Abstract
Odonata is considered a “flagship” group of insects and its investigation is of primary importance especially for protected areas where freshwater ecosystems occur. In this study, we focused on Odonate fauna in the “Cansiglio Forest” (Veneto, Italy), a karst area where the only checklist available dates back more than 40 years ago. To update the Odonate adult distribution in the area, we selected 21 ponds that were sampled monthly, from May to August, during a 2-year survey. In total, 21 species (belonging to 14 genera and 5 families) have been recorded: we confirmed 15 species from the previous species list and we added to the whole species list 6 new species. Dominant families were represented by Libellulidae (33%) and Aeshnidae (23%), the most common genus was Sympetrum (19%), and the most frequent species was Coenagrion puella (63%). In term of patterns of species richness, highly grazed and pastured ponds exhibited the lower number of species and individuals, as a probable response to the high level of animal disturbance on the vegetation and due to the eutrophication processes. Our results are important also in terms of conservation and management of freshwater sites belonging to Natura 2000 site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Odonata (Insecta: Zygoptera, Anisoptera) represent focal organisms for conservation purposes (Samways 2008; Clausnitzer et al. 2009; Uboni et al. 2020) in freshwater ecosystems, where they can be considered as a “flagship” indicator group (Sharma et al. 2007; Balzan 2012; Hart et al. 2014).
One of the most important ecological roles of the Odonata is that they are among the major invertebrate predators in terrestrial (during the adult phase) and aquatic (during the larvae phase) ecosystems, occupying a high level along the trophic food chain (Askew 1988; Corbet 1993; Samways and Steytler 1996; Clark and Samways 1996; Reece and Mcintyre 2009). Since they are also sensitive to environmental changes (Balzan 2012), they have been often used as bioindicator in both aquatic and terrestrial habitats (Corbet 1993; Sahlén and Ekestubbe 2001; Willigalla and Fartmann 2012; Jacob et al. 2017; Uboni et al. 2020).
The distribution of Odonata species in the environment is largely determined by the presence of suitable habitats able to maintain viable populations (McPeek 2008; Balzan 2012), and it is known that local abiotic and biotic factors (temperature, water chemistry, abundances of predators or parasites) can influence the survival, growth, and fecundity of individuals (Askew 2004). Nowadays, ecological requirements and autecological factors limiting species distributions in habitats are still unclear, and for these reasons, Odonata is a continually fascinating studying group (McPeek 2008; Balzan 2012).
The Cansiglio Forest is a protected site belonging to Natura 2000 Network (IT3230077), located in the northeastern part of Italy (it belongs to the provinces of Treviso, Belluno and Pordenone) (Buffa and Lasen 2010). It is mainly occupied by forest, pastures, hills and old ponds, and it represents a priority area for nature and biodiversity conservation. Furthermore, the scarce anthropization characterizing the area allowed the preservation of the Alpine traditional landscape, otherwise very rare in all the other Alps. The study site is a plateau in the Pre-Alps, with mean altitude of 1000 m and it is surrounded by reliefs ranging from 1400 to 1600 m a.s.l. Interestingly, the Cansiglio Forest lies in a karstic area, where ponds and temporary torrents are the only form of surface hydrography (there are no permanent waterways such as rivers, lakes or springs): these scarce water sources present the only natural watering places for local fauna. The climate is, on average, cool with fresh summers. Temperature extremes range from 30° to – 30 °C (T lower than – 25 °C have been recorded); the average annual rainfall is about 1800 mm and the atmospheric humidity is very high during the whole year; the area is often filled with thick mist formed by the daily heat excursion (http://old.ortarzo.it/aaoc2014/en/forest-cansiglio/).
The importance of this area lies in the contemporary presence of traditional alpine rural structures mixed to high naturalistic and cultural value elements, where extensive and dense forests intermix with meadow-pastures. This mosaic of habitats hosts a multitude of ecological niches fostering a high biodiversity of fauna and flora. These factors lead to a great value for natural balance and ecosystems dynamics (Tables 1, 2, 3, 4, 5 and 6).
