Abstract
Electrocatalytic glucose oxidation reaction (GOR) has attracted much attention owing to its crucial role in biofuel cell fabrication. Herein, we load MoO3 nanoparticles on carbon nanotubes (CNTs) and use a discharge process to prepare a noble-metal-free MC-60 catalyst containing MoO3, Mo2C, and a Mo2C–MoO3 interface. In the GOR, MC-60 shows activity as high as 745 µA/(mmol/L cm2), considerably higher than those of the Pt/CNT (270 µA/(mmol/L cm2)) and Au/CNT catalysts (110 µA/(mmol/L cm2)). In the GOR, the response minimum on MC-60 is as low as 8 µmol/L, with a steady-state response time of only 3 s. Moreover, MC-60 has superior stability and anti-interference ability to impurities in the GOR. The better performance of MC-60 in the GOR is attributed to the abundant Mo sites bonding to C and O atoms at the MoO3–Mo2C interface. These Mo sites create active sites for promoting glucose adsorption and oxidation, enhancing MC-60 performance in the GOR. Thus, these results help to fabricate more efficient noble-metal-free catalysts for the fabrication of glucose-based biofuel cells.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose (C6H12O6) is one of the most common biomasses. Among various reactions related to glucose, the electrocatalytic glucose oxidation reaction (GOR) has attracted great attention [1]. The GOR plays a crucial role in constructing glucose-based biofuel cells for solving energy and environmental concerns [1, 2]. The GOR is also a key reaction in the fabrication of sophisticated devices that detect trace glucose in blood and food [2,3,4,5]. Noble metal catalysts, e.g., Au- and Pt-based catalysts, are the most widely used catalysts for the GOR because of their high catalytic activity, which is usually evaluated by the electronic current (μA) originating from detecting and converting 1 mmol/L (mM) glucose on a 1 cm2 electrode coated by catalysts. For example, the PdCuPt nanocrystal catalyst with multibranches exhibited an activity of 378 µA/(mmol/cm2) in the GOR. However, noble metal catalysts still suffer from poor response to trace glucose and low anti-interference ability to impurities [2]. Additionally, high prices and scarce resources of noble metals limit the large-scale commercialization of noble metal catalysts. Efficient noble-metal-free catalysts for the GOR are thereby highly desired.
Many noble-metal-free catalysts have been developed for the GOR, e.g., transition metal oxides, transition metal phosphates, transition metal sulfides, and carbon nanofibers [6,7,8,9,10,11,12]. Wang et al. [7] fabricated a NiCo–NiCoO2/carbon hollow cage catalyst for the GOR and obtained an improved GOR activity of 340.4 µA/(mmol/L cm2) and good long-term stability. This good performance was attributed to the high electronic conductivity, large surface area, and fast ion/electron transfer at the electrode and electrolyte–electrode interface on the NiCo-NiCoO2/carbon hollow cage catalyst [7]. Mao et al. [8] filled NiS/Ni(OH)2 nanosheets in hollow interiors of ammonium polyacrylate-functionalized polypyrrole nanotubes (NH4PA/PPyNTs) to form NiS/Ni(OH)2/NH4PA/PPyNTs catalyst. In the GOR, NiS/Ni(OH)2@NH4PA/PPyNTs exhibited improved performance, with a detection limit of only 3.1 µmol/L (the lowest glucose concentration that the catalyst can respond to) [8]. Furthermore, ionizable NH4PA and good conductive PPyNTs promoted electronic transmission as well as the transport of glucose and products, whereas NiS/Ni(OH)2 nanosheets provided more abundant catalytic active sites [8]. This characteristic was shown to be the origin of the improved GOR performance of NiS/Ni(OH)2@NH4PA/PPyNTs [8]. Peng et al. [11] reported that the activity of the carbon-layer-coated tungsten oxide nanosphere catalyst in the GOR was higher than that of the pure tungsten oxide nanosphere catalyst without carbon. Coating a carbon layer with a thickness of 15 nm on a tungsten oxide nanosphere created oxygen vacancies on the surface of tungsten oxide nanospheres, and these vacancies enhanced electron transfer between the catalyst and reactants (H2O and glucose), promoting glucose oxidation by O or/and OH groups formed from H2O dissociation [11]. This result was proposed to be the origin of the higher activity of the carbon-layer-coated tungsten oxide nanosphere catalyst in the GOR [11]. Despite extensive studies, the performance of the noble-metal-free catalyst in the GOR, including activity, response time, stability, and anti-interference ability to impurities, are still below the requirements of commercialization and need further improvements.
