation process qualitatively. For each yarn and weathering time, five spectra were collected using 64 scans with a resolution of 4 cm−1. Subsequently, the spectra were equalized by standard normal variate (SNV) normalization and averaged using Spectragryph software [24]. For better visibility of changes in the carbonyl region, the spectra of non-weathered yarns were subtracted from the spectra of weathered yarns. The spectra were viewed comprehensively, but the functional groups from Table 1 are discussed.
2.7 Differential Scanning Calorimetry
DSC curves were obtained using a Netzsch DSC 214 Polyma calorimeter (Netzsch Holding, Selb, Germany). Yarn samples were heated at a rate of 10 °C/min from 20 to 250 °C in an inert atmosphere. The degree of crystallinity \({X}_{\mathrm{c}}\) was calculated using the formula:
where \({\Delta H}_{\mathrm{m}}\) is the melting enthalpy of a sample calculated as the integration of the DSC curve between 130 and 235 °C with linear baseline, and \({\Delta H}_{m}^{0}\) is the melting enthalpy of 100% crystalline nylon taken as 240 J/g [20]. Mean values and sample standard deviations were calculated from three measurements for each yarn and weathering time.
2.8 Energy Dispersion X-Ray Analysis
Fluorine concentration in coated yarns was measured in an FEI Quanta 200 3D scanning electron microscope (Thermo Fisher Scientific, Hillsboro, OR, US) using the energy dispersive x-ray detector EDAX Pegasus XM4 (Ametek, Inc., Berwyn, PA, US). An accelerating voltage of 3 kV was chosen, which showed the strongest fluorine signal. The yarns were analyzed in a low vacuum mode with a chamber pressure of 0.15 mbar, a magnification of 3000 × , and a spot size of 7.
2.9 Scanning Electron Microscopy
The fiber surface was analyzed using an FEI Quanta 200 3D scanning electron microscope (Thermo Fisher Scientific, Hillsboro, OR, US). The yarns were coated with gold using an Agar Sputter Coater AGB7340 (Agar Scientific Ltd, Stansted, UK) with a chamber pressure of 0.1 mbar, a current of 30 mA, and an application time of 60 s. The microscopy was conducted under a high vacuum, using an accelerating voltage of 7 kV and an Everhart–Thornley detector of secondary electrons.
3 Results and Discussion
3.1 Tensile Test
The force–elongation curves (Fig. 2) indicated that weathering led to significant changes in mechanical behavior. Breaking force and elongation at break decreased during weathering, as seen in Fig. 3. On average, uncoated yarns lost more breaking force than coated yarns (Fig. 3a). However, the differences were insignificant due to the high standard deviations caused by the effect of dyes (Fig. 3b). The results are in contrast to our previous publication, which reported a higher decrease in coated yarns as a result of exposure to outdoor weathering. The cause may be temperature differences between artificial and outdoor weathering. During accelerated weathering, temperatures ranged between 50 and 60 °C, compared to an average temperature of 10 °C (min − 5 °C, max + 25 °C) during outdoor weathering. Bernstein and Gillen [30] showed that humidity is not a critical aging factor below 50 °C. Thus, a hydrophobic coating can slow down hydrolytic degradation during periods of 100% humidity in accelerated weathering.
Nevertheless, the main factor determining the extent of degradation is not the coating, but the dye used. For example, the breaking force of uncoated white yarns decreased by 65% after 1 week of weathering compared to only 18% for uncoated dark blue yarns. The underlying mechanism for such significant differences in degradation for different colors is presented in Sect. 3.3.
3.2 Molecular Weight Analysis
Viscosity measurements confirmed chain scission (Fig. 4). The viscosity-average molecular weight linearly correlated with the maximum breaking force (Fig. 5a), and second–order polynomial correlated to elongation at break (Fig. 5b). The yarns did not show signs of a critical molar mass leading to a sudden change in elongation at break during degradation, as is presented for semicrystalline polymers [31]. This points to the fact that the degradation was not homogeneous through the thickness of the yarns. Coated yarns showed higher breaking strength for a given molecular weight. This suggests that the coating, which does not contribute to the mechanical properties of the yarn, degrades by chain scission. Further indications of the decomposition of the coating are presented in Sects. 3.6 and 3.7.
3.3 UV–Vis Spectroscopy
3.3.1 Visible Region
The visible region between 400 and 750 nm (Fig. 6) revealed that the colors that appear to be the same for coated and uncoated yarns (light blue and purple) have different chemical origins. Therefore, a direct comparison of coated and uncoated yarns without other factors affecting degradation is impossible, as the different chemical origins of the same colors cannot be ignored. However, to compare coated and uncoated yarns as accurately as possible in Sect. 3.4, light blue yarns were chosen for this purpose.