In this fascinating context, the only data about Odonate fauna (Insecta: Zygoptera, Anisoptera) dated back to 40 years ago (Bucciarelli 1978) while no recent information are available regarding the distribution of this ecologically important group on the site. For this reason, and considering the uniqueness of this area, we decided to update of the old Odonate checklist. Moreover, we explicitly evaluated grazed and ungrazed ponds aiming at investigating if and how species abundances and richness were influenced by cattle disturb.
With this study, we aim at increasing the knowledge on the ecology, diversity and distribution of this Insect group in the Pre-Alps and, at the same time, to provide an important reference for the habitat management in the Natura 2000 site.
2 Materials and methods
2.1 Study area
The study area is almost completely located inside the "Cansiglio Forest-Veneto Region" SIC-ZPS (IT 3,230,077), an area of 5060 ha with an average altitude of 1189 m a.s.l. (Natura 2000, Standard form). It represents part of the Cansiglio-Cavallo massif, the westernmost portion of the Friulian Prealps (Cancian et al. 1985). The site falls entirely inside the Alpine biogeographical region bordering the Dinaric–Balkan area. The characteristic soils of the wetland habitats belong to the Cutanic Alisol (acidic pH) and Luvic Phaeozems (close to neutrality) types, and they are mainly developed on marly limestone type in the Cretaceous age, on which the karstic phenomenon is still active (Garlato and Borsato 2016). The plateau of the Cansiglio has the shape of a large basin, a “polje”, resulting from the fusion of minor karst units (“uvala”): Pian Cansiglio, Pian di Valmenera, Pian di Cornesega, Pian delle Code. In this limestone plateau are situated many dolines and ponors. The macroclimate of the study area is the same of the mountain regions, characterized by humid conditions and cold winter; the cold air descending from the internal slopes hangs in the basin causes the characteristic phenomenon of heat inversion: the temperature decreases as it passes from the surrounding hills to the lower central areas (ARPAV 2013). In this plateau the most common vegetation is the beech-wood (Dentario pentaphylli-Fagetum sylvaticae) (Del Favero 2004), but there are also many humid habitats represented by ponds, peat bogs and humid grasslands of secondary origin. The ponds, named “lame” or "lamarazzi", are located inside the sinkholes whose bottom is naturally waterproofed with the residue of the dissolution of the marl limestone (De Nardi 1978) or with various materials used by humans to create water reserves essential for watering livestock and wild animals. The ponds are often of temporary character with a variable water level (they can completely dry up during summer); due to the wild and domestic animals watering activity, ponds usually present a high content of nutrients. The pond structure is mostly characterized by the water mirror in the center, surrounded by the riparian vegetation and a humid transitional edge, often dominated by Deschampsia cespitosa. If ponds dry up, they are incorporated into the surrounding grasslands and can happen that the evolution let them to become peat bogs. Sometimes, peat bogs become inactive bogs due to natural or anthropogenic changes.
2.2 Sampling design and data collection
In total, 21 ponds have been selected in the Cansiglio Forest for fieldwork activities (ML4, ML5, AF5, AF6, AF7, AF11, AF12, AF18, G5, RV1, RV2, AC2, AL2, VA4, LF14, LP, CC, LCM, GB, LM, BM, Fig. 1).
Samplings on Odonata were conducted monthly starting from the beginning of May 2013 and ending in August 2015. At each sampling site, adults were searched along a predetermined transect of 30–40 m in length (along the ponds’ banks) and 5 m of width situated at the ponds’ banks; adults were searched from 10 am to 6 pm during sunshine days when temperatures were higher than 20 °C and with low wind speed (Buchwald 1994). Individuals were caught with an entomological net, identified following Dijkstra and Lewington (2006), photographed and then released. No individuals were collected. We followed the systematics according to Dijkstra and Schröter (2020).