Herein, we coat MoO3 nanoparticles on carbon nanotubes (CNTs) to form a noble-metal-free MC-60 catalyst by a discharge process under an argon (Ar) atmosphere (Figs. 1a, S1, and S2). In the GOR, MC-60 exhibits an activity of 745 µA/(mmol/L cm2). This activity is higher than those on a CNT-supported Pt catalyst (Pt/CNT) (270 µA/(mmol/L cm2)) and a CNT-supported Au catalyst (Au/CNT) (110 µA/(mmol/L cm2)). Moreover, MC-60 has superior stability and anti-interference ability to impurities in the GOR. The improved performance of MC-60 in the GOR is due to the abundant Mo sites bonding to C and O atoms at the MoO3–Mo2C interface, which serve as active sites for glucose adsorption and oxidation.
Experimental Section
Catalyst Preparation
Figure 1a illustrates the preparation process of catalysts. Ammonium molybdate (AMT, 170 mg) was first loaded on CNT (200 mg) via wet impregnation. Second, the sample obtained in the first step was dried at 60 °C for 12 h, forming the CNT-supported AMT (AMT/CNT). Third, AMT/CNT was treated by the discharge process for 20 min, affording a catalyst MC-20. Elongating the discharge process conducted on AMT/CNT to 60 min produced the MC-60 catalyst. For comparison, we also prepared a Mo2C/CNT catalyst using the following steps. First, AMT (170 mg) and glucose (510 mg) were loaded on CNTs (200 mg) via wet impregnation. Second, the impregnated sample was dried at 60 °C for 12 h. Third, the dried sample was treated by the discharge process for 60 min, forming the Mo2C/CNT catalyst.
The set-up of the discharge process is shown in Figs. S1 and S2. In the discharge process, a 500 mg sample was first placed in the discharge chamber. Subsequently, Ar was introduced into the discharge chamber. Next, 100 V was applied to the electrodes of the discharge chamber to trigger the discharge process. The discharge process proceeds at atmospheric pressure without heating. The temperature of the discharge process is below 150 °C, lower than the temperature traditionally used for dissociating AMT (> 200 °C) [13,14,15,16]. During the discharge process, abundant electrons with energies of 5−10 eV are produced from Ar ionization and move at high speeds in the discharge chamber. Collisions of the electrons with other species can efficiently dissociate metal salts into metal oxides, reduce metal ions into metal atoms, control the transfer and aggregation of metal atoms to form metal nanoparticles with uniform sizes, and trigger various reactions among different species [13,14,15,16]. Further details regarding the discharge process have been reported in our previous work [16,17,18].
Catalyst Characterization
A PANalytical X’Pert powder X-ray diffraction meter with Cu Kα radiation was applied to observe the X-ray diffraction (XRD) patterns of catalysts. Raman spectra were obtained on equipment (Jobin–YvonLabRam HR800) with an Ar laser (514 nm). X-ray photoelectron spectroscopy (XPS) observations were conducted on an AXIS UltraDLD spectrometer. All binding energies were calibrated using the C 1s peak at 284.8 eV. Specific surface areas of catalysts were measured on a micromeritics ASAP 2020 adsorption analyzer and calculated using the Brunauer–Emmett–Teller (BET) method. Transmission electron microscopy (TEM) observations, including high-resolution TEM (HRTEM), high-angle annular dark field, and elemental mapping images, were performed on a transmission electron microscope (FEI Titan G2 80-200 Chemi STEM).
Electrochemical Measurements
A workstation (CHI760E, CH Instrument, Inc.) with a three-electrode system comprising a working electrode, counter electrode, and reference electrode was used to perform electrochemical measurements at room temperature. Loading the catalyst on a circular glassy carbon electrode with a diameter of 0.3 cm produced the working electrode. A Pt plate electrode (0.5 mm × 0.5 mm) and a saturated calomel electrode (SCE) were the counter and reference electrodes, respectively. To activate these electrodes and stabilize the reaction system, 20 cycles of cyclic voltammetry (CV) scans were performed at 100 mV/s from − 1 to 1 V (vs. SCE) before the experiments. The electrocatalytic oxygen evolution reaction (OER) from H2O was performed in a 100 mL aqueous solution containing 0.1 mol NaOH. The potential was calculated using Eq. (1):
ESCE and ERHE were obtained against the SCE and calibrated versus the reversible hydrogen electrode (RHE), respectively. Linear sweep voltammetry (LSV) polarization curves were recorded at 5 mV/ s with a 90% iR (internal resistance) compensation. The overpotential at 10 mA/cm2 (η10) was calculated by subtracting 1.23 V from the potential (ERHE) at 10 mA/cm2 on the LSV curve. To calculate electrochemical double-layer capacitance (CDL), Δj was first obtained from Eq. (2):
Here, joxidation and jreduction are current densities on the oxidation and reduction parts of the CV curves, respectively; Δj was then plotted as a function of the scan rate and linear fitting of the Δj-(scan rate) curve obtained CDL. Linear fitting on the Tafel curve deduced from the LSV curve led to the Tafel slope.