3.3.2 Ultraviolet region
It is evident from Fig. 6 that significant differences in absorption in the UVA and UVB parts of the spectrum between the yarns are present. The absorption in the UVB and UVA regions was plotted against the relative loss of yarn strength after 1 week of weathering to investigate the screening effect of the dyes (Fig. 7). Absorption in the UVB region was only weakly correlated with relative strength loss after 1 week of weathering (R2 = 0.61), while UVA absorption showed a strong correlation with strength loss after 1 week of weathering (R2 = 0.86). The uncoated dark blue yarn was excluded from the correlation as an outlier, for which there are two explanations. First, it is possible that the screening effect of dyes affects the weathering process only to a certain extent and that when a certain degree of UV screening is achieved, other mechanisms, such as auto-oxidation and hydrolysis, take over the key role in weathering. Second, the dark blue dye, on the one hand, absorbs significantly more UV radiation than other dyes, but on the other hand, initiates degradation due to the formation of radicals.
3.4 Infrared Spectroscopy
To describe the differences between coated and uncoated yarns, spectra of light blue yarns are discussed.
3.4.1 Hydroxy/Hydroperoxy Region
No signs of an increase in the concentration of hydroxy and hydroperoxy groups were found (Fig. 8). The hydroxy groups of carboxylic acids formed as a product of hydrolysis are visible in the FTIR spectra in a study by Deshoules et al. [32], in which they weathered nylon 6 in water with and without oxygen, resulting in a similar decrease in molecular weight as in our study. This suggests that the hydrolytic reaction (Eq. (9) in Scheme 1) probably does not play a significant role in the weathering conditions in this study, although as described in the next Sect. 3.4.2, it cannot be neglected entirely.
FTIR spectra of uncoated a and coated b light blue yarns after 0, 1, and 2 weeks of weathering. The carbonyl region is enlarged in Fig. 9
3.4.2 Carbonyl Region
A decrease in carbonyl intensity was observed in both coated and uncoated yarns, although the effect was more pronounced in uncoated yarns (Fig. 9). This is contrary to most published literature, which typically reports an increase in carbonyl intensity upon oxidative degradation [23, 27, 28, 33, 34]. However, a decrease in carbonyl intensity has been observed previously as a result of the photolysis of thermally oxidized nylon [35]. This decrease may be due to the formation of gaseous products, such as carbon monoxide and carbon dioxide, caused by chain scission reactions [26]. These products migrate into the surrounding atmosphere when formed and can only be detected by chromatographic or mass spectrometry methods. Our study highlights the importance of considering multiple degradation pathways and their associated measurements when analyzing the degradation of nylon 6.
Additionally, our analysis revealed that non-weathered yarns (both coated and uncoated) showed a major peak centered at 1735 cm−1 and a second peak centered at 1715 cm−1, which is typically attributed to the presence of imides and ketones, respectively. Since such degradation products are not typically formed during industrial production [36, 37], it must be either a result of the autoclave treatment of nylon yarns during production [38] or the oxidation process during storage. The main difference between coated and uncoated yarns was in the peak centered around 1698 cm−1, which was present only in the coated yarns. This peak is unlikely to be associated with the coating since the carbonyl group of the acrylate polymer, which is the chemical basis of the coating, should be centered around a higher wavenumber of about 1732 cm−1 [39]. Instead, it may be a degradation product due to heat treatment that the coated ropes undergo during manufacture to stabilize the hydrophobic coating [38], such as conjugated carbonyls or α,β-unsaturated aldehydes.
Moreover, after 1 week of weathering, the yarns showed a decrease in the imide peak at 1735 cm−1, which is known to hydrolyze to the primary amide and carboxylic acid (Eq. (1) in Scheme 1) [32]. This was confirmed by the formation of a peak centered at 1710 cm−1 attributed to the carboxylic acid. We also observed an increase in other carbonyl compounds, such as aldehydes (1725 cm−1) from Eq. (2) in Scheme 1 and α,β-unsaturated ketones or aldehydes (1690 cm−1), as evident in the subtracted spectra in Fig. 9c, d. After 2 weeks of weathering, both coated and uncoated yarns developed a peak centered at 1700 cm−1 associated with conjugated carbonyls and α,β-unsaturated aldehydes (Fig. 9c, d).
3.4.3 Amide I and II Bands
No significant changes were found, only slight deviations with a random character (Fig. 8). Both increase [40, 41] and decrease [23, 32] of amide I and II bands due to degradation are reported in the literature.