2.3 Statistical analysis
In 5 out of the 21 explored ponds, we did not record any Odonata species. These ponds were thus excluded from the following analyses. Aiming at describing diversity patterns in our data, individual based rarefaction curves have been calculated: this method can be easily used to describe the efficiency of the sampling effort as well as the completeness of the species inventories (Bacaro et al. 2012, 2016).
To evaluate Odonata species diversity, the H’ Shannon–Wiener information index (Pielou 1975) was calculated as
where Pi is the relative abundance of the ith species (for i from 1 to S where S is the total number of species).
To examine the relationship between S (species richness), H (information – the Shannon–Wiener diversity index) and E (evenness as measured using the Shannon–Wiener evenness index, otherwise known as Pielou J), we used the SHE analysis. This method explores the contribution of species number and equitability to changes in diversity. SHE analysis can be easily used to determine how these parameters change by increasing the sampling effort (Seaby and Henderson 2007).
To test the effect of animal disturbance (measured by the presence/absence of grazing activities close to the studied ponds) on Odonata species richness and to describe variation in species composition based on the frequency of grazing, the following analyses have been performed:
- a one-way analysis of variance (ANOVA) was performed considering species richness as the response variable and disturbance as the predictor (a factor with three levels: G = grazed all the year; Ga = grazed after mowing; UN = ungrazed).
- second, principal component analysis (PCA) was used to describe patterns in species composition; afterwards, the level of disturbance was overlaid on the PCA ordination biplot. Before running the PCA, the Hellinger transformation was applied to species abundance data (Legendre and Legendre 2012; Ricotta 2021).
Rarefaction analyses were performed using the Rarefy package (Thouverai et al. 2021), for the ANOVA the function “aov” in the R base package (R Core Team, 2021) was used while for the PCA, the FactoMineR package (Le et al. 2008) was used. For the SHE analysis, the program PAST was used (Palaeontological Statistics; Hammer 2012).
3 Results
In total, 1851 individuals belonging to 21 different species (Zygoptera: 7 spp., Anisoptera: 14 spp.) (Table 4 in Appendix) were recorded in 16 out of the 21 selected sampling sites (Table 5 in Appendix). Five sites (AF12, AF18, AL2, LF14, LM) dried up at the beginning of the sampling activity, and for this reason no individuals were recorded.
Sampled species represents the 22.1% of the whole Italian Odonata fauna, that counts 95 species in total (Dijkstra and Schröter 2020), and 14.6% of the 144 species recorded in Europe (Boudot and Kalkman 2015; Lopez et al. 2020).
Individual-based rarefaction curves approached the asymptote for almost all the sampled sites (Fig. 2), suggesting that the sampling effort was adequate to collect the whole species diversity at the pond level.
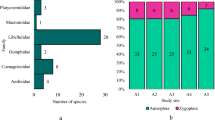
The updated checklist of the Odonata for the “Cansiglio Forest” is thus presented in Table 1. Sympetrum was the most abundant genus (19%) represented by 4 species, followed by Aeshna (14%) represented by 3 species, Anax (9%) and Libellula (9%) both represented by 2 species, as shown in Table 6 in Appendix. The two rarest species in this study area were Ceriagrion tenellum and Somatochlora flavomaculata, recorded only once in two different sites. The most common species was Coenagrion puella, with 1167 individuals recorded in 16 out of the 21 sampling sites.
Interestingly, we detected a notable difference in species diversity among the 16 ponds: the highest number of species (12) was found in GB, the lowest (1) in ML5 and LCM. The highest number of individuals (393) was found in ML4, whereas only 1 individual was detected in LCM. For the set of surveyed ponds, the Shannon–Weaver diversity index (Hʹ) ranged between 0 (LCM, ML5) and 1.86 (GB), with a mean value of 0.96. The corresponding evenness index (Heven) ranged between 0.24 and 1, with a mean value of 0.54 (Table 2).
Using the SHE analysis, it was possible to determine how species richness (S) and the information index (H) tend to increase by increasing the sampling effort, while the contrary was observed for the evenness (E) (Fig. 3).
