Abstract
The aim of this study is to separate PET flakes by applying floatation technique, initially from binary polymer blends and later from the mixture of five common polymers such as polypropylene, polyethylene, poly(vinyl chloride), polstyrene and poly(ethylene terephthalate). A connection line between polymer and fiber is presumed in this study, because the dyeing of recovered polyester with three disperse dyes followed. The separation of plastics was performed in a Plexiglas air floatation column. The Lutensol® TO 8 surfactant of non-ionic origin was used to selectively wet the plastics. The parameters studied were pH values, surfactant concentration and treatment time. The dyeing of the recovered PET was performed with Serilene Blue RL, Serisol Crimson ECF and Serisol Yellow ECF dyes at 90 °C, 110 °C, 130 °C and pH = 4.6. The results obtained showed a good separation of polyester from both the binary mixtures and the mixture of five polymers with a recovery rate of up to 95 ± 2%. The influence of pH and concentration of surfactant is significantly demonstrated, while the effect of the treatment time is considered negligible. Finally, recovered polyester showed very good dyeing properties with all three disperse dyes, since the color exhaustion rate reached approximately 95% for the temperature of 130 °C, which is the usual dyeing temperature of polyester.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The European Commission launched the Circular Plastics Alliance in 2018 to help plastics’ chains boost the EU market for recycled plastics up to 10 million tons by 2025. Achieving this target requires producing an additional 3.4 million tons of recycled plastics in Europe by 2025, compared to 2020. Therefore, sorting capacities should increase by at least 4.2 million tons by 2025 and recycling capacities by at least 3.8 million tons [1].
The main problem associated with plastic recycling has its roots in the screening or presence of different types of plastic waste, which needs greater pretreatment before recycling, compared to glass or metal. They must often be of nearly identical composition to be processed efficiently. Alternative initiatives on plastic waste management reveal that mechanical recycling should be favored instead of restricted resource-drilling solutions often discussed [2,3,4]. Post-consumer plastic waste commonly consists of mixed plastics of unknown composition, which makes it a more complex stream to recycle. No matter how efficient the recycling scheme is, primer separation of plastics is the most important step in the recycling process. The steps involved are usually the following: cutting/shredding, contaminant separation, plastic sorting and melting extrusion. Thereby, the development of plastic separation techniques is a growing necessity [5,6,7].
A number of promising technologies for the separation of mixed thermoplastics are under investigation and include air classification, hydrocycloning, sink/flotation, turbocharging, depolymerization/purification/repolymerization, selective dissolution, sorting-based on infrared analysis or laser scanning of polymers and, finally, incorporation of chemical markers into different polymers. Up to now, only gravity separation and sink/flotation techniques have been used on industrial scale. The lack of ability to separate a complex mixture of plastics poses limits in the recycling and reuse of most polymers [8, 9]. This untreated waste could generate a primary route for microplastics that are commonly found in the environment [10], another serious issue revealed lately.
Flotation originally developed for mineral separation, contributed enormously to the economy of industrialized countries. However, the process is also beneficial to a large variety of dissolvable chemical species, such as metals and dyes’ ions. Today, flotation processes are frequent into paper manufacturing for deinking and waste paper recycling, potable water treatment, removal of emulsified oil from various industrial waste-waters and the separation of used plastics [11,12,13].
Therefore, froth flotation is an attractive technique for the plastics recycling industry as shown by the increase in research activities in this area. The basis of froth flotation relies on the different wettability of particles. It is a physicochemical process based on the selective adhesion of some particles to the air (hydrophobic particles) or the water (hydrophilic particles) in a solid/water dispersion. The separation takes place in a container (flotation cell), where water and particles are put together, and then air is continuously injected giving rise to the formation of air bubbles. This mixture is previously conditioned with the controlled addition of small quantities of specific chemical reagents (collectors, bubbling agents, modifiers) to promote the selective formation of aggregates between solid particles, of a certain composition, and air bubbles. After collision, hydrophobic particles adhere to the air bubbles, moving upwards to the top of flotation cell where they are recovered as the floated product part. Hydrophilic particles settle in the mixture and become the non-floated product. Plastics are hydrophobic by nature, therefore in the separation of plastic mixtures by froth flotation, the surface of one or more species should become hydrophilic, while the others are kept hydrophobic [8, 14].