3.4.4 FTIR Summary
Infrared spectroscopy analysis shows that the degradation pathways for coated and uncoated yarns are similar and share common mechanisms, differing only in the extent of these reactions. Although the hydrolytic reaction products were detected, their concentration levels were relatively low. These products are likely the result of imide hydrolysis rather than direct hydrolysis of nylon. Notably, the undetectability of hydroxyl groups suggests that hydrolysis (Eq. (9) in Scheme 1), imide decomposition (Eq. (1) in Scheme 1), and the Russell mechanism (Eq. (6) in Scheme 1) are unlikely to be the dominant degradation pathways. However, due to the observed decrease in absorption in the carbonyl region, it is likely that the primary degradation pathway is photooxidative degradation through a Norrish type I reaction (Eq. (7) in Scheme 1), leading to chain scission and the formation of gaseous products.
3.5 Differential Scanning Calorimetry
The results from DSC showed a subtle increase in nylon crystallinity during weathering. However, due to relatively small changes in crystallinity and high standard deviations, no significant differences could be observed between coated and uncoated yarns or individual colors (Fig. 10). The increase in crystallinity is because the degradation occurs mainly in the amorphous region [26]. In addition, the higher mobility of polymer molecules after the scission of molecular chains can increase crystallinity directly [42].
3.6 Energy Dispersive X-Ray Analysis
The fluorine concentration decreased on average by 30 wt.% after 1 and 2 weeks of weathering (Fig. 11). However, due to significant variability in fluorine concentration along the yarns, a more precise analysis was not possible. To contextualize these findings, Washington and Jenkins [43] estimated the half-life of hydrolytic degradation of polyacrylate polymer with perfluoroalkyl side chains, based on C8 technology, to be 55 years for a pH range of 5 to 8. Therefore, it is reasonable to assume that the observed decrease in fluorine concentration was not primarily due to hydrolysis, but rather photo-induced degradation. This is consistent with Isbilir et al. [44], who found that a fluorinated hydrophobic coating was resistant to 85 °C temperature and humid air, as well as freeze–thaw cycles, but experienced fluorine loss when exposed to artificial UV light and outdoor environment. In their artificial weathering study, the loss of fluorine was approximately 35 at.% after exposure to a total UV light dose of 54,000 kJ/m2, which is higher than the doses used in our study (17,470 and 34,930 kJ/m2). The degradation mechanism of the coating is likely to be similar to that of polymethacrylate. This involves random homolytic scission of the molecular backbone and photolysis of the ester side group [3, p. 138].
3.7 Scanning Electron Microscopy
Interestingly, no cracking of the fiber surface, as often reported in the literature [25, 41], was observed even in the most degraded yarns. However, an uneven surface can be seen on the surface of non-weathered coated yarns (Fig. 12), which according to EDX, is caused by the hydrophobic coating. After 1 and 2 weeks, the yarns had a smoother surface, indicating coating degradation. Isbilir et al. [44] reported the observation of loss of coating nanoparticles in their study, which was discussed in the Sect. 3.6. The authors further highlighted a direct correlation between the decrease in fluorine content and the loss of the coating nanoparticles.
4 Conclusion
In this paper, the degradation of dyed nylon yarns with and without a perfluorinated hydrophobic coating was studied, as it was suggested in the literature that this coating might accelerate the degradation of nylon. Results indicate that the coating is unlikely to accelerate nylon degradation, with no significant difference in the average loss of breaking force observed between coated and uncoated yarns. However, the coating decomposed during weathering, resulting in a decreased concentration on the fiber's surface. The type of dye used was found to have the greatest impact on degradation, with a threefold difference in strength loss observed between the most and least resistant yarns. These differences were attributed to the screening effect of dyes in the UVA region. Therefore, careful selection of dyes, and not the application of the coating, is crucial for the development of products that maintain both mechanical resistance and visual appearance.
Data availability
The data that support the findings of this study are openly available on Mendeley Data at https://www.data.mendeley.com/datasets/t8j4fnmwww/1.