Notably, no statistically significant differences in species richness was observed among ponds with different disturbance levels and grazing intensity (Fig. 4, ANOVA F(2,13) = 0.291, p value = 0.752).
Conversely, via principal component analysis (PCA), two main groups of ponds (Fig. 5) were separated along the first and second axis: along the first axis fell ponds characterized by the lowest level of animal disturbance (UN) while Grazed ponds were mainly ordered along the second axis. In detail, it is possible to observe the clear separation among (1) seven ponds (ML4, ML5, RV1, RV2, VA4, LM, LP) that are grazed during all the season, characterized by pond banks often completely damaged; (2) four ponds (AF5, AF7, AF11, CC) where grazing activity started every year after grass mowing in August; (3) five ponds not grazed at all (G5, LCM, AF6, GB, BM) (Table 3). Moreover, the number of species increased from (1) to (3) following the less disturbance gradient in both suborders. Furthermore, it is possible to observe that C. aenea, E. lindenii, S. fusca and S. sanguineum were strictly connected with ungrazed ponds, whereas L. depressa and C. puella were more concentrated in the disturbed habitats.
Principal component analysis (PCA) on Hellinger transformed species abundance data. Ponds have been classified according to their disturbance level where G grazed, UN ungrazed, GA grazed after mowing (in August). Species abbreviations are described in Appendix Table 4
4 Discussion
The Cansiglio Forest is situated in Veneto Region, where an overall of 65 species of Odonata are reported (Pizzo 2009). However, only few studies were ever conducted in this Region to assess the diversity of Odonata. The most recent ones are: (1) 21 species (8 Zygoptera, 13 Anisoptera) detected in two sites belonging to the province of Treviso from 2005 to 2007; (2) 46 species detected in the Province of Belluno in 2009; (3) the “Atlas of the dragonflies of the eastern Veneto plain” published by Zanetti (2015) where 43 species are reported. More in detail, only one study regarding Odonate’s species (Bucciarelli 1978) was ever conduct in Cansiglio Forest, resulting in 24 species detected; although 22 sampling sites were investigated, it was impossible to understand their location since the names were not traceable and geographic coordinates were not available.
In our study, we found a relatively high number of species (21) in a small area (about 2 hectares), with most of the species (71%) that are widespread in whole Italy. Surprisingly, respect to the general results obtained by Bucciarelli (1978), Libellulidae remained the most common family detected, Sympetrum was confirmed the most common genus and Coenagrion puella the most common species. The predominant presence of the family Libellulidae (represented by 7 species) could be the result of many widespread generalists living in the mosaic of forest and anthropized areas (cattle livestock), where fast and agile flying dragonflies predominant, as already found by Renner et al. (2015).
In detail, we confirmed the following species: Lestes barbarus, Sympecma fusca, Enallagma cyathigerum, Coenagrion scitulum, C. puella, Aeshna cyanea, Aeshna affinis, Anax imperator, Libellula depressa, Libellula quadrimaculata, Crocothemys erythraea, Sympetrum striolatum, Sympetrum meridionale, Sympetrum fonscolombei, Sympetrum sangiuneum. On the contrary, we did not confirm the following species: Ischnura elegans, Aeshna mixta, Othetrum cancellatum, Orthetrum brunneum, Sympetrum vulgatum, Sympetrum flaveolum, Leucorrhinia pectoralis. Considering the last two species, their present lack in the study can be justified with the major threats connected with their ecology. It is known that S. flaveolum populations are in decline due to intensive grazing activity that damage pond banks especially during the reproductive period, and for grazing animal dejections that contribute to the phenomena of water eutrophication (Riservato et al. 2014); for this reason, the species is considered as Vulnerable in the Italian Red List (Riservato et al. 2014). The same decline is evident for L. pectoralis, a species that suffers from large-scale conversion of fenlands and peat systems for agricultural use and from eutrophication (Kalkman 2014). In the study area, we detected both grazing and eutrophication, that cause damaging on ponds and natural habitats.
