Abstract
Brown seaweed Sargassum latifolium produces a sufficient amount of alginate. It is derived from the cell wall of seaweed as a natural anionic polysaccharide. In the present study, alginate was extracted from S. latifolium, its physicochemical properties, the potential for silver nanoparticles formation, characterization of alginate silver nanoparticles, and its antibacterial activity was studied. Physical analysis of extracted sodium alginate as colour (pale yellow), yield (25.34%), water content (13.17%), ash content (15.5%), and pH (8.6) were investigated. In addition, UV scan analyses of extracted alginate illustrated that the bioactive compounds separated in the range of 200–400 nm. On the other hand, ultraviolet–visible spectroscopy (UV–Vis) scan analysis of synthesized alginate silver nanoparticles gave a maximum peak at 413 nm. Besides that, Fourier Transforms Infrared spectroscopy (FT-IR) analysis of alginate, and alginate silver nanoparticles revealed a variety of functional groups, such as –OH, –NH, –CH, –COOH, CO, and C–C, and thermogravimetric analysis of extracted sodium alginate demonstrated thermal degradation occurs in two steps; Dehydration followed by degradation to Na2CO3 and a carbonized substance that breaks down slowly between 600 and 750 °C in N2. Biochemical contents, protein, and carbohydrate were receptively, respectively, and were 0.89 and 78.56 mg/g dry wt. Alginate silver nanoparticles recorded maximal antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumonia, Escherichia coli, Bacillus subtilis, and Bacillus cereus more than alginate. On the other hand, alginate has no activity against E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sargassum sp. is categorized as an industrial seaweed. However, few people know that it is one type of seaweed currently in great demand, because it is relatively abundant but not always used to its full potential. It produces a sufficient quantity of alginate. Sodium alginate is a natural anionic polysaccharide derived from the cell walls of Sargassum sp. and contains high fiber and vital minerals (Mokoginta et al. 2019; Szekalska et al. 2016). “Alginate” is the name for alginic acid salts, although it can also refer to all alginic acid derivatives and alginic acid itself; in several publications, instead of “alginate,” the word “algin” is used (Pereira 2018). Alginates are composed of D-mannuronic (M) and L-guluronic (G) acids that are organized into M-block and G-block sections, as well as alternate sequence blocks (MG blocks) (Chakravartula et al. 2019). The production of sodium alginate is cheap, safe for the environment, and widely accessible. Besides, alginates are biocompatible, biocompostable, and nontoxic, they also developed in the food and biomedical industries (Sharma et al. 2012). Due to its antiseptic nature, water-retaining capacity, and optimum water vapour transmissivity, alginate polymer is employed in various dressing treatments. It also has antibacterial characteristics against pathogens of therapeutic concern (Raguavaran et al. 2022).
For the manufacture of metal nanoparticles, numerous physical and chemical procedures have been developed (Kumar and Yadav 2009), but these approaches are costly and need for the employment of harmful chemicals as lowering and/or capping agents (Li et al. 2009). While synthetic biogenic nanoparticles have a wide range of activities and can be used in biological applications, green biosynthesis is cheaper, easier, and less risky than conventional methods (Morones et al. 2005). The green biosynthesis of nanoparticles can be achieved by selecting a solvent acceptable to the environment with eco-friendly reducing and stabilizing agents (Jegadeeswaranet al. 2012). Therefore, valuable alternatives to physical and chemical approaches to nanoparticle manufacturing have been proposed using biological methodologies (Mohanpuria et al. 2008).
Recent years have seen a significant number of studies on the use of polysaccharide-based nanoparticles for biomedical applications, such as biosensors, drug delivery, wound healing, and tissue engineering. These plentiful, biocompatible, and tissue regeneration-capable polysaccharides nanoparticles, which also comprise chitosan, alginate, and starch, are attracting much attention (El-Sayed et al. 2021; Dalavi et al. 2022).