According to the European Plastics Organization the commercial demand aims mainly to thermoplastics and includes polypropylene (PP, 19.4%), high density polyethylene (HDPE, 12.4%), poly(vinyl chloride) (PVC, 10%), poly(ethylene terephthalate) (PET, 7.9%), polystyrene (PS, 6.7%) regarding polymer types [3]. Based on that information, along with the PET percentages of the solid waste composition, plus the chemical structure of the polymer, it is concluded that PET is the polymer type that should be incorporated in this study.
Poly(ethylene terephthalate), more commonly known as PET in the packaging industry and referred to as “polyester” in the textile industry, is an indispensable material with immense applications owing to its excellent physical and chemical properties. On the other hand, due to its increasing consumption and extremely slow biodegradability, PET waste disposal has created serious environmental and economic concerns. Thus, management of PET waste has become an important social issue [15]. In fact, Code 1 was given to PET as the predominant recyclable plastic, the first one that has participated in serious efforts of recycling and whose products are valuable in chemical industry. After all, the connection between PET polymer and fibers is crossing through recycling and sustainable development. Recycled PET may not be easily induced in food packaging (although that has started to change), because of residues or contamination concerns, yet it is highly valuable and applicable in textile industry. After cleansing and melting of polyester, it may be treated as fabric, so its dyeing capability is a serious concern. Under that perspective, the two major applications of PET are met, in a recycling, reusing sense.
Since under normal conditions most plastics are hydrophobic, to separate, selective wetting of one or more of them is necessary. As plastics belong to solids with low surface energy, their wetting by water can be achieved by chemical modification of their surface with suitable reagents, known as wetting agents. These are surfactants which consist of molecules capable of being adsorbed on the surface of plastic particles and of groups that can make them hydrophilic [16]. The disperse agent Lutensol® TO 8, particularly, is a non-ionic surfactant agent of the formula RO(CH2CH2O)xH, where R = iso-C13H27 and x = 8.
Disperse dyes are non-ionic compounds with minimal solubility in water, thus most suitable to dye hydrophobic fibers such as polyester. For reasons of effective diffusion, the particles of disperse dyes should be as thin as possible, with low molecular weight molecules ranging from 400 to 600. It is essential that disperse dyes be able to withstand dyeing conditions such as pH and temperature [17, 18]. Due to their hydrophobic properties and low solubility in water, these dyes can penetrate inside the polyester fibers, especially if the dyebath is held at high temperatures [19]. The factors that affect the disperse dyeing process are the chemistry of dispersing agent (mostly azo dyes), the particle size in dispersion, the pH of the dyebath or its temperature. Dyeing agents, when found in small concentrations in dye solutions, increase its rate.
In the present work, the flotation separation of PET particles either from bi-component mixtures or from a blend of the five thermoplastics waste mixture (PP, HDPE, PVC, PET, PS) was investigated, followed by the study of the dyeability characteristics of retrieved PET. The effect of the wetting agent Lutensol® TO 8 was investigated for the first time on the separation of polymers without alkaline treatment. The novelty of the current research is the combination of PET packaging material reprocessing and the valorization of the waste by changing its end-of-use, treated as possible polyester fabric. Thus, dyeing behavior study of the particles in second phase is determinant.
2 Experimental
2.1 Materials
PET, PVC, PP, HDPE, PS samples were taken by commercial soft drink bottles, wastewater pipes, plastic cups, lids from soft drink bottles, and drinking cups, respectively. In that way, the feedstock reflects the actual state of municipalities wastes. The samples were cleaned thoroughly before application. The plastics were shredded in mill with a sieve in the exit and a size fraction 1–3 mm was finally used. Plastics were of different colors which made easier the analysis eventually.
Additionally, the common solutions of CH3COOH, CH3COONa, NaOH, HCl, were used in various experiments, along with Na2S2O4 as reducing agent (all reagents supplied by Merck), Lutensol® TO 8 as floating agent, Kahatex TE (ΜW = 600) as dispersing agent, pine oil as frothing agent and Alcosperse® AD (naphthalene formaldehyde sodium salt condensation product, (C10H8O3S·CH2O)x·xNa, MW = 261.25), Optinol MBF (mixture of benzoate carriers, phosphate and sulfonated anionic dispersing and levelling agents as benzyl benzoate, n-butyl benzoate, ethoxy oleyl alcohol phosphate ester, sulfonated oleic acid potassium salt, 2-(2-butoxyethoxy)-ethanol) as dyeing modifiers. The three disperse dyes are commercially available under the labels Serilene Blue RL (MW = 356), Serisol Crimson ECF (MW = 348), Serisol Yellow ECF (MW = 269) corresponding to blue, red and yellow colors, respectively.