On the other side, we implemented the species list with 6 species, Anax parthenope, Ceriagrion tenellum, Erythromma lindenii, Ischnura pumilio, and the family Cordulidae, with Cordulia aenea and Somatochlora flavomaculata. All these “new species” were found in a maximum of three sites respectively, mostly in common among the species. More, it must be underlined that these sites were characterized by none or at least slightly cattle grazing. A. parthenope is a common species in the Mediterranean countries and local in central Europe, with a range that is expanding since the 1990s (Askew 2004; Dijkstra and Lewington 2006). In our study area the species was found in the site CC, one of the less disturbed and grazed ponds among the area, at 1192 m a.s.l. In the same site, we also found I. pumilio and C. tenellum. The latter species is widespread mostly in southwest Europe, and it is typical of stagnant waterbodies, small streams and slowly flowing ditches, upland peat bogs, marshes, seepages (Askew 2004; Dijkstra and Lewington 2006; Ferreira and Samraoui 2010) and its main threats are effectively livestock impacts on the breeding areas, especially on vegetation, and water extraction (Ferreira and Samraoui 2010). Even if the species is considered as Least Concern both in Italy (Riservato et al. 2014) and in Europe (Kalkman et al. 2010), it is close to a Near Threatened status due to the degradation and the loss of its breeding habitats. E. lindenii is another southern Europe species that is slowly expanding it range northwards (Dijkstra and Lewington 2006), and in the study area was found only once in GB, a well-managed pond. Considering the new sampled family Cordulidae, it is important to underline that probably both C. aenea and S. flavomaculata were already present in the study area also in the past, but simply had not been spotted. C. aenea is a northern Eurasia species, with strong populations mainly restricted to lowland and in the southern Europe mostly found in mountain lakes (Askew 2004; Dijkstra and Lewington 2006). This species is considered as Nearly Threatened (NT) in Italy, especially due to the to the invasion of the alien species Procambarus clarkii, the deterioration and the eutrophication of its breeding place (mainly converted to fishing ponds) (Riservato et al. 2014). S. flavomaculata is a typical species of temperate valleys and lowlands at low altitude, often in rich, cultivated land (Askew 2004; Dijkstra and Lewington 2006) that in our study area was found at 1001 m a.s.l.
It must be noted that GB was the site with the highest species number and the highest value of Shannon–Weaver diversity index. This site is situated inside the “Giangio Lorenzoni” Alpine Botanical Garden and it is positively affected by the pond management conducted within the structure. The site with highest number of individuals is ML4 (1065 m a.s.l.), a pond characterized by a modest grazing activity that never dries up during the year. On the contrary, the sites with the smallest number of species and individuals were ML5 and LCM: the first has completely damaged pond banks because it is used as a watering pond for cattle; the second is situated in the forest, in a shaded area at 1286 m a.s.l.
Interestingly, in the site LP situated at the highest altitude (1535 m a.s.l.) we detected 9 species: A. cyanea, A. juncea, C. puella, C. erythraea, E. cyathigerum, L. depressa, L. quadrimaculata, S. fonscolombii, S. striolatum. These data confirmed the attitude of A. juncea, a typical species of medium–high altitude wetlands (Dijkstra and Lewington 2006), characterized by a Holartic distribution and confined to mountainous areas in the south of its areal distribution (Askew 2004). On the other side, the presence of S. fonscolombii and C. erythraea at such altitude is noteworthy and it confirms the moving forward behaviour of many species (Ott 2010), since the first is an Afro-asiatic species widespread largely the Mediterranean area (Askew 2004) and the second is an Afro-Mediterranean species typical of lowland areas (Askew 2004).