The reaction solution for the GOR was similar to that for OER (aqueous solution with 0.1 mol/L NaOH, 100 mL), except that a certain amount of glucose was added to the solution. The relationship between current density and glucose concentration was deduced according to CV scans obtained at different glucose concentrations. Before the GOR, the reaction solution was stirred at a speed of 200 r/min for 2 min to ensure fast and uniform dispersion of glucose. All tests were performed in a static solution without stirring. The current density as a function of time and the immediate response were obtained at a 500 mV potential (vs. SCE).
Results and Discussion
Catalyst Characterization
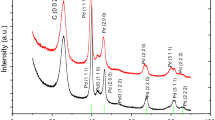
As depicted in Fig. 1b, MC-20 exhibits XRD peaks at 12.6°, 14.1°, 21.9°, 23.2°, 25.5°, 27.3°, 28.6°, 29.4°, 32.9°, 33.6°, 35.2°, 38.7°, 39.4°, 45.6°, 46.0°, 49.2°, 52.7°, 53.9°, 54.9°, 56.2°, 57.4°, 58.7°, 64.3°, 69.5°, 72.8°, 76.3°, and 78.6°, with the peak appear at 25.5° being attributed CNTs (PDF#75-1621) and other peaks being attributed MoO3 (PDF#76-1003). In the XRD pattern of MC-60 (Fig. 1b), in addition to the peaks of CNTs and MoO3, peaks at 18.4°, 34.6°, 38.1°, 42.4°, 44.7°, 48.6°, 49.7°, and 67.3° are attributed to Mo2C (PDF#75-1621). This result indicates that MC-20 is composed of CNTs and MoO3, while MC-60 contains CNTs, MoO3, and Mo2C. Abundant electrons are produced in the discharge process during catalyst preparation [13,14,15,16,17,18]. When conducting the discharge process to treat AMT/CNTs for 20 min, collisions of the electrons produced during the discharge process with AMT result in the dissociation of AMT to MoO3, producing MC-20 containing CNTs and MoO3 [13,14,15,16,17,18]. When the discharge process is increased to 60 min, in addition to the dissociation of AMT from MoO3, collisions of the electrons produced during the discharge process with Mo species and CNTs create Mo–C bonds, thus affording MC-60 that contains CNTs, MoO3, and Mo2C [13,14,15,16,17,18]. N2 adsorption–desorption experiments (Fig. 1c) reveal that surface areas of MC-20 (70.95 m2/g) and MC-60 (70.55 m2/g) are similar. The Raman spectra of MC-20 and MC-60 (Fig. 1d) are almost identical and show peaks at 1332 and 1581 cm−1, respectively, due to CNTs [16,17,18].
As per the TEM images (Figs. 2 and S3), MoO3 nanoparticles are dispersed on CNTs on MC-20 and MC-60. Lattice fringes with a distance of 0.340 nm observed on MC-20 and MC-60 are due to the (002) plane of CNTs (Fig. 2a and b) [16]. MoO3 nanoparticles on MC-20 have an average size of (12.1 ± 0.8 nm) (Fig. S3). In addition to their presence on CNTs, lattice fringes with a distance of 0.233 nm are also found on MC-20 and attributed to the (131) plane of MoO3 (Fig. 2a). The average size of the MoO3 nanoparticles on MC-60 (11.6 ± 0.7 nm) (Fig. S3) is close to that on MC-20. In addition to appearing on CNTs, lattice fringes with distances of 0.233, 0.212, and 0.201 nm are also observed on MC-60 and are caused by MoO3(131) (PDF#76-1003), Mo2C(112) (PDF#75-1621), and Mo9O26(020) (PDF#05-0441) planes, respectively (Fig. 2b). This result reveals Mo2C formation on MC-60. When treating metal oxides with the discharge process, collisions of discharge process electrons with metal oxides create oxygen vacancies on metal oxides [13,14,15,16]. Thus, oxygen vacancies are formed on MoO3 of MC-60 during the discharge process. This outcome could be the origin of Mo9O26 on MC-60. Elemental mapping patterns confirm the presence of C, Mo, and O on MC-20 and MC-60 (Fig. 2c and d).