2.2 Frothed Flotation Experiments
The bench-scale flotation cell was a Perspex column (internal diameter 4 cm and total height 60 cm). A cylindrical ceramic porous diffuser, with a range of pore diameters 16–40 μm, was used as a gas sparger [11]. The investigated parameters were pH, wetting agent concentration, and conditioning time. Suspensions of 0.5 L tap water bearing 1 g of each polymer were conditioned by agitation (500 rpm for 20 min in total on a magnetic stirrer Heidolph MR 3001 K) after the addition of the wetting agent Lutensol® TO 8 and pH adjustment (with calibrated pH-meter METROM 605) by adding NaOH or HCl solutions. Pine oil (20 mg/L) was added as frothing agent. Subsequently, the suspension was transferred into the flotation column where the flotation experiment was initiated by feeding air at a flow rate of 100 mL/min for 5 min. After then, the floated and sinked products were collected separately, rinsed, dried, and weighed (Precisa 180 A balance, ± 0.0001 g). Plastics recovery was calculated using dry weights of concentrates and tailing products by employing Eq. (1),
where \({m}_{C}\) and \({m}_{T}\) are the dry mass of the concentrate and tailing products, respectively.
2.3 Dyeing Experiments
Dyeing was carried out in a Rotadyer apparatus (John Jeffreys Ltd., Rochdale Banbury), at pH 4.6 for 30 min, at three temperatures 90, 110, 130 °C at a solid to liquor ratio of 1:25. The dyeing pots of 100 mL volume, contained 1 g of PET particles, the dyestuff Serilene Blue RL or Serisol Crimson ECF or Serisol Yellow ECF (C.I Disperse Blue 56, Disperse Red 13, Disperse Yellow 3, Fig. 1) and the buffer solution 0.2 M CH3COOH/0.2 M CH3COONa along with the additives: Alcosperse® AD as dispersing agent at 1 g/L, Optinol MBF as levelling agent at 1 g/L concentrations. The samples after dyeing were washed with water and a reduction cleansing washing was performed to remove surface deposited dyestuff, by employing a solution with the dispersing agent Kahatex TE 1 g/L, 1.5 g/L NaOH, and Na2S2O4 as reducing agent 3 g/L at 343 K for 20 min.
Dyestuff concentration in solutions after dyeing (after solutions cooled down to room temperatures) was evaluated spectrophotometrically by monitoring the absorbance of the solution using a UV–Vis spectrophotometer (U-2000, Hitachi, range 400–800 nm), at the λmax of 635 nm for blue, 517 nm for red and 435 nm for yellow disperse dyes. The amount of dye adsorbed at time t, qt (mg/g), and at equilibrium qe (mg/g), was calculated using the following equations, respectively:
where \(V\): solution volume (L); \(m\): mass of PET (g); \({C}_{0}\), \({C}_{t}\), \({C}_{e}\): dye concentration initially, at time t and at equilibrium (mg/L), respectively.
Dye solutions of 0, 2, 4, 6, 8, 10, 15 and 20 mg/L were prepared to create the standard curves by UV absorptions. Each dye solution was measured at the appropriate wavelength, then an absorption–concentration diagram was constructed. The addition of 1 g/L Alcosperse® AD and 1 g/L Optinol MBF auxiliary agents in standard solutions, for all temperatures, was maintained to mimic the experimental conditions. Taking in consideration the dilutions of the samples, the calculations followed for the exhaustion values.
Kinetic experiments were also carried out with 800 mg/L as initial dye concentration and varying the dyeing time (0–1 h) for each case. At certain time intervals and until equilibrium was restored, a dye cartridge was removed from the machine and cooled with tap water. The contents of the ampoule were then filtered in Buchner funnel to separate the painted PET particles from the paint solution. The experimental data were fitted to pseudo-first-order kinetic model (Eq. (4)) and pseudo-second-order kinetic model (Eq. (5)) equations [20]. The parameters of the aforementioned kinetic models: \({k}_{1}\) (min−1), \({k}_{2}\) (min−1(mg/g)−1) are the rate constants of the pseudo-first order and pseudo-second-order equations, respectively.
Equilibrium experiments were also carried out by varying the initial dye concentration (200–2400 mg/L) in all three temperatures and dyes, by keeping constant the mass of PET at 1 g. The experimental data were fitted to Langmuir isotherm (Eq. (6)) and Freundlich isotherm (Eq. (7)) (linear forms of isotherms) [20].
where \({q}_{\mathrm{max}}\) (mg/g) is the maximum amount of adsorption; \({K}_{L}\) (L/mg) is the Langmuir adsorption equilibrium constant; \({K}_{F}\) (mg1−1/nL1/ng−1) is the Freundlich constant representing the adsorption capacity, n is the constant depicting the adsorption intensity.