PCA analysis confirmed that grazing activity intensity influenced the species distribution in the study area, with a clear distinction between more tolerant species (e.g., C. puella and L. depressa) recorded in the most grazed ponds, and species strictly connected with ungrazed ponds (C. aenea, E. lindenii, S. fusca, S. sanguineum). In this context, C. puella was the only species that increased in terms of individual in the highly grazed sites, remaining the only Zygoptera species flying in those sites, and confirming its known ecological plasticity, as it can survive and breed also in small waterbodies with eutrophic character (Askew 2004). Even if A. affinis, C. tenellum, L. barbarus, S. fonscolombii, S. meridionale were observed in grazed ponds, these species were recorded only where grazing activity did not impact largely on the ponds’ banks. However, it must be noted that A. affinis, L. barbarus, S. fonscolombii were probably less correlated to the ponds’ banks condition and more to the ephemeral characteristics of the sites, that dried up partially or completely during the summer.
Overall, it is important to consider that adults can move from one pond to another and when grazed and ungrazed ponds are very close to each other, grazed ones could act as a trap for the most mobile species.
Finally, in ungrazed ponds more species were sampled for both Anisoptera and Zygoptera (Table 3), confirming that Odonate community diversities are reduced by livestock grazing (Foote and Hornung 2005; Mazzacano et al. 2014).
5 Conclusions
In conclusion, the first updated checklist of Odonata in the karst area of the Cansiglio Forest was realized, with the result of 1851 individuals belonging to 21 species detected. This result underlines the important role of wetland habitats at low and medium elevation in the Alps in preserving biodiversity, especially because these habitats are often menaced and degraded by human activities and infrastructures. Respect to previously published data, we confirmed the presence of 16 species, we added 6 species, and we observed the lack of other 6 species, probably due to different management in the area. With the aim of biodiversity conservation, our study has confirmed that grazing activity can negatively influence Odonate communities, in both Anisoptera and Zygoptera, reducing number of species and number of individuals. More, in the most grazed ponds, only generalist species were observed. These results are in line with the idea that especially in anthropized wetlands, it is important to create different habitats with different ecological niche to allow more species to breed and survive. Finally, in the light of the results obtained, it would be important to re-evaluate the intensity of grazing in the area, especially where it is too intensive and leads to complete damage ponds’ banks. It will be also important to plan a proper management of those ponds that are completely dried up on one side due to the natural succession and on the other site to their completely abandonment that let the shrubs invade the entire pond surface. These suggestions would be necessary to preserve and eventually increment the species richness of the area.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
ARPAV (2013) Le principali variabili meteorologiche del Cansiglio. Servizio meteorologico, Ufficio Validazione dati e Climatologia
Askew RR (1988) The dragonflies of Europe. Harley Books, Colchester
Askew RR (2004) The Dragonflies of Europe, revised. Harley Books, Colchester
Bacaro G, Rocchini D, Ghisla A, Marcantonio M, Neteler M, Chiarucci A (2012) The spatial domain matters: a R code for spatially constrained species rarefaction. Ecol Complex 12:63–69