Full-range survey XPS spectra confirm the presence of C, O, and Mo on MC-20 and MC-60 (Fig. S4). Thus, the C 1s XPS spectrum of MC-20 exhibits four peaks at 284.8, 286.9, 290.2, and 291.8 eV (Fig. 3a). The peak at 284.8 eV is due to C–C and C=C bonds in CNTs, and the peak at 286.9 eV is caused by the C–O bond formed between C atoms and OH groups on CNTs [17,18,19,20,21,22,23]. Peaks at 290.2 and 291.8 eV are due to O=C–O bonds in COOH groups on CNTs [17,18,19,20,21,22,23]. The O 1s XPS spectrum of MC-20 has two peaks at 531.1 and 532.4 eV, characteristic of O atoms in the lattice of MoO3 (Olatt) and OH or/and COOH groups on CNTs (Oads) [17,18,19,20,21,22,23,24,25]. The Mo 3d XPS spectrum of MC-20 can be fitted into two peaks at 233.2 and 236.3 eV (Fig. 3c), which are due to Mo6+ in MoO3 [22, 23]. The C 1s XPS spectrum of MC-60 (Fig. 3d) is similar to that of MC-20, indicating that a longer discharge process for catalyst preparation did not influence CNT features. Compared with those of MC-20, the O 1s XPS peaks of MC-60 (Fig. 3e) shift to lower binding energies. This shift could be due to the electronic interactions between MoO3 nanoparticles and CNTs. Mo 3d XPS peaks due to Mo6+ in MoO3 on MC-60 are located at 232.9 and 236.1 eV, lower than those on MC-20. This comparison further implies electronic interactions between MoO3 nanoparticles and CNTs on MC-60. In the Mo 3d XPS spectrum of MC-60, in addition to the peaks of Mo6+ in MoO3, a peak appears at 228.1 eV and is assigned to Mo2+ in Mo2C (Fig. 3f) [22,23,24,25]. These XPS spectra demonstrate that Mo2C is negligible on MC-20 but present on MC-60.
MC-20 is prepared by conducting a 20 min discharge process on AMT/CNT (Fig. 1a). As depicted by XPS spectra (Fig. 3a–c), the Mo-containing species on MC-20 is MoO3, and no other Mo-containing species such as AMT and Mo2C are on MC-20. Thus, the 20 min discharge process can completely dissociate AMT into MoO3 but cannot produce Mo2C. Increasing the discharge process time from 20 to 60 min leads to Mo2C formation, as reflected by XPS spectra (Fig. 3d–f). Mo2C could be produced from the reaction of Mo atoms of MoO3 with C atoms of CNTs, rather than the reaction of AMT with CNTs, as AMT was completely dissociated into MoO3 in the 20-min discharge process. To explore how the time of the discharge process affects Mo2C formation, Mo 3d XPS spectra of catalysts prepared using 20, 40, 60, 90, and 120 min discharge processes are compared (Fig. S5). By increasing the discharge process time from 20 to 120 min, the Mo 3d XPS peak of Mo2+ of Mo2C becomes stronger. According to Mo 3d XPS spectra, the ratios of Mo2C/MoO3 on catalysts prepared using 20, 40, 60, 90, and 120 min discharge processes are calculated to be 0, 0.06, 0.11, 0.15, and 0.18, respectively. Thus, Mo2C content on the catalyst increases when increasing the discharge process time for catalyst preparation from 20 to 120 min. Mo2C is formed in two ways. First, during a discharge process longer than 20 min, collisions of discharge process electrons with MoO3 nanoparticles and CNTs trigger the reaction of Mo atoms of MoO3 nanoparticles and C atoms of CNTs at the MoO3–CNT interface, producing Mo2C there. Second, during a discharge process longer than 20 min, collisions of discharge process electrons with CNTs induce a transfer of C atoms from CNTs to MoO3 nanoparticles, triggering the reaction of Mo atoms with C atoms. This produces Mo2C on MoO3 nanoparticles. Mo2C formations on MC-60, at the MoO3–CNT interface or on MoO3 nanoparticles, create the Mo2C–MoO3 interface on MC-60.