References
R. Shamey, K. Sinha, Rev. Prog. Color. Relat. Top. 33, 93 (2003). https://doi.org/10.1111/j.1478-4408.2003.tb00147.x
E. Richaud, O. OkambaDiogo, B. Fayolle, J. Verdu, J. Guilment, F. Fernagut, Polym. Degrad. Stab. 98, 1929 (2013). https://doi.org/10.1016/j.polymdegradstab.2013.04.012
J.F. Rabek, Polymer Photodegradation, 1st edn. (Springer, Dordrecht, 1995). https://doi.org/10.1007/978-94-011-1274-1
V. Venoor, J.H. Park, D.O. Kazmer, M.J. Sobkowicz, Polym. Rev. 61, 598 (2021). https://doi.org/10.1080/15583724.2020.1855196
D. Sedláček, M. Roso, L. Viel, N. Perotto, B. Caven, M. Hasler, W. Nachbauer, Process. Inst. Mech. Eng. Part P J. Sport. Eng. Technol. (2021). https://doi.org/10.1177/17543371211062816
W.D. Schindler, P.J. Hauser, Repellent finishes. In: Chemical Finishing of Textiles. Elsevier, Amsterdam, pp. 74–86 (2004). https://doi.org/10.1533/9781845690373.74
J. Glüge, M. Scheringer, I.T. Cousins, J.C. DeWitt, G. Goldenman, D. Herzke, R. Lohmann, C.A. Ng, X. Trier, Z. Wang, Environ. Sci. Process. Impacts 22, 2345 (2020). https://doi.org/10.1039/D0EM00291G
A.B. Spierings, A. Ritter, O. Henkel, U. Holzdoerfer, Text. Res. J. 78, 886 (2008). https://doi.org/10.1177/0040517507087667
C. Lassen, J. Kjølholt, S.H. Mikkelsen, M. Warming, A.A. Jensen, R. Bossi, I.B. Nielsen, Polyfluoroalkyl substances (PFASs) in textiles for children, The Danish Environmental Protection Agency (2015). https://www2.mst.dk/Udgiv/publications/2015/04/978-87-93352-12-4.pdf. Accessed 10 Jan 2023
D. De Smet, D. Weydts, M. Vanneste, Sustainable Apparel (Elsevier, Amsterdam, 2015), pp.3–33. https://doi.org/10.1016/B978-1-78242-339-3.00001-7
D.K. Setua, G.D. Pandey, R. Indusekhar, G.N. Mathur, J. Appl. Polym. Sci. 75, 685 (2000). https://doi.org/10.1002/(SICI)1097-4628(20000131)75:5%3c685::AID-APP11%3e3.0.CO;2-0
T.J. Turton, J.R. White, Polym. Degrad. Stab. 74, 559 (2001). https://doi.org/10.1016/S0141-3910(01)00193-8
G.A. Horsfall, Text. Res. J. 52, 197 (1982). https://doi.org/10.1177/004051758205200307
P.P. Klemchuk, Polym. Photochem. 3, 1 (1983). https://doi.org/10.1016/0144-2880(83)90042-8
F.M. Tera, M.N. Michael, A. Hebeish, Polym. Degrad. Stab. 17, 13 (1987). https://doi.org/10.1016/0141-3910(87)90044-9
N.S. Allen, M. Ledward, G.W. Follows, Polym. Degrad. Stab. 38, 95 (1992). https://doi.org/10.1016/0141-3910(92)90001-L
N.S. Allen, Polym. Degrad. Stab. 44, 357 (1994). https://doi.org/10.1016/0141-3910(94)90095-7
J.C.V.P. Moura, A.M.F. Oliveira-Campos, J. Griffiths, Dyes Pigm. 33, 173 (1997). https://doi.org/10.1016/S0143-7208(96)00050-2
C. Fleischmann, M. Lievenbrück, H. Ritter, Polymers (Basel). 7, 717 (2015). https://doi.org/10.3390/polym7040717
Q. Deshoulles, M. Le Gall, C. Dreanno, M. Arhant, D. Priour, P.-Y. Le Gac, Polym. Degrad. Stab. 183, 109435 (2021). https://doi.org/10.1016/j.polymdegradstab.2020.109435
ISO 4892-3:2006, Plastics—methods of exposure to laboratory light sources—part 3: fluorescent UV lamps. International Organization for Standardization, Geneva, 2006. https://www.iso.org/standard/33343.html. Accessed 10 Jan 2023
P. Brennan, C. Fedor, Sunlight, UV and accelerated weathering, Q-Lab Tech. Bullentin LU-0822. (1994). http://q-lab-corporation.ru/doc/Weathering-LU-0822.pdf. Accessed 10 Jan 2023
K. Shi, L. Ye, G. Li, J. Therm. Anal. Calorim. 126, 795 (2016). https://doi.org/10.1007/s10973-016-5523-6
F. Menges, Spectragryph—optical spectroscopy software (2020). http://www.effemm2.de/spectragryph/. Accessed 10 Jan 2023
M. Moezzi, J. Yekrang, M. Ghane, M. Hatami, J. Ind. Text. 50, 240 (2020). https://doi.org/10.1177/1528083718825316