Finally, some experiments for the color fastness have been conducted to control the color endurance of dyed PET flakes, retrieved after floatation. The painted PET flakes were placed in plastic bags which were adjusted to have dimensions of 1 × 6 cm. They were then placed in a metal case side by side with Blue Wool Standards (James H. Head Co Ltd, Halifax England, batch# 000932). In this experimental part, 8 strips of blue wool control fabric were placed fixed onto an opaque cardboard “half-masked”, numbered as 1–8 (very low to very high light fastness, dimensions 1 × 6 cm too) and introduced in the ageing device, in the Q-SUN Xenon chamber for 48 h at 50 °C, emitting at 340 nm. The observation was conducted in an observation chamber (Mulder's VeriVide®) with the application of D65 lamp, which mimics the average natural daylight by naked eye.
The washing resistance experiments were performed on PET samples that were painted at 130 °C and with dye solutions of concentrations of 800, 1600, and 2400 mg/L. In a metal ampoule 0.4 g of PET particles were added along with multi-standard fabric (size 4 × 10 cm). Then 3 g/L of soap solution and 1 g/L Na2CO3 were added to a 50 mL bath. The ampoules were rotated in the sprayer for 40 min at a temperature of 50 °C. The multi-standard fabric was then removed and rinsed twice with deionized water and allowed to dry. The color fastness was assessed visually too, using the standard grey contrast scale (the scale 5 to 1 points out gradual contrast increase, as described in ISO 105-A02) to detect the intensity of color changes in terms of contrast on polymer flakes. For all cases the same experienced experimenter made the bare-eye visual observations, standing in the same position, under the same light conditions.
3 Results and Discussion
3.1 Flotation Separation of the bi-component Mixture PET-PVC
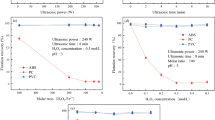
To investigate the effect of pH on the selective separation of the bi-component mixture PET–PVC, the particles were subjected to flotation experiments without any auxiliary reagent (Fig. 2a). The effect of conditioning time was found negligible and for this reason it was kept constant at 20 min (tcond = 20 min, tflot = 5 min, CWA = 0 mg/L). In similar experimental works tcond is kept in lower values, yet, in spite of good results retrieved for < 20 min, a longer tcond was chosen to assure strong retention of agent on every polymer. The floatabilities of PET and PVC are quite similar in the whole pH range. In the pH range 4.0–9.0, PET and PVC particles were floated almost completely indicating hydrophobic characteristics but at higher pH values were almost sunk indicating hydrophilic characteristics. The most noteworthy point is that at pH = 4.0, PVC recovery is slightly depressed with respect to PET which indicates a potential separation.
Previous publications mention that selective flotation separation of the PET–PVC mixture is impossible without changing the surface properties of these polymers. Both polymers exhibit almost the same degree of hydrophobicity. It is evident that significant chemical changes at the surface of either PVC or PET are required to make a selective flotation separation of PET–PVC mixtures [8]. Some researchers applied strong alkaline treatment before flotation to destroy hydrophobicity of plastics. On this basis, the method involves treatment of alkaline solutions followed by plasticizer conditioning and then froth flotation. Unlike these studies, some researchers applied directly froth flotation without any previous alkaline treatment. In the latter, calcium lignin sulfonate or tannic acid was used as wetting agents [21].
Plastic bi-component mixtures were treated, at neutral pH to avoid consumption of chemicals, with the wetting agent at various concentrations and the results are shown in Fig. 2b. It is clear that increasing the wetting agent dosage from 0 to 80 mg/L there is no significant effect and both particles remain floatable. However, higher concentrations caused a steep decline in PVC recovery and a more gradual in PET’s one. The dosage of 160 mg/L wetting agent, resulted to a floated product with ~ 100% PET content but at ~ 30% recovery. This could be attributed to the fact that some polymers as PET and PVC contain some polar lateral groups which include electronegative atoms such as Cl or O, respectively, and enhance the adsorption of wetting agent [22].