GOR Performance of Catalysts
During the GOR process, OER inevitably occurs [2,3,4,5,6,7,8,9,10, 17]. Moreover, O atoms and OH groups formed from OER are key species for glucose oxidation in the GOR. Thus, before exploring the performance of catalysts in the GOR, their performance in OER is first studied. Figure 4a illustrates CV curves for CNTs, MC-20, and MC-60 in OER. Pure CNT has no activity to OER. MC-20 and MC-60 are active to OER, with the activity of MC-60 higher than that of MC-20. According to LSV polarization curves (Fig. 4b), η10 for MC-20 and MC-60 is calculated to be 450 and 410 mV, respectively. Thus, to produce a 10 mA/cm2 current, 410 mV and 450 mV potentials are needed on MC-60 and MC-20, respectively. This result confirms that MC-60 has a higher activity to OER than MC-20. The electrochemical active surface area (ECSA) is evaluated by measuring CDL in a potential range without a Faradic process at scan rates of 20, 40, 60, 80, 100, and 120 mV/s (Fig. S6). As shown in Fig. 4c, the CDL of CNT, MC-20, and MC-60 are 2.0, 8.8, and 10.0 μF/cm2, respectively. Thus, the ECSA of CNT, MC-20, and MC-60 are 0.05, 0.22, and 0.25, respectively. The larger ECSA could be responsible for the higher OER activity of MC-60. The Tafel slopes of MC-20 and MC-60 are measured to be 210 and 168 mV/dec, respectively (Fig. 4d). The smaller Tafel slope reveals that the reaction rate is faster on MC-60 than on MC-20. These results show that MC-60 has higher activity and faster kinetics in OER than MC-20.
The reaction system for the GOR has a certain amount of added glucose, but otherwise is similar to that for OER. We first explore the performances of MC-20 and MC-60 in the GOR in an aqueous solution (0.1 mol NaOH) containing 5 mmol/L glucose, as depicted in Fig. 5a. Compared with those obtained in the absence of glucose (Fig. 4a), the CV curves of MC-20 and MC-60 with 5 mmol glucose added to the reaction solution exhibit evident peaks from 1.35 to 1.55 V, which are caused by the reduction and oxidation of glucose [5,6,7,8,9,10,11]. This comparison reveals that the GOR is triggered by MC-20 and MC-60. Compared with those of MC-20, the reduction and oxidation peaks of glucose in the CV curves of MC-60 are stronger. Thus, MC-60 has higher activity in the GOR than MC-20. We next explore the CV curves of MC-20 and MC-60 at different glucose concentrations (Cglucose) (Fig. S7). The current density (j) at the top of the glucose oxidation peak on the CV curve in Fig. S7 is plotted as a function of Cglucose in Fig. 5b. The j–Cglucose relationships are well fitted by straight lines for MC-20 (Eq. (3)) and MC-60 (Eq. (4)):
GOR performance of catalysts. a CV curves; b Current density as a function of Cglucose; c Activity of catalysts prepared using different discharge times; d Current density measured when adding different Cglucose into reaction solution at different times for MC-60; e Current density measured when continuously adding glucose (0.1 mmol/L), urea (0.1 mmol/L), glutamic acid (0.1 mmol/L), oxalic acid (0.1 mmol/L), citric acid (0.1 mmol/L), amylum (0.1 mmol/L), chitosan (0.1 g/L) and NaCl (0.1 mmol/L) into reaction solution at different times (0.5 V vs. SCE) for MC-60. f Stability of MC-20 and MC-60 in GOR
The slope of the j–Cglucose line corresponds to the activity of the catalyst in the GOR, i.e., the electronic current (μA) originating from detecting and converting 1 mmol glucose on a 1 cm2 electrode coated by the catalyst. According to the j–Cglucose lines in Fig. 5b, the activity of MC-60 in the GOR is 745 µA/(mmol/L cm2), 28.6-fold higher than that of MC-20 (26 µA/(mmol/L cm2)).