P.N. Thanki, R.P. Singh, Polymer 39, 6363 (1998). https://doi.org/10.1016/S0032-3861(97)10390-1
P. Cerruti, M. Lavorgna, C. Carfagna, L. Nicolais, Polymer 46, 4571 (2005). https://doi.org/10.1016/j.polymer.2005.03.065
W. Dong, P. Gijsman, Polym. Degrad. Stab. 95, 1054 (2010). https://doi.org/10.1016/j.polymdegradstab.2010.02.030
G. Socrates, Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd edn. (Wiley, Chichester, 2001)
R. Bernstein, K.T. Gillen, Polym. Degrad. Stab. 95, 1471 (2010). https://doi.org/10.1016/j.polymdegradstab.2010.06.018
B. Fayolle, E. Richaud, X. Colin, J. Verdu, J. Mater. Sci. 43, 6999 (2008). https://doi.org/10.1007/s10853-008-3005-3
Q. Deshoulles, M.L. Gall, C. Dreanno, M. Arhant, D. Priour, P.Y.L. Gac, Polym. Degrad. Stab. 197, 109851 (2022). https://doi.org/10.1016/j.polymdegradstab.2022.109851
L.H. Cai, Z.G. Qi, J. Xu, B.H. Guo, Z.Y. Huang, Chin. Chem. Lett. 28, 949 (2017). https://doi.org/10.1016/j.cclet.2016.11.017
I. Ksouri, O. De Almeida, N. Haddar, J. Polym. Res. 24, 133 (2017). https://doi.org/10.1007/s10965-017-1292-6
C.H. Do, E.M. Pearce, B.J. Bulkin, H.K. Reimschuessel, J. Polym. Sci. A Polym. Chem. 25, 2409 (1987). https://doi.org/10.1002/pola.1987.080250908
P. Gijsman, G. Meijers, G. Vitarelli, Polym. Degrad. Stab. 65, 433 (1999). https://doi.org/10.1016/S0141-3910(99)00033-6
K.H. Su, J.H. Lin, C.C. Lin, J. Mater. Process. Technol. 192–193, 532 (2007). https://doi.org/10.1016/j.jmatprotec.2007.04.056
R. Karrer, The perfect rope - Production and Use, Seon, 2002. http://personal.strath.ac.uk/andrew.mclaren/Turin2002/CD%20congresso/The%20perfect%20dynamic%20rope%20-%20production%20and%20use.pdf. Accessed March 24, 2023
G. Duan, C. Zhang, A. Li, X. Yang, L. Lu, X. Wang, Nanoscale Res. Lett. 3, 118 (2008). https://doi.org/10.1007/s11671-008-9123-7
V. Fernández-González, J.M. Andrade, B. Ferreiro, P. López-Mahía, S. Muniategui-Lorenzo, Spectrochim. Acta A Mol. Biomol. Spectrosc. 263, 120162 (2021). https://doi.org/10.1016/j.saa.2021.120162
J.-M. Lee, R. Busquets, I.-C. Choi, S.-H. Lee, J.-K. Kim, L.C. Campos, Water 12, 3551 (2020). https://doi.org/10.3390/w12123551
P.N. Thanki, C. Ramesh, R.P. Singh, Polymer 42, 535 (2001). https://doi.org/10.1016/S0032-3861(00)00374-8
J.W. Washington, T.M. Jenkins, Environ. Sci. Technol. 49, 14129 (2015). https://doi.org/10.1021/acs.est.5b03686
K. Isbilir, F. Lisco, G. Womack, A. Abbas, J.M. Walls, 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion, Waikoloa, HI, USA (2018), p. 3426–3431. https://doi.org/10.1109/PVSC.2018.8547272
Acknowledgements
We thank Univ.-Prof. Dr. Thomas Bechtold for providing valuable advice during the research.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. This work was supported by the European Regional Development Fund [project ITAT1027] and the Vice-Rectorate for Research at the University of Innsbruck.
Author information
Authors and Affiliations
Contributions
DS: Conceptualization, Methodology, Investigation, Visualization, Writing—Original Draft, MR: Conceptualization, Methodology, Investigation. APM: Validation.
Corresponding author
Ethics declarations
Conflict of interest
Author Daniel Sedláček declares that in the course of this research he started working on another research project with Mammut Sports Group AG.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sedláček, D., Roso, M. & Manian, A.P. The Effect of a Hydrophobic Coating on the Photodegradation of Dyed Nylon 6 Yarns. Fibers Polym 24, 3889–3900 (2023). https://doi.org/10.1007/s12221-023-00311-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00311-8