Figure 2c displays the effect of wetting agent at pH = 4.0, which previously showed a possibility of separation. The change of pH keeps the behavior of PVC recovery almost the same with respect to pH = 7.3, while causes the complete recovery of PET by the higher wetting agent dosage. The pH values in the alkali range do not promote separation between PET and PVC particles, i.e., both types of polymers achieve great recoveries. This is why we chose pH = 7.3 as a satisfactory environment for just PET recovery. The addition of 160 mg/L, led to a floated product with ~ 100% PET recovery and ~ 85% content.
3.2 Flotation Separation of the bi-component Mixture PET-PS
The effect of pH on flotation separation of the bi-component mixture PET–PS is illustrated in Fig. 3a. The floatability of PS is complete (100%) for the whole pH range which favors the separation of the mixture in the strong alkaline region. The pH = 10 results to a floated product with ~ 85% PS content and ~ 100% recovery.
The addition of 160 mg/L wetting agent makes possible the separation of mixture at pH = 7.3, as shown in Fig. 3b, by rendering mainly PET hydrophilic. PS particles were separated from PET particles with 99.5% recovery and 85% content. At pH = 10.0 less wetting agent (120 mg/L) is needed to succeed the same results as at pH = 7.3 (Fig. 3c). By employing 160 mg/L, leads to a lower separation because both particles become hydrophilic. PS is a non-polar polymer, known for creating static electricity around its particles, due to its chemical structure.
3.3 Flotation Separation of the bi-component Mixture PET-PP
Figure 4a, b illustrates the effect of pH and wetting agent concentration at pH = 7.3 on flotation separation of the bi-component mixture PET–PP. The behavior of flotation has the same trend with the bi-component mixture PET–PS (Fig. 3a, b). At pH = 10.0 a wetting agent concentration 160 mg/L is needed to succeed flotation of PP particles with 99.5% recovery and 87% content, as shown in Fig. 4c. PP as a polyolefin bears no hydrophilicity in its macromolecules.
3.4 Flotation Separation of the Bi-component Mixture PET-HDPE
It is obvious that, the effect of pH and wetting agent at pH = 7.3 and 10.0 on flotation separation of the bi-component mixture PET–HDPE, as illustrated in Fig. 5, has the same trend with the bi-component mixture PET–PS. The chemical affinity between PP and PE is shown in many characteristics of those two industrial polymers, where they show similar behavior. At pH = 7.3 the wetting agent concentration 160 mg/L is needed to succeed flotation of HDPE particles with 99.5% recovery and 80% content, as shown in Fig. 5b. The similar behavior of PS–PP–HDPE polymers could be attributed to the lack of polar groups in their structure, which enhance the adsorption of wetting agent [22].
3.5 Flotation Separation of PET from the five-component Mixture PET-PVC-PS-PP-HDPE
The effect of pH, in the presence of 160 mg/L wetting agent concentration, is given in Fig. 6. PET recovery is almost complete at pH = 4.0 and after then presents a steep descent at pH = 7.3 succeeded by complete sink at pH = 10.0. Although several options could be developed for the separation of PET from the five-component mixture, the flotation at pH = 4.0 was chosen and followed, which keeps PVC in the underflow and all the other plastics in the froth. It is crucial to determine the conditions where PET and PVC act differently to separate them. After this step, the flotation separation of PET from the four-component mixture PET–PS–PP–HDPE was realized at neutral pH as shown in Fig. 7. A wetting agent concentration of 160 mg/L was proved to succeed complete flotation of PS–PP–HDPE particles while PET remains complete unfloatable and could be removed from the bottom of the flotation column.
3.6 Dyeing of Recovered PET by Three Disperse Dyes
Figure 8a depicts the kinetic study of recovered PET coloration by Serilene Blue RL at three isothermal conditions 90, 110, 130 °C, which shows the variations of the sorption capacity \({q}_{t}\) (mg/g) vs time. For all the temperatures, there is a steep increase of the sorption capacity within 20 min followed by a more gradual one. The data were fitted to pseudo-first order kinetic (Eq. (4)) and pseudo-second order kinetic (Eq. (5)) models. The resulting kinetic parameters along with the correlation coefficient (R2) are given in Table 1. It is clear from R2 values and the estimated \({q}_{e}\) capacities that the adsorption process follows pseudo-second-order kinetic model better than first-order kinetic model. This suggests that the rate-limiting step of this sorption system may be chemical sorption. The \({q}_{e}\) values are calculated as described in part 2.3 and describe quite sufficiently the kinetic of the PET dyeing.
The previous results are displayed as color exhaustion percentage in Fig. 8b. As it can be seen, color removal is very fast, almost complete (~ 90%) and can be finished within 40 min. It should be stressed that since the same experiments have been realized with virgin PET without any significant differences, means that recovered PET can be effectively used and replace virgin PET in several applications of moderate demands.