This paper focused on the extraction of sodium alginate from brown seaweed Sargassum latifolium, physicochemical characterization of extracted alginate, utilization of extracted alginate to form silver nanoparticles, and the antibacterial activity of alginate and alginate silver nanoparticles were studied.
2 Materials and methods
2.1 Extraction of sodium alginate from S. latifolium biomass
Sargassum latifolium was collected from Ras Sudr on the Red Sea coast in the Gulf of Suez at a longitude of 32° 43′ East and latitude 29° 35′ North. Sargassum latifolium was identified and described by Abu Ahmed et al. (2022). The alginates extracted in water-soluble salts form by the method of Truus et al. (2001). 20 g air-dried S. latifolium powder was boiled in 300 ml of distilled water for 30 min, followed by filtration, and the precipitate was boiled in 0.5% (W/V) aqueous CaCl2 solution for a further 30 min. after that, the mixture was left to cool to room temperature, this step followed by filtration and boiling the precipitate in 300 ml of 0.5% Na2CO3 for 1 h with continuous stirring. Alginic acid was transformed into soluble sodium salt during this stage, and it is still present in the extract. After that, in addition to the insoluble algal residue, a highly viscous solution was produced. A cloth filter was used to filter the mixture by squeezing. Finally, ethanol was added to the filtrate to precipitate the sodium alginate. The resulting alginate was air dried, collected, crushed into powder, and stored safely until additional research.
2.2 Characterization of alginate extracted from S. latifolium
2.2.1 Chemical characterization of sodium alginate
Dubois et al. (1956) method was used to calculate carbohydrate content and Lowry et al. (1951) method was used to evaluate protein content.
2.2.2 Physical characterization of sodium alginate
2.2.2.1 Alginate yield
Alginate yield was estimated as follows:
2.2.2.2 Water content
The water content of sodium alginate was obtained using the Omar et al. (1988) method.
2.2.2.3 Ash content
Three samples of dry alginate (5 g each) were placed in crucibles and dried for 30 min at 105 °C in the oven to determine the ash content. Ash content was determined by the following equation:
2.2.2.4 Hydrogen ion concentration (pH)
pH was determined using a horizon ecology Co pH meter 5995.
2.2.2.5 UV–Vis scan analysis of extracted alginate
ANTI UNICAM-UV VISIBLE VISION SOFTWARE V3.20 was used to determine sodium alginate aqueous solution UV absorbance spectrum between 190 and 590 nm.
2.2.2.6 Fourier transform infrared spectrometry (FT-IR)
The Mattson 5000 FT-IR spectrometer was used to record FT-IR spectrum. The dry sodium alginate was crushed with KBr powder and pressed into pellets for FT-IR spectroscopy in the 400–4000 cm−1 frequency range (Wang et al. 2004).
2.2.2.7 Thermogravimetric analysis (TGA) of extracted alginate
The TGA was carried on a thermoanalyzer of the type 50-H. The TGA was obtained in the range of 25–800 °C, under a nitrogen atmosphere with a flow rate of 20 ml min−1 at a rise of 10 °C min−1. The sample was previously dried at 60 °C for 1 h and placed into a platinum sample pan. The graph was plotted as weight loss (%) vs. temperatures.
3 Preparation of alginate silver nanoparticles
At room temperature, 10 mg of alginate was dissolved in 100 ml of a 1 mM AgNO3 aqueous solution while being stirred. pH of the solution was adjusted (10–11) using KOH. Then, it was left on a magnetic stirrer under continual heating (70 °C) for 30 min. The reduction of Ag+ ions to Ag0 (silver nanoparticles) was observed visually by the change of colour to brown in solution and was apparent immediately after the beginning of the reaction (Yugay et al. 2020). The synthesized silver nanoparticles were centrifuged several times at 20,000×g for 30 min (5430R, Eppendorf) and washed with sterile Milli-Q® water before further analysis. To calculate the concentration of the reduced Ag0 crystals, aliquots (1 ml) of each specimen were dried under vacuum and weighed using an analytical electrical balance.