Catalysts prepared using different discharge process times exhibit different activities in the GOR. As illustrated in Fig. 5c, when increasing the discharge process time from 20 to 120 min, the GOR activity of the catalyst first increases from 26 to 745 µA/(mmol/L cm2) and then decreases to 232 µA/(mmol/L cm2), with the highest GOR activity being achieved on MC-60. We also examine the activity of the Mo2C/CNT catalyst in the GOR. The Mo 3d XPS spectrum of Mo2C/CNT (Fig. S8) shows Mo2C peaks but not MoO3 peaks, indicating that MoO3 is negligible on Mo2C/CNT. Moreover, the GOR activity of Mo2C/CNT is µA/(mmol/L cm2), which is much lower than that of MC-60. Additionally, in our previous work, we explored the performance of Pt/CNT and Au/CNT catalysts in the GOR [11, 17, 26]. The activity of MC-60 in the GOR is also higher than those of Pt/CNT (270 µA/(mmol/L cm2)) and Au/CNT (110 µA/(mmol/L cm2)). The detection limit (response minimum) reflects the sensitivity of the catalyst in the GOR and is measured by recording the amperometric response (Fig. 5d). A lower detection limit indicates that the catalyst has a higher sensitivity in the GOR. By successively adding a certain amount of glucose to the reaction solution, the current density on MC-60 shows step-like changes representing the response of MC-60 to the GOR. The step-like current density response is still evident at a Cglucose as low as 8 μmol/L (μM) and disappears when Cglucose is lower than 8 μmol/L. Thus, the detection limit of MC-60 in the GOR is 8 μmol. The steady-state response time of MC-60 at a Cglucose of 8 μmol/L is only 3 s. The detection limit on MC-20 is 200 μmol, 25-fold larger than that of MC-60 (Fig. S9). Moreover, the steady-state response time of MC-20 (10 s, Fig. S9) is much longer than that of MC-60. This comparison further demonstrates the higher efficiency of MC-60 in the GOR.
To measure the anti-interference ability of the catalyst to impurities during the GOR, we continuously add glucose (0.1 mmol), urea (0.1 mmol), glutamic acid (0.1 mmol), oxalic acid (0.1 mmol), citric acid (0.1 mmol), amylum (0.1 mmol), chitosan (0.1 g/L), and NaCl (0.1 mmol) to the reaction solution. When glucose is added to the reaction solution, MC-60 shows a fast and strong response; when other species are added, there is no response (Fig. 5e), revealing the excellent anti-interference ability of MC-60 to impurities during the GOR. The current density remains almost unchanged after proceeding with the GOR for 30 h on MC-60, but it decreases by 20% after proceeding with the GOR for 10 h on MC-20. This comparison indicates that MC-60 has a higher stability in the GOR (Fig. 5f).
Discussion
As previously mentioned, MC-60 containing MoO3 and Mo2C considerably outperforms the MoO3/CNT catalyst without Mo2C (MC-20) in the GOR. Next, we discuss the origin of this difference in performance. The morphologies, MoO3 nanoparticle sizes, and surface areas of MC-60 and MC-20 are similar and, therefore, not the origins of the difference. An evident difference between MC-60 and MC-20 is that Mo2C is negligible on MC-20 but present on MC-60. The presence of Mo2C on MC-60 at the MoO3-CNT interface or on MoO3 nanoparticles creates a Mo2C–MoO3 interface on MC-60. This interface could influence glucose adsorption and oxidation on MC-60.
To elucidate the influence of the Mo2C–MoO3 interface on glucose adsorption and oxidation, we explore the glucose adsorption and oxidation on pure MoO3, the MoO3/Mo2C composite with Mo–C bonds at the MoO3–Mo2C interface, and pure Mo2C using density functional theory (DFT) calculations. Details of the DFT calculation method and structures for modeling these systems are shown in supplementary information (Fig. S10). The adsorption energy (Ea) of glucose on a catalyst is calculated using Eq. (5):
Positive and negative Ea values represent endothermic and exothermic adsorption, respectively. Therefore, a more negative Ea indicates a more favorable adsorption of glucose on the catalyst. On pure MoO3 and pure Mo2C, the Mo site is most favorable for glucose adsorption, with Ea of − 0.12 and − 0.25 eV, respectively (Fig. S10). On the MoO3/Mo2C composite with Mo–C bonds at the MoO3–Mo2C interface, Mo site bonding to C and O atoms at this interface is most favorable for glucose adsorption, with an Ea of − 0.68 eV (Fig. S10). This result indicates that the MoO3/Mo2C composite with Mo–C bonds at the MoO3–Mo2C interface promotes glucose adsorption, compared with pure MoO3 and pure Mo2C.
Previous studies have shown that during the GOR, the product of glucose oxidation by O or/and OH groups produced from H2O dissociation is gluconate anion, and glucose oxidation to gluconate anion proceeds along with the formation of intermediates I1–I3 (Fig. 6) [17, 27,28,29,30]. Figure 6 shows the oxidation process as well as the DFT-calculated reaction energy (Er) and energy barrier (Eb) of each step on pure MoO3, the MoO3/Mo2C composite with Mo–C bonds at the MoO3–Mo2C interface, and pure Mo2C. As shown by Er and Eb in Fig. 6, glucose oxidation is more favorable on the MoO3/Mo2C composite with Mo–C bonds at the MoO3–Mo2C interface than on pure MoO3 and pure Mo2C, thermodynamically and kinetically. Thus, the MoO3/Mo2C composite with Mo–C bonds at the MoO3–Mo2C interface promotes glucose oxidation, compared with pure MoO3 and pure Mo2C.