Bacaro G, Altobelli A, Cameletti M, Ciccarelli D, Martellos S, Palmer MW, Ricotta C, Rocchini D, Scheiner SM, Tordoni E, Chiarucci A (2016) Incorporating spatial autocorrelation in rarefaction methods: Implications for ecologists and conservation biologists. Ecol Ind 69:233–238
Balzan M (2012) Associations of dragonflies (Odonata) to habitat variables within the Maltese Islands: A spatiotemporal approach. J Insect Sci 12: 87. Available online:insectscience.org/12.87. https://doi.org/10.1673/031.012.8701
Boudot J-P, Kalkman VJ (ed) (2015) Atlas of the European dragonflies and damselflies. KNNV Publishing, The Netherlands
Bucciarelli I (1978) Odonati della foresta demaniale del Cansiglio (Veneto) (IX contributo alla conoscenza degli Odonati). Soc Ven Sc Nat 3:19–27
Buchwald R (1994) Vegetazione e Odonatofauna negli ambienti acquatici dell’Italia Centrale. Braun-Blanquetia 11:3–77
Buffa G, Lasen C (2010) Atlante dei siti Natura 2000 del Veneto. Regione del Veneto-Direzione Pianificazione Territoriale e Parchi, Venezia, pp 394
Cancian G, Ghetti S, Semenza E (1985) Aspetti geologici dell’Altopiano del Cansiglio, Lavori Soc. Ven Sc Nat 10:79–90
Clark TE, Samways MJ (1996) Dragonflies (Odonata) as indicators of biotype quality in the Kruger National Park, South Africa. J Appl Ecol 33:1001–1012. https://doi.org/10.2307/2404681
Clausnitzer V, Kalkman VJ, Ram M, Collen B, Baillie JEM, Bedjanic M, Darwall WRT, Dijkstra KDB, Dow R, Hawking J, Karube H, Malikova E, Paulson D, Schütte K, Suhling F, Villanueva R, von Ellenrieder N, Wilson K (2009) Odonata enter the biodiversity crisis debate: the first global assessment of an insect group. Biol Cons 142:1864–1869. https://doi.org/10.1016/j.biocon.2009.03.028
Corbet P (1993) Are Odonata useful as bioindicators? Libellula 12(3/4):91–102
Dijkstra KDB, Lewington R (2006) Field Guide to the Dragonflies of Britain and Europe. Bloomsbury Publishing
De Nardi A (1978) Il Cansiglio Cavallo, lineamenti geologici e morfologici. Doretti UD
Del Favero R (2004) Biodiversità e indicatori nei tipi forestali del Veneto, Multigraf, Spinea (VE)
Ferreira S, Samraoui B (2010) Ceriagrion tenellum. The IUCN Red List of Threatened Species 2010: e.T165495A6040578. Downloaded on 10 May 2018
Foote AL, CLR (2005) Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecolog Enthomol 30(3):273–283. https://doi.org/10.1111/j.0307-6946.2005.00701.x
Garlato A, Borsato V (2016) I suoli del SIC-ZPS IT3230077 “Foresta del Cansiglio”, Lavori Soc. Ven Sc Nat 41:115–120
Hammer O (2012) PAST: Paleontological Statistics, Version 2, 16. Natural History Museum, University of Oslo, Olso, Reference manual
Hart LA, Bowker MB, Tarboton W, Downs CT (2014) Species Composition, Distribution and Habitat Types of Odonata in the Simangaliso Wetland Park, KwaZulu-Natal, South Africa and the Associated Conservation implications. PLoS ONE 9(3):e92588. https://doi.org/10.1371/journal.pone.0092588
Jacob S, Thomas AP, Manju EK (2017) Odonata (Dragonflies and Damselflies) as bio indicators of water quality. Int J Innov Res Sci Eng Technol https://doi.org/10.15680/IJIRSET.2017.0609144
Kalkman VJ (2014) Leucorrhinia pectoralis. The IUCN Red List of Threatened Species 2014:2019. https://doi.org/10.2305/IUCN.UK.2014-1.RLTS.T165486A19167032.en.Downloadedon26February
Kalkman VJ, Boudot J-P, Bernard R, Conze K-J, De Knijf G, Dyatlova E, Ferreira S, Jović M, Ott J, Riservato E, Sahlén G (2010) European Red List of Dragonflies. Publications Office of the European Union, Luxembourg
Le S, Josse J, Husson F (2008) FactoMineR: An R Package for Multivariate Analysis. J Statist Software, 25(1): 1–18. https://doi.org/10.18637/jss.v025.i01
Legendre and Legendre (2012) Numerical Ecology. Elsevier, Amsterdam
López-Estrada EK, Barona Fernández J, Cardo-Maeso N, Teruel Montejano S, Díaz-Martínez C (2020) Onychogomphus cazuma sp. nov. from Spain: Molecular and morphological evidence supports the discovery of a new European dragonfly species (Odonata: Gomphidae). Odonatologica 49:125–154. https://doi.org/10.5281/zenodo.3823337