4 Characterization of alginate silver nanoparticles
4.1 Ultraviolet (UV) scan analysis of alginate silver nanoparticles
UV–Vis absorption was used to observe how the colour of the reaction medium changed colour. The UV–Vis absorbance spectrum of alginate silver nanoparticles was recorded individually using ANTI UNICAM-UV VISIBLE VISION SOFTWARE V3.20 between 200 and 800 nm.
4.2 Fourier transform infrared spectrometry (FT-IR)
FT-IR spectrum was recorded on The Mattson 5000 FT-IR spectrometer. The dried alginate silver nanoparticles were crushed individually with KBr powder and pressed into pellets for FT-IR spectra measurement in the frequency range of 400–4000 cm−1 (Wang et al. 2004).
4.3 Transmission electron microscope of alginate silver nanoparticles
Physical and morphological properties of obtained nanoparticles were characterized using transmission electron microscopy, TEM (JEOL TEM-2100) at Electron Microscope Unit, Central Laboratory, Fact. of Agric., Mansoura Univ., Mansoura, Egypt (El-Refaiet al. 2018).
5 Bioassay of antibacterial activity
5.1 Preparation of inoculum
The solution of sodium alginate was prepared by the dissolution of sodium alginate powder (10 mg) in 100 ml distilled water. Sodium alginate silver nanoparticles were prepared by the dissolution of sodium alginate (10 mg) in 100 ml AgNo3 (method previously mentioned). Ciprofloxacin was used asa positive control. Distilled water and silver nitrate were used asa negative control.
5.2 Pathogenic bacteria
Six different pathogenic bacterial strains, three strains of gram-positive bacteria (Staphylococcus aureus, Bacillus cereus, and Bacillus subtilis), and three strains of gram-negative bacteria (Escherichia coli, Klebsiella pneumonia, and Pseudomonas aeruginosa) were used in this study. Bacterial species (Gram-positive and Gram-negative) were obtained from the laboratory of Bacteriology, Faculty of Science, Damietta University, Egypt.
5.3 Agar well-diffusion method
The antibacterial potentiality of alginate and alginate silver nanoparticles were tested by the standard agar well-diffusion method. 15 ml agar media were poured into sterile Petri-dishes (All of the sets were set in triplicates) and seeded with 0.1 ml of an 18 h old nutrient broth culture of the tested bacterium. Under aseptic conditions, Wells were made using a sterile cork borer (6 mm), and 10 µl of each inoculum was introduced into each well. Plates were then incubated for 24 h at 37 °C. Using a ruler, the inhibitory zone was measured and expressed in millimetres (mm) (Prabhuet al. 2010).
6 Results and discussion
6.1 Chemical characterization of extracted alginate
Sodium alginate extracted from Sargassum latifolium had relatively low protein content (0.89%) and high carbohydrate content (78.56%). Similar findings were found by Rashedy et al. (2021), who reported that the protein content of sodium alginate extracted from five different species of brown seaweed from Red Sea shores ranged from 0.57% to 1.4%, and carbohydrate content ranged from 76.3% to 83.1%. In addition, Viswanathan and Nallamuthu (2014) reported that the protein content of sodium alginate extracted from six different species of brown algae ranged from 1.4% to 7.17%, and carbohydrate content ranged from 75.56% to 93.21%. In addition, Dave and Parekh (1975) reported that this shift could be either spatial or temporal. It is, however, mostly due to the quality of the surrounding water.