The adsorption isotherms are depicted in Fig. 8c. It demonstrates the correlation between residual dye concentrations in the liquid phase vs the dye concentrations in the solid phase for various initial dye concentrations. Data showed an enhancement in the amount of dye adsorbed, when the initial dye concentration and temperature was increased.
The experimental data were fitted to the Langmuir and Freundlich models and the resulting parameters are given in Table 2. As noticed, the higher the temperature occurs, the higher the adsorption capacity is shown. The temperature plays a key role in such physicochemical phenomena: the rise of the temperature facilitates the motion of the particles and molecules, the development of numerous forces among them, thus the appearance of extent adsorption in the system. The higher correlation coefficient of the Langmuir model revealed that the particular isotherm provides adequate theoretical correlation.
Working analogously for the other two disperse dyes, coloring red and yellow PET flakes, we have gathered the results of the UV-absorption measurements and afterwards calculated the capacities of dyes absorbed by the polymer. Later the equations were employed. The charts formed are shown in Fig. 9 for Serisol Crimson ECF dye and Fig. 10 for Serisol Yellow ECF dyes. For each case, it seems that the dye absorbance capacity increases with increasing temperature, since results at 130 °C are more satisfactory than at 90 °C. Αt higher temperatures the coherence between polymeric chains and increases the kinetic energy of the dye molecules. Moreover, it was noticed that in that dyeing temperature the addition of one modifier is sufficient, Alcosperse® AD. The colour exhaustion is very fast for all three dyes used, almost complete within the first 40 min of the process.
As occurring in blue coloration, the kinetic parameters for other two disperse dyes follow the same pattern: the adsorption process follows pseudo-second-order kinetic model better than pseudo-first-order kinetic model. Thus, Table 3 was filled down, providing the data calculated for the case of Serisol Crimson ECF and Serisol Yellow ECF dyes at three dyeing temperatures. The three dyes exhibit similar behavior (at pH = 4.6, dyeing time = 1 h), with the raise in the initial concentration of dyes increasing the amount adsorbed on PET flakes. The same trend is observed with the raise of dyebath temperature.
Regarding thermodynamic equilibrium, the experimental data appear to follow the Langmuir–Freundlich. Those show that the isotherms provide satisfactory correlation between experimental and theoretical data. The adsorption isotherm of dyeing represents the equilibrium state in the system, while the shape of the adsorbent curve gives information about the mechanism of the dye and the type of bonds developing.
As far as endurance of dyeing is concerned, Fig. 11 shows the light resistance of dyes PET samples after 2 days exposure in Q-SUN Xenon chamber (340 nm). The results demonstrate the excellent colour fastness of all three dyes, which once again reveals the in-depth adsorption of the dye on the PET particles. In the picture we may observe all three dyes (blue, red and yellow colours) and all dye percentages tested, have been proved stable. The flakes (shaped in bags) have remained unchanged over time exposure, despite the alteration of blue wool standards until the 5th strip.
The washing resistance of the dyes PET samples in 2%, 4% and 6% o.m.f. with all three disperse dyes is presented in Table 4. The scale 5 out of 5 indicates no contrast differences among the initially dyed sample and the washed dyed samples. Thus, the color has retained its intensity. The indication among 4–5 out of 5 shows a slight difference on the washed particles. The results obtained show the great stability of the colors in the wash for all three tones of dyeing. This fact shows that the adsorption of the disperse dyes is not superficial on the polyester and demonstrates the reliable results.
3.7 Perspectives on the Flotation Separation of Plastics
In many communities, plastic recycling rates appear low due to the complexity of sorting and processing. Usually, the reported rate of plastics recycling is regarded as unrealistically high by most recycling professionals with experience in their cycled plastics markets. Oil and gas are important feedstocks for plastics, and plastics production requires large amounts of energy, eight times more to make virgin plastic than recycled plastic, for some types. More plastics would be recycled if tax payers did not subsidize oil and energy prices [23].
Artificially low virgin materials prices discourage materials-efficient design, recycling, reuse, and other resource-conserving systems and practices. Most recycling commodity prices are pegged to be below the level for comparable virgin raw materials. Furthermore, low virgin materials prices drive down prices for recyclable materials as well, making it more difficult for recyclers to cover costs and stay in business. Apart that, the concerns on human health and safety dominate in the management of plastic wastes [23,24,25].