Based on the DFT calculations, bonding of Mo with C and O at the MoO3–Mo2C interface promotes adsorption and oxidation. Mo2C formation on MC-60 at the MoO3–CNT interface or on MoO3 nanoparticles creates abundant Mo sites, wherein Mo bonds to C and O atoms to promote glucose adsorption and oxidation. This occurrence could be the origin of the better performance of MC-60 in the GOR. However, excess Mo2C on CNT-supported MoO3 nanoparticle catalysts decreases the number of MoO3, thus decreasing the amount of Mo sites bonding to C and O atoms. This occurrence could be the origin of the decreased GOR performance of catalysts that have more Mo2C including catalysts prepared using the 90 and 120 min discharge processes as well as the Mo2C/CNT catalyst (Fig. 5c). The ratio of Mo2C/MoO3 in MC-60 is 0.11. Therefore, on the CNT-supported MoO3 catalysts, this ratio should be controlled near 0.11.
Conclusion
In summary, we loaded MoO3 nanoparticles on CNTs and used a discharge process to form a noble-metal-free MC-60 catalyst containing a Mo2C-MoO3 interface. In the GOR, MC-60 shows activity as high as 745 µA/(mmol/L cm2), 28.6-fold higher than that on catalysts without a Mo2C–MoO3 interface (26 µA/(mmol/L cm2)) and higher than those on the Pt/CNT catalyst (270 µA/(mmol/L cm2)) and the Au/CNT catalyst (110 µA/(mmol/L cm2)). In the GOR, the response minimum on MC-60 is as low as 8 µmol/L, with a steady-state response time of only 3 s. Moreover, MC-60 has superior stability and anti-interference ability to impurities in the GOR. The better performance of MC-60 in the GOR is due to abundant Mo sites bonding to C and O atoms at the MoO3–Mo2C interface. The Mo sites bonding to C and O atoms at the MoO3–Mo2C interface create active sites promoting glucose adsorption and oxidation, thus enhancing the performance of MC-60 in the GOR. These results help fabricate more efficient noble-metal-free catalysts for glucose-based biofuel cells.
References
Bag S, Baksi A, Nandam SH et al (2020) Nonenzymatic glucose sensing using Ni60Nb40 nanoglass. ACS Nano 14(5):5543–5552
Fu S, Zhu C, Song J et al (2016) PdCuPt nanocrystals with multibranches for enzyme-free glucose detection. ACS Appl Mater Interfaces 8(34):22196–22200
Wang C, Nie XG, Shi Y et al (2017) Direct plasmon-accelerated electrochemical reaction on gold nanoparticles. ACS Nano 11(6):5897–5905
Rafaïdeen T, Baranton S, Coutanceau C (2019) Highly efficient and selective electrooxidation of glucose and xylose in alkaline medium at carbon supported alloyed PdAu nanocatalysts. Appl Catal B Environ 243:641–656
Chen M, Cao X, Chang K et al (2021) A novel electrochemical non-enzymatic glucose sensor based on Au nanoparticle-modified indium tin oxide electrode and boronate affinity. Electrochim Acta 368:137603
Li Q, Shao Z, Han T et al (2019) A high-efficiency electrocatalyst for oxidizing glucose: ultrathin nanosheet co-based organic framework assemblies. ACS Sustain Chem Eng 7(9):8986–8992
Wang L, Yu H, Zhang Q et al (2021) NiCo–NiCoO2/carbon hollow nanocages for non-enzyme glucose detection. Electrochim Acta 381:138259
Mao H, Cao Z, Guo X et al (2019) Ultrathin NiS/Ni(OH)2 nanosheets filled within ammonium polyacrylate-functionalized polypyrrole nanotubes as an unique nanoconfined system for nonenzymatic glucose sensors. ACS Appl Mater Interfaces 11(10):10153–10162
Wang Y, Shao Y, Matson DW et al (2010) Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4(4):1790–1798
Shu Y, Li B, Chen J et al (2018) Facile synthesis of ultrathin nickel-cobalt phosphate 2D nanosheets with enhanced electrocatalytic activity for glucose oxidation. ACS Appl Mater Interfaces 10(3):2360–2367
Peng X, Nie X, Zhang L et al (2020) Carbon-coated tungsten oxide nanospheres triggering flexible electron transfer for efficient electrocatalytic oxidation of water and glucose. ACS Appl Mater Interfaces 12(51):56943–56953