6.2 Physical characterization of extracted alginate
Physical characterization of extracted sodium alginate, including the parameters of moisture content, ash content, pH, colour, and yield, which aimed to detect alginate quality produced by S. latifolium recorded in Table 1. The results demonstrated that the colour of extracted alginate was pale yellow, pH was 8.6, moisture content was 13.71%, ash content was 15.5%, and the yield of alginate was 25.34%. Similar physicochemical results were obtained by Rashedy et al. (2021), who reported that sodium alginate yield of Padina boergesenii, Turbinaria triquetra, Homophyysa cuneformis, Dictyota ciliolata and Sargassum aquifolium collected from red sea shores were 17%, 22.2%, 13.3%, 20%, and 19%, respectively. The color of the extracted alginate for all of these species was pale-yellow, and also pH was 9.7, while water content was 13.1%, 12%, 6.4%, 9.7%, and 12.5% receptively. In addition, Viswanathan and Nallamuthu (2014) found that water content of alginate from six different species of brown algae ranged from 15.62% to 28.35%, and ash content ranged from 23.01% to 12.78%.
6.3 Ultraviolet scan analysis of alginate extracted from S. latifolium
Ultraviolet scan analysis was used to confirm the structure of alginate, as shown in Fig. 1. The extracted alginate showed UV maximum absorption spectra at λmax 200–400 nm. The peaks at 220, 233, and 276 nm can be attributed to flavonoids and phenolics and their derivatives (Rajeswari and Jeyaprakash 2019). Proteins and amino acids were identified in aromatic and poly-aromatic compounds by absorption bands between 260 and 400 nm (Husseinet al. 2019).
6.4 Fourier transform infrared scan analysis of extracted alginate
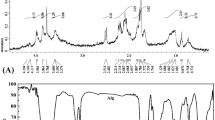
The FT-IR of sodium alginate (Fig. 2) reveals characteristic functional groups showing peaks at 3901 cm−1, 3755 cm−1, 3676 cm−1, 3652 cm−1, 3450 cm−1, 2923 cm−1, 2854 cm−1, 2733 cm−1, 2476 cm−1, 2053 cm-1, 1654 cm−1, 1546 cm−1, 1524 cm−1, 1431 cm−1, 1323 cm−1, 1246 cm−1, 1163 cm−1, 1030 cm−1, 673 cm−1, 616 cm−1 and 504 cm−1. The bands between 4000 and 3400 cm−1 were attributed to hydroxyl group (El-Rafie et al. 2013; Kannan 2014; Mota et al. 2015), and also spectral peaks in this range are due to alcohol and acids (Flórez-Fernández et al. 2019).
The weak absorption bands in the range 2920–2800 cm−1 are unique bands of C–H stretching vibration of CH2 or assigned to the secondary amines (Aprilliza 2017; El-Rafie et al. 2013; Stojanovic et al. 2012).
The strong peaks between 1640 and 1320 cm−1 are related to the symmetric and asymmetric stretching vibration of the carboxylate group (Flórez-Fernández et al. 2019; Pereira et al. 2003; Sheng et al. 2004). The bands at 1030 and 1033 cm−1 are also related to CO–C stretching (Esposito et al. 2020; Iqbal et al. 2020). The bands that occur in the region 700 − 500 cm−1 were Skeletal deformation bands (Socrates 2004).
6.5 Thermogravimetric (TGA) analysis of extracted alginate
The thermal degradation (TGA) of material is investigated by estimated the weight loss of a sample as a function of time or temperature in a varied and controlled atmosphere. The weight loss and derivative of weight loss of sodium alginate are presented in Fig. 3. Sodium alginate in the N2 atmosphere degrades in two steps in the first step, alginate showed a weight loss of 15.24% and the second stage showed 27.61%. These findings show that the salt broke down through dehydration, followed by degradation to Na2CO3 and a carbonized substance that breaks down slowly at 600–750 °C in N2. According to Newkirk and Aliferis (1958), the sample holder and atmosphere employed are said to be important factors in the breakdown of Na2CO3. In the present study, the decomposition of alginate appeared above 800 °C is in agreement with the findings of Soares et al. (2004) using N2 atmosphere.