Flotation is undoubtedly the most important and versatile mineral-processing technique. Compared with mineral flotation originally patented in 1877, plastics flotation is just in its infancy. Plastic flotation research began in the 1970s but there are few full-scale applications of this technique in industry at present. However, more and more attention is now being addressed to this subject [22].
Although a great number of projects have been initiated on plastics flotation, fundamental studies reported on this subject are insufficient, and different authors suggested different or even inconsistent mechanisms [26,27,28,29,30]. More particularly, mixtures of PET–PE–PP, PET–PS and PET–POM–PVC [21] have been investigated, mixtures of PET–PE and PET–PP have been separated with high selectivity [27], PET flotation was applied for separation from PS too (using sodium lignosulfonate, arabic gum and tannic acid as wetting agents) [14]. In the case of the separation of PET–POM and PVC, Al2(SO4)3 and calcium lignosulfonate [29] were applied. Most researchers have used the flotation technique to separate PET-PVC mixtures [21], while Shent et al. tried to explain the mechanism by which this separation occurs [22]. All of the above studies recorded quite satisfactory results in alkaline environments, though they used way different equipment and chemical environments. More specifically, Fraunholcz et al. showed that the combination of alkaline treatment followed by adsorption of surfactant had a synergistic effect with improved separation and lower reagent consumption. This is because alkaline treatment promotes the hydrolysis of the polyester, increasing the number of hydrophilic groups (hydroxyl- and carboxylic groups) and the surface roughness [28].
It is, thus, understood that flotation is a very promising technique in the separation of plastic waste, since it has become the focus of many studies and presents advantages such as satisfactory performance and low cost [31]. Therefore, further research on mechanism of adsorption of wetting agents on plastic surface is necessary. In addition, there exist several problems in the current situation with respect to the technique, including mismatch between industrial need and academic work, how to rapidly evaluate plastic floatability and lack of online analysis. Therefore, more efforts are necessary for industrial application of plastics flotation.
The contribution of this publication, according to the previous results, is that the floating of binary mixtures polymers, the pH and concentration of the surfactant has been proved to significantly affect their separation. For this reason, the floating of the mixture of the five polymers PET–HDPE–PP–PS–PVC, is proposed under optimum conditions CWA = 160 mg/L, tcond = 20 min, tflot = 5 min. At pH = 4.0 (Fig. 6) the floatation of PET is almost complete, while decreasing sharply at pH = 7.3 and vanishing at pH = 10. At the same time at pH = 4.0 there is a complete precipitation of PVC with all the rest polymers to float. In these conditions, PVC is completely separated from the other polymers. Afterwards, a second float cycle follows with the four remaining polymers PET-HDPE-PP-PS, under proposed conditions tcond = 20 min, tflot = 5 min, pH = 7.3. As it seems (Fig. 7), for surfactant concentration CWA = 160 mg/L the three HDPE–PP–PS polymers remain unaffected and float while the PET is almost completely precipitated and can be removed from the bottom of the float column. In other words, complete separation of PET is achieved.
4 Conclusion
This study was designed for the separation of PET from plastics mixture by flotation and valorization of retrieved PET particles by dyeing. The floatation was effective in a non-alkaline environment while it was found that the floatation time is not that influential factor. Under the studied experimental conditions, it can be drawn that the separation of PET from the bi-component mixture PET–PVC could be succeeded by flotation at pH = 4.0 and wetting agent concentration 160 mg/L. Regarding the separation of PET from the bi-component mixtures with PP or HDPE, it could be also succeeded in the case PET–PS. Finally, the separation of PET from the five-component mixture PET–PVC–PS–PP–HDPE could be realized in two steps in non-alkaline conditions, as usual. In the first at pH = 4.0, PVC is sinking and removed from the suspension in the presence of wetting agent (160 mg/L). In the second at pH = 7.3, PET is sinking and removed from the suspension in the presence of wetting agent (160 mg/L).
On the other hand, the valorization of polymer waste was checked in the field of fibers. The recovered PET flakes were effectively colored in the study and thus potentially be used by the polyester industry. Regarding the dyeing properties of recycled PET, it is proved that it can be dyed satisfactorily with all three disperse dyes, for the fundamental color shades, blue, red and yellow. The best results are presented at the temperature of 130 °C, which is the usual dyeing temperature of polyester. The fitting of dye exhaustion experimental data indicated a pseudo-second-order of kinetic model which suggests that the rate-limiting step of this sorption is similar to chemical sorption.