Mazzacano C, Paulson D, Abbott J (2014) Backyard ponds: guidelines for creating and managing habitat for dragonflies and damselflies. Migratory Dragonfly Partnership. Portland, OR., pp 22
McPeek MA (2008) Ecological factors limiting the distributions and abundances of Odonata. Dragonflies & Damselflies. In: Córdoba-Aguilar A, Editor. Model organisms for ecological and evolutionary research. Oxford University Press. pp 51–62
Ott J (2010) Dragonflies and climatic changes - recent trends in Germany and Europe. In 2010. Monitoring Climatic Change With Dragonflies, ed. J Ott Biorisk 5:253–286. https://doi.org/10.3897/biorisk.5.857
Pielou EC (1975) Ecological diversity. Wiley, New York, pp. 165
Pizzo L (2009) Boll Mus civ St. nat. Venezia, 59
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Reece BA, Mcintyre NE (2009) Community assemblage patterns of odonates inhabiting a wetland complex influenced by anthropogenic disturbance. Insect Conserv Divers 2(2):73–80. https://doi.org/10.1111/j.1752-4598.2008.00044.x
Renner S, Périco E, Sahlén G, dos Santos DM, Consatti G (2015) Odonates from the Taquari River valley, RS, Brazil, Check List 11(5): 1740 https://doi.org/10.15560/11.5.1740
Ricotta C (2021) From the Euclidean distance to compositional dissimilarity: What is gained and what is lost. Acta Oecologica 111:103732. https://doi.org/10.1016/j.actao.2021.103732
Riservato E, Fabbri R, Festi A, Grieco C, Hardersen S, Landi F, Utzeri C, Rondinini C, Battistoni A, Teofili C, Utzeri C (2014) Lista Rossa IUCN delle libellule Italiane. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Roma
Sahlén G, Ekestubbe K (2001) Identification of dragonflies (Odonata) as indicators of general species richness in boreal forest lakes. Biodivers Conserv 10:673–690. https://doi.org/10.1023/A:1016681524097
Samways MJ (2008) Dragonflies as focal organisms in contemporary conservation biology. In: Córdoba-Aquilar A (ed) Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research. Oxford University Press, Oxford, pp 97–108
Samways MJ, Steytler NS (1996) Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management. Biol Cons 78:279–288. https://doi.org/10.1016/S0006-3207(96)00032-8
Seaby RMH, Henderson PA (2007) SDR-IV Help Measuring and understanding biodiversity. Lymington, Hampshire
Sharma G, Sundararaj R, Karibasvaraja LR (2007) Species diversity of Odonata in the selected provenances of Sandal in Southern India. Zoos Print J 22 (7):2765–2767. https://doi.org/10.11609/JoTT.ZPJ.1593.2765-7
Thouverai E, Pavoine S, Tordoni E, Rocchini D, Ricotta C, Chiarucci A, Bacaro G (2021) Rarefy: Rarefaction Methods. R package version 1.1. https://CRAN.R-project.org/package=Rarefy
Uboni C, Jugovic J, Tordoni E, Pizzul E, Riservato E, Bacaro G (2020) Dragonfly (Odonata) diversity patterns in mixohaline coastal wetlands. Estuaries Coasts 43:375–386
Willigalla C, Fartmann T (2012) Patterns in the diversity of dragonflies (Odonata) in cities across Central Europe. Eur J Entomol 109:235–245
Zanetti M (2015) Atlante delle libellule della Pianura Veneto Orientale. Adle, Associazione Naturalistica Sandonatese. 176
Acknowledgements
We would like to thank Reparto Carabinieri per la Biodiversità di Vittorio Veneto, Giardino Botanico Alpino del Cansiglio, Golf Club Cansiglio, Luca Dorigo, and Veneto Agricoltura. We would like to thank the two anonymous reviewers for their suggestions and comments.
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uboni, C., Borsato, V. & Bacaro, G. Odonate fauna assemblages in the “Cansiglio Forest” (Insecta: Odonata). Rend. Fis. Acc. Lincei 32, 899–910 (2021). https://doi.org/10.1007/s12210-021-01029-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-021-01029-6