6.6 UV–Vis scan analysis of alginate silver nanoparticles
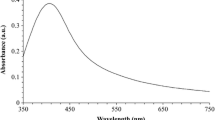
After 48 h, the color of the reaction mixture changed to brownish yellow was visually evident of AgNO3 reduction (Fig. 4). The intensity of the brown color increased as the incubation period increased. On the other hand, there is no change in the color of the silver nitrate solution without sodium alginate (control). The characteristic absorption peak of synthesized alginate silver nanoparticles recorded by Ultraviolet scan analysis at 413 nm (Fig. 5). Numerous studies of the green production of silver nanoparticles revealed similar surface plasmon resonance (SPR) peaks. For example, alginate and fucoidan extracted from Fucus evanescens promoted silver nanoparticles with surface plasmon resonance peaks at 408 and 402 nm receptively, and also fucoidan and laminarian extracted from Sargassum cichorioides showed Ag-NPs with SPR peaks at 412 nm and 418 nm receptively (Yugay et al. 2020). Deepak et al. (2018) reported that he addition of Sargassum wightii aqueous extract caused the reduction of silver ions to create silver nanoparticles, with a high surface plasmon resonance measured at 420 nm. When several seaweed polysaccharides were introduced to AgNO3 solution, pH-adjusted, and heated, the colour of the reaction mixture changed from colourless to brownish almost instantly. For different types of polysaccharides, the brown colour intensity increased quickly and then stayed consistent after 1 h of observation. Because of their characteristic excitation of surface plasmons in the 400–490 nm region, Ag-NPs colloids have a brown colour (Gilaki 2010). As a result, the change in colour of the reaction mixture from clear to brown indicates the formation of silver nanoparticles (Vanaja et al. 2013).
6.7 Fourier transform infrared spectrometry of alginate silver nanoparticles
Figure 2 illustrates the functional groups of alginates responsible for the reduction of silver ions and stabilization of reduced silver ions. Figure 6 shows the FT-IR spectra obtained from alginate nanoparticles.
Table 2 shows the bands attributed to alginate, and alginate nanoparticles.
The functional groups of any organic compound are responsible for its biological activity. FTIR analysis of alginate revealed a broad band of –OH stretching at 4000–3400 cm−1, CH– stretch around 2920–2800 cm−1, symmetric and asymmetric of COO– at 1640–1320 cm−1, antisymmetric stretch of C–O–C at around 1095–1025 cm−1. Therefore, FTIR analysis confirmed the presence of hydroxyl, amine, carboxylic, and alkanes groups that might be responsible for the capping of silver ions to form silver nanoparticles. Alginate and AgNPs may interact by a Vander Waals reaction. Silver nanoparticles may be absorbed by anionic alginate via electrostatic attraction. (Asthana et al. 2016). FTIR and Table 2 should be illustrated these bands 1624 cm−1 in the extracted alginate related to carbonyl (C=O) group in alginate. 1031 cm−1 correspond to guluronic (G) units (El-Sayed et al. 2021).
6.8 Transmission electron microscope of alginate silver nanoparticles
Transmission electron microscopy (Fig. 7) was applied to characterize the size and shape of the nanoparticles. Nanoparticles were confirmed to be present, and morphological details of typical silver nanoparticles were revealed by electron microscope investigation. With TEM, particles were seen to be spherical, distinct, and regular. The particle size for alginate silver nanoparticles was about 4.34–13.77 nm. Our results agreed with El-Rafie et al. (2013), who found that the formed silver nanoparticles from polysaccharides extracted from Ulva faciata, Pterocladia capillacae, Jania rubins, and Colpmenia sinusa were polydispersed and predominantly spherical with maximum diameter size of 7, 7, 12 and 20 nm receptively (Table 3).