Liang T, Zou L, Guo X et al (2019) Rising mesopores to realize direct electrochemistry of glucose oxidase toward highly sensitive detection of glucose. Adv Funct Mater 29(44):1903026
Rui N, Zhang X, Zhang F et al (2021) Highly active Ni/CeO2 catalyst for CO2 methanation: preparation and characterization. Appl Catal B Environ 282:119581
Wang Z, Zhang Y, Neyts EC et al (2018) Catalyst preparation with plasmas: how does it work? ACS Catal 8(3):2093–2110
Kortshagen UR, Sankaran RM, Pereira RN et al (2016) Nonthermal plasma synthesis of nanocrystals: fundamental principles, materials, and applications. Chem Rev 116(18):11061–11127
Liu P, Niu L, Men YL et al (2023) Overturning photoreduction product of CO2 by defect- and COOH-functionalized multi-wall carbon nanotubes. Appl Catal B Environ 320:121985
Men YL, Liu P, Liu Y et al (2022) Noble-metal-free WO3-decorated carbon nanotubes with strong W–C bonds for boosting an electrocatalytic glucose oxidation reaction. Ind Eng Chem Res 61(12):4300–4309
Zhao B, Zhao Y, Liu P et al (2023) Boosting the adsorption and removal of dye from water by COOH-functionalized carbon nanotubes. Green Chem Eng 4(1):88–98
Ali S, Rehman SAU, Shah IA et al (2019) Efficient removal of zinc from water and wastewater effluents by hydroxylated and carboxylated carbon nanotube membranes: Behaviors and mechanisms of dynamic filtration. J Hazard Mater 365:64–73
Usman Farid M, Luan HY, Wang Y et al (2017) Increased adsorption of aqueous zinc species by Ar/O2 plasma-treated carbon nanotubes immobilized in hollow-fiber ultrafiltration membrane. Chem Eng J 325:239–248
Hong S, Lee DM, Park M et al (2020) Controlled synthesis of N-type single-walled carbon nanotubes with 100% of quaternary nitrogen. Carbon 167:881–887
Basiuk EV, Huerta L, Basiuk VA (2019) Noncovalent bonding of 3d metal(II) phthalocyanines with single-walled carbon nanotubes: a combined DFT and XPS study. Appl Surf Sci 470:622–630
Bensghaïer A, Lau Truong S, Seydou M et al (2017) Efficient covalent modification of multiwalled carbon nanotubes with diazotized dyes in water at room temperature. Langmuir 33(27):6677–6690
Garland BM, Fairley N, Strandwitz NC et al (2022) A study of in situ reduction of MoO3 to MoO2 by X-ray photoelectron spectroscopy. Appl Surf Sci 598:153827
Zhang F, Dong X, Cheng X et al (2019) Enhanced gas-sensing properties for trimethylamine at low temperature based on MoO3/Bi2Mo3O12 hollow microspheres. ACS Appl Mater Interfaces 11(12):11755–11762
Wang Z, Liu Y, Cheng Y et al (2022) Fast and efficient electrocatalytic oxidation of glucose triggered by Cu2O–CuO nanoparticles supported on carbon nanotubes. Front Chem 10:998812
Beden B, Largeaud F, Kokoh KB et al (1996) Fourier transform infrared reflectance spectroscopic investigation of the electrocatalytic oxidation of D-glucose: identification of reactive intermediates and reaction products. Electrochim Acta 41(5):701–709
Holade Y, Servat K, Napporn TW et al (2016) Highly selective oxidation of carbohydrates in an efficient electrochemical energy converter: cogenerating organic electrosynthesis. Chemsuschem 9(3):252–263
Lei HW, Wu B, Cha CS et al (1995) Electro-oxidation of glucose on platinum in alkaline solution and selective oxidation in the presence of additives. J Electroanal Chem 382(1–2):103–110
Ganji MD, Bakhshandeh A (2013) Electronics and structural properties of single-walled carbon nanotubes interacting with a glucose molecule: ab initio Calculations. Commun Theor Phys 60(3):341–347
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 82170426 and 22078193) and Double Thousand Plan of Jiangxi Province (Nos. 461654, jxsq2019102052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Men, YL., Dou, N., Zhao, Y. et al. Boosted Electrocatalytic Glucose Oxidation Reaction on Noble-Metal-Free MoO3-Decorated Carbon Nanotubes. Trans. Tianjin Univ. 30, 63–73 (2024). https://doi.org/10.1007/s12209-023-00378-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-023-00378-7