References
Research and Development Agenda 2020, https://ec.europa.eu/growth/industry/strategy/industrial-alliances/circular-plastics-alliance_en. Accessed 10 Jun 2022
E. MacArthur Foundation and McKinsey & Company, Τhe New Plastics Economy: Rethinking the future of plastics & catalysing action, https://ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics-and-catalysing. Accessed 10 Jun 2022
Plastics Europe, Plastics - the facts 2021, https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/. Accessed 10 Jun 2022
Z. Wen, Y. Xie, M. Chen, C.D. Dinga, Nat. Commun. 12, 425 (2021)
S.M. Al-Salem, P. Lettieri, J. Baeyens, Waste Manag. 29, 2625 (2009)
A. Brems, J. Bayens, R. Dewil, Therm. Sci. 16, 669 (2012)
N. Singh, D. Hui, R. Singh, I.P.S. Ahuja, L. Feo, F. Fraternali, Compos. Part B-Eng. 115, 409 (2017)
J. Drelich, J.H. Kim, T. Payne, J.D. Miller, R.W. Kobler, Sep. Purif. Technol. 15, 9 (1999)
B.M. Rodrigues, C. Saron, Int. J. Environ. Sci. Technol. 19, 1293 (2022)
P. Muthukumaran, P. Suresh Babu, M. Kamaraj, J. Aravind, Int. J. Environ. Sci. Technol. 20, 1081 (2023)
D. Zamboulis, E.N. Peleka, N.K. Lazaridis, K.A. Matis, J. Chem. Technol. Biotechnol. 86, 335 (2011)
L.M. Shengo, W.N.C. Mutiti, Int. J. Environ. Sci. Technol. 13, 2505 (2016)
H.B. Ortiz-Oliveros, R.M. Flores-Espinosa, Int. J. Environ. Sci. Technol. 16, 3679 (2019)
F. Pita, A. Castilho, Waste Manag. 60, 91 (2017)
L. Bartolome, M. Imran, B.G. Cho, W.A. Al-Masry, D.H. Kim, in Material recycling - Trends and perspectives. ed. by D.S. Achilias (InTechOpen, Croatia, 2012), pp.1–22
S. Saisinchai, Eng. J. 18(1), 45 (2014)
J.R. Aspland, Text. Chem. Color. 24(12), 18 (1992)
J.R. Aspland, Text. Chem. Color. 25(1), 21 (1993)
G.A.F. Roberts, Color. Technol. 112(2), 62 (1996)
H. Patel, Int. J. Environ. Sci. Technol. 19, 10409 (2022)
Ü. Yenial, F. Burat, A.E. Yüce, A. Güne, M.O. Kangal, Min. Proc. Ext. Met. Rev. 34, 412 (2013)
H. Shent, R.J. Pugh, E. Forssberg, Resour. Conserv. Recycl. 25, 85 (1999)
Grassroots Recycling Network, Welfare for waste, ed. Susan Kinsella, 1999, https://archive.grrn.org/reports/w4w/w4w.pdf. Accessed 10 Jun 2022
R. Shamsi, G.M.M. Sadeghi, H. Vahabi, J. Seyfi, R. Sheibani, P. Zarrintaj, F. Laoutid, M.R. Saeb, J. Polym. Environ. 27, 2490 (2019)
T.S. Gomes, L.L.Y. Visconte, B.A.V. Elen, E.B.A.V. Pacheco, J. Polym. Environ. 27, 533 (2019)
H. Shen, E. Forssberg, R.J. Pugh, Resour. Conserv. Recycl. 33, 37 (2001)
G. Dodbiba, N. Haruki, A. Shibayama, T. Miyazaki, T. Fujita, Int. J. Miner. Process. 65, 11 (2002)
N. Fraunholcz, Miner. Eng. 17, 261 (2004)
S. Pongstabodee, N. Kunachitpimol, S. Damronglerd, Waste Manag. 28, 475 (2008)
M.T. Carvalho, C. Ferreira, L.R. Santos, M. ConceiçãoPaiva, Polym. Eng. Sci. 52, 157 (2012)
H. Wang, C. Wang, J. Fu, G. Gu, Waste Manage. 34, 309 (2014)
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mpika, S.A., Nikolaidis, N.F., Bikiaris, D.N. et al. Study on PET Recovery from Polymeric Mixtures, Using the Floatation Technique, and Valorization of the Collected Particles by Dyeing Them. Fibers Polym 24, 1995–2008 (2023). https://doi.org/10.1007/s12221-023-00173-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00173-0