6.9 Antibacterial activity of extracted alginate and alginate silver nanoparticles
In the present study, alginate silver nanoparticles showed bactericidal activity more than alginate (Figs. 8 and 9A, B) against P. aeruginosa, S. aureus, K. pneumonia, E. coli, B. subtilis, and B. cereus. On the other hand, alginate has no activity against E. coli (Table 3). Previous research revealed that silver nanoparticles have a lot higher antibacterial activity than silver ions (Ajayi and Afolayan 2017). Our current research also indicated that the synthesized silver nanoparticles from alginate have more bactericidal potential than both silver ions and alginate. In comparison with chemically produced nanoparticles, seaweed-mediated silver nanoparticles were revealed to be more effective bactericidal agents (Mittal et al. 2017). It is really interesting that the Gram-negative bacteria were discovered to be more responsive to all treatments than the Gram-positive strains. Gram –ve bacteria have a peptidoglycan layer (2–3 nm) between the outer membrane and cytoplasmic membrane, according to research (Murray et al. 1965). On the other hand, Gram +ve bacteria lack the outer membrane but contain a thick layer of peptidoglycan (30 nm thickness) (Shockman and Barren 1983). A possible explanation for why Gram +ve bacteria are less vulnerable than Gram –ve bacteria is Gram +ve bacteria have more peptidoglycan than Gram –ve bacteria; this stimulates an electrostatic effect, which results in more entrapment of silver by the peptidoglycan layer in Gram +ve bacteria than in Gram –ve bacteria (Kawahara et al. 2000). In addition, Sahayaraj and Rajesh (2011) reported that The use of silver nanoparticles as new antimicrobial agents represents a viable alternative to inhibit the growth of many pathogenic species. Three most common mechanisms were suggested on the activity of nano-silver on bacteria up to now: (1) uptake of free silver ions followed by disruption of ATP production and DNA replication, (2) formation of Reactive Oxygen species (ROS), and (3) direct damage to cell membranes.
A Antibacterial activity of alginate and alginate silver nanoparticles against (a–a*) K. pneumonia—its control, (b–b*) B. subtilis—its control (c–c*) B. cereus—its control. B Antibacterial activity of alginate and alginate silver nanoparticles against (d–d*) P. aeruginosa—its control, (e–e*) S. aureus–its control and (f–f*) E. coli—its control
7 Conclusions
The results obtained in our study confirmed the efficiency of alginate silver nanoparticles as antibacterial agents. In addition, green synthesis of metallic nanoparticles is cheaper, easier, and less risky than conventional methods. UV and FTIR results indicate that extracted alginate can be used as a capping agent for the synthesis of silver nanoparticles. TEM was used to characterize the size and shape of synthesized silver nanoparticles.
References
Abu Ahmed SE, Deyab MA, Hassan NI, El-Sheekh MM (2022) Physicochemical characterization of Sargassum latifolium at Ras Sudr Shores–Red Sea Coast of Egypt. Egypt J Bot 62(3):1920
Ajayi E, Afolayan A (2017) Green synthesis, characterization and biological activities of silver nanoparticles from alkalinized Cymbopogon citratus Stapf. Adv Nat Sci Nanosci Nanotechnol 8(1):015017
Aprilliza M (2017) Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Publishing, p 012019
Asthana A, Verma R, Singh AK, Susan MABH, Adhikari R (2016) Silver nanoparticle entrapped calcium-alginate beads for Fe (II) removal via adsorption. Wiley Online Library, pp 42–51
Chakravartula SSN, Soccio M, Lotti N, Balestra F, Dalla Rosa M, Siracusa V (2019) Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials 12(15):2454
Dalavi PA, Anil S, Murugan SS, Prabhu A, Venkatesan J (2022) Polysaccharide-based nanoparticles for dentistry applications. Polysaccharide nanoparticles. Elsevier, pp 329–341
Dave M, Parekh R (1975) Protein content of green seaweeds from Saurashtra coast. Salt Res Indi 11(2):41–44
Deepak P, Amutha V, Birundha R, Sowmiya R, Kamaraj C, Balasubramanian V, Balasubramani G, Aiswarya D, Arul D, Perumal P (2018) Facile green synthesis of nanoparticles from brown seaweed Sargassum wightii and its biological application potential. Adv Nat Sci Nanosci Nanotechnol 9(3):035019
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
El-Rafie H, El-Rafie M, Zahran M (2013) Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohyd Polym 96(2):403–410
El-Refai AA, Ghoniem GA, El-Khateeb AY, Hassaan MM (2018) Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J Nanostruct Chem 8(1):71–81
El-Sayed AAM, Abouzeid FM, Ismail MM, ElZokm GM (2021) Characterization and utilization of Sargassum linifolium and Stypopodium schimperi polysaccharides as blue inhibitors for steel electopolishing. Water Sci Technol 83(2):409–424
Esposito L, Barbosa AI, Moniz T, Costa Lima S, Costa P, Celia C, Reis S (2020) Design and characterization of sodium alginate and poly (vinyl) alcohol hydrogels for enhanced skin delivery of quercetin. Pharmaceutics 12(12):1149
Flórez-Fernández N, Domínguez H, Torres M (2019) A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int J Biol Macromol 124:451–459
Gilaki M (2010) Biosynthesis of silver nanoparticles using plant extracts. J Biol Sci 10(5):465–467
Hussein MH, Hamouda RA, Elhadary AMA, Abuelmagd MA, Ali S, Rizwan M (2019) Characterization and chromium biosorption potential of extruded polymeric substances from Synechococcus mundulus induced by acute dose of gamma irradiation. Environ Sci Pollut Res 26(31):31998–32012
Iqbal DN, Tariq M, Khan SM, Gull N, Iqbal SS, Aziz A, Nazir A, Iqbal M (2020) Synthesis and characterization of chitosan and guar gum based ternary blends with polyvinyl alcohol. Int J Biol Macromol 143:546–554
Jegadeeswaran P, Shivaraj R, Venckatesh R (2012) Green synthesis of silver nanoparticles from extract of Padina tetrastromatica leaf. Dig J Nanomater Biostruct 7(3):991–998
Kannan S (2014) FT-IR and EDS analysis of the seaweeds Sargassum wightii (brown algae) and Gracilaria corticata (red algae). Int J Curr Microbiol App Sci 3(4):341–351
Kawahara K, Tsuruda K, Morishita M, Uchida M (2000) Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent Mater 16(6):452–455
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84(2):151–157
Li H, Carter JD, LaBean TH (2009) Nanofabrication by DNA self-assembly. Mater Today 12(5):24–32
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Madkour F, Elshoubaky GA, Ebada MA (2019) Antibacterial activity of some seaweeds from the Red Sea coast of Egypt. Egypt J Aquatic Biolo Fish 23(2):265–274
Mittal J, Jain R, Sharma MM (2017) Phytofabrication of silver nanoparticles using aqueous leaf extract of Xanthium strumerium L. and their bactericidal efficacy. Adv Nat Sci Nanosci Nanotechnol 8(2):025011
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10(3):507–517
Mokoginta M, Indriati N, Dharmayanti N, Nurbani S (2019) Extraction and characterization of sodium alginates from Sargassum polycystum for manufacturing of tuna (Thunnus sp.) meatballs. IOP Publishing, p 012047
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346
Mota R, Pereira SB, Meazzini M, Fernandes R, Santos A, Evans CA, De Philippis R, Wright PC, Tamagnini P (2015) Effects of heavy metals on Cyanothece sp. CCY 0110 growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles. J Proteomics 120:75–94
Murray R, Steed P, Elson H (1965) The location of the mucopeptide in sections of the cell wall of Escherichia coli and other gram-negative bacteria. Can J Microbiol 11(3):547–560
Newkirk A, Aliferis I (1958) Drying and decomposition of sodium carbonate. Anal Chem 30(5):982–984
Omar S, Ahmad N, Ahmad F (1988) Composition of alginate from brown seaweeds, Sargassum and Padina spp. Pertanika (malaysia) 11(1):79–85
Pereira L (2018) Biological and therapeutic properties of the seaweed polysaccharides. Int Biol Rev 2(2):1–50. https://doi.org/10.18103/ibr.v2i2.1762
Pereira L, Sousa A, Coelho H, Amado AM, Ribeiro-Claro PJ (2003) Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol Eng 20(4–6):223–228
Prabhu AS, Jubery TZN, Freedman KJ, Mulero R, Dutta P, Kim MJ (2010) Chemically modified solid state nanopores for high throughput nanoparticle separation. J Phys Condens Matter 22(45):454107
Raguavaran R, Mondal D, Sharma D, Jithin M, Yadav N (2022) Biomedical applications of alginate nanoparticles. Polysaccharide nanoparticles. Elsevier, pp 311–328
Rajeswari R, Jeyaprakash K (2019) Bioactive potential analysis of brown seaweed Sargassum wightii using UV-VIS and FT-IR. J Drug Delivery Therapeut 9(1):150–153
Rashedy SH, Abd El Hafez MS, Dar MA, Cotas J, Pereira L (2021) Evaluation and characterization of alginate extracted from brown seaweed collected in the Red Sea. Appl Sci 11(14):6290
Sahayaraj K, Rajesh S (2011) Bionanoparticles: synthesis and antimicrobial applications. Sci against Microbial Pathogens 23:228–244
Sharma S, Sanpui P, Chattopadhyay A, Ghosh SS (2012) Fabrication of antibacterial silver nanoparticle—sodium alginate–chitosan composite films. RSC Adv 2(13):5837–5843
Sheng PX, Ting Y-P, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275(1):131–141
Shockman GD, Barren JF (1983) Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol 37(1):501–527
Soares JdP, Santos J, Chierice G, Cavalheiro E (2004) Thermal behavior of alginic acid and its sodium salt. Eclética Química 29(2):57–64
Socrates G (2004) Infrared and Raman characteristic group frequencies: tables and charts. Wiley
Stojanovic R, Belscak-Cvitanovic A, Manojlovic V, Komes D, Nedovic V, Bugarski B (2012) Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads. J Sci Food Agric 92(3):685–696
Szekalska M, Puciłowska A, Szymańska E, Ciosek P, Winnicka K (2016) Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int J Polym Sci 2016:7697031. https://doi.org/10.1155/2016/7697031
Truus K, Vaher M, Taure I (2001) Algal biomass from Fucus vesiculosus (Phaeophyta): investigation of the mineral and alginate components. Proc Estonian Acad Sci Chem 50(2):95–103
Vanaja M, Gnanajobitha G, Paulkumar K, Rajeshkumar S, Malarkodi C, Annadurai G (2013) Phytosynthesis of silver nanoparticles by Cissus quadrangularis: influence of physicochemical factors. J Nanostruct Chem 3(1):1–8
Viswanathan S, Nallamuthu T (2014) Extraction of sodium alginate from selected seaweeds and their physiochemical and biochemical properties. Int J Innov Res Sci Engin Technol 3(4):10998–11003
Wang Y, Zhang M, Ruan D, Shashkov AS, Kilcoyne M, Savage AV, Zhang L (2004) Chemical components and molecular mass of six polysaccharides isolated from the sclerotium of Poria cocos. Carbohyd Res 339(2):327–334
Yugay Y, Usoltseva R, Silant’ev V, Egorova A, Karabtsov A, Kumeiko V, Ermakova S, Bulgakov V, Shkryl Y (2020) Synthesis of bioactive silver nanoparticles using alginate, fucoidan and laminaran from brown algae as a reducing and stabilizing agent. Carbohyd Polym 245:116547
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sheekh, M.M., Deyab, M.A., Hassan, N.I. et al. Green biosynthesis of silver nanoparticles using sodium alginate extracted from Sargassum latifolium and their antibacterial activity. Rend. Fis. Acc. Lincei 33, 867–878 (2022). https://doi.org/10.1007/s12210-022-01102-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-022-01102-8