Abstract
The interest in CO2 conversion to value-added chemicals and fuels has increased in recent years as part of strategic efforts to mitigate and use the excessive CO2 concentration in the atmosphere. Much attention has been given to developing two-dimensional catalytic materials with high-efficiency CO2 adsorption capability and conversion yield. While several candidates are being investigated, MXenes stand out as one of the most promising catalysts and co-catalysts for CO2 reduction, given their excellent surface functionalities, unique layered structures, high surface areas, rich active sites, and high chemical stability. This review aims to highlight research progress and recent developments in the application of MXene-based catalysts for CO2 conversion to value-added chemicals, paying special attention to photoreduction and electroreduction. Furthermore, the underlying photocatalytic and electrocatalytic CO2 conversion mechanisms are discussed. Finally, we provide an outlook for future research in this field, including photoelectrocatalysis and photothermal CO2 reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excessive emissions of carbon dioxide (CO2) from the burning of fossil fuels have led to serious and potentially irreversible impacts on climate and the environment, such as global warming, rising sea levels, ocean acidification, and glacial ablation. As the atmospheric CO2 concentration is predicted to increase from 404 × 10–6 in 2017 to 600 × 10–6 by 2100, an urgent need has arisen to reduce the carbon footprint generated by human activities [1, 2]. To mitigate this ever-growing CO2 concentration, significant efforts have been dedicated to developing different technologies, including CO2 capture and storage, suppression of CO2 emission at the source, and direct CO2 conversion into value-added fuels and chemicals. Among these emerging technologies, the transformation of CO2 gas into more valuable fuels and chemicals, known as the CO2 reduction reaction (CO2RR), is an attractive approach to alleviating excessive CO2 emissions while generating a series of valuable C1 (CO, HCOOH, CH3OH, and CH4) and C2+ (CH3COOH, C2H4, C2H5OH, and C3H7OH) products [3,4,5]. Given their energy-efficient, low-cost, sustainable, and mild operating conditions, photocatalytic and electrocatalytic CO2RRs have emerged as the most promising methods for converting CO2 into value-added products. Photocatalytic CO2 reduction (PCR) harvests intermittent light from solar energy to drive direct chemical conversion of atmospheric CO2 into solar fuels with the aid of water [6, 7]. Although PCR can be performed under mild operating temperatures and conditions, the low efficiency due to rapid electron–hole pair recombination hinders its practical relevance [8, 9]. Moreover, the product selectivity of PCR is difficult to control, and thus, the products generally consist of two or more of the aforementioned chemicals and fuels [7, 10]. On the other hand, electrocatalytic CO2 reduction (ECR) offers higher product yields and conversion efficiency, particularly when connected to renewable electricity sources, yet it is a complicated reaction because it involves multiple elementary reactions along with the transfer of coupled proton–electron pairs to produce diverse products [11, 12]. Furthermore, the sluggish reaction kinetics due to the stable C=O bond and the competition with the hydrogen evolution reaction (HER) are fundamental challenges faced by ECR [13,14,15,16]. Therefore, exploring an efficient catalyst that can overcome these limitations is of significance to the real-world implementation of PCR and ECR technologies.
Two-dimensional (2D) transition metal carbides and nitrides, also known as MXenes, are a new family of 2D materials, having a general chemical formula of Mn+1XnTx (n = 1–4; M = early transition metal; X = C and/or N; Tx = surface-terminated group) [17, 18]. They are produced by selective etching of A layers (A represents a IIIA/IVA group element) from the respective MAX phases [19, 20]. The chemical composition of MXenes is largely diverse, where more than one M element can be in their layered structure, forming solid-solution and ordered double structures [21]. Even high entropy MXene, which contains more than five metals, can be successfully synthesized [22]. Therefore, hundreds of possible MXene compositions have been predicted to date. Since their introduction in 2011 [23], MXenes have revolutionized many research areas in the 2D material systems, including the sustainability field [24]. The excellent electronic, mechanical, chemical, and physical properties, as well as controllable surface groups and large surface area, make them promising as high-performance catalysts for energy conversion reactions [25,26,27]. Numerous works are available on the functionalization of MXene-based catalysts for photocatalytic and electrocatalytic CO2 reduction due to their highlighted properties [28, 29]. As pristine MXenes cannot generally absorb light energy, they act as co-catalysts in tandem with other photocatalysts because of their impressive electrical conductivity and abundant active sites [30, 31]. In this regard, MXenes serve as robust support and 2D platform for photocatalyst growth to promote efficient photoinduced electron–hole pair separation and transfer processes [30]. Their abundant functional groups also enhance the CO2 molecular adsorption and activation, enabling higher conversion efficiency [32]. Meanwhile, the knowledge and research development of MXenes as catalysts for electrochemical CO2 reduction is relatively inadequate because it is still a growing field [33]. Most of this work mainly focuses on computation-guided discovery to screen the most optimized composition and structure of MXenes [29, 34] as well as to obtain deeper knowledge on CO2 capture and reduction pathways and the fundamental reaction mechanisms converting CO2 into chemicals and fuels [35]. Despite the progress made in this emerging field, further theoretical and experimental studies are still needed to gain deep insight into the structure–activity relationships of MXene-based photocatalysts and electrocatalysts for CO2 reduction.
In this review, we have primarily summarized the recent advances of MXene-based photocatalysts and electrocatalysts for CO2 reduction applications. This review is divided into three major parts: (1) a mechanistic understanding of photocatalytic and electrocatalytic CO2 reduction; (2) research progress on photocatalytic CO2 conversion over MXene-based nanocomposites; and (3) research progress on MXene-based electrocatalysts for CO2 reduction. Photoelectrocatalysis (PEC) and photothermal CO2 reduction are also briefly discussed. Finally, the remaining challenges and future directions in developing MXene-based CO2 reduction catalysts are provided to spur further progress.
Fundamental Mechanisms of PCR and ECR
As CO2 reduction involves multiple electron and proton transfer processes, understanding the chemical reaction pathway is essential in developing efficient photo(electro)catalysts to select the preferred products. Photoreduction and electroreduction of CO2 can occur under ambient conditions; thus, they should compete with the traditional CO2 hydrogenation. PCR generally involves the three stages shown in Fig. 1, which will occur if the illumination energy is higher than the photocatalyst bandgap energy (Eg) [36]. First, the electron–hole pair is generated through photon absorption, wherein the electron is excited to the conduction band, and the hole circulates around the upper level in the valence band in Step (1) [37]. Step (2) involves charge transport and spatial separation toward the photocatalyst surface, which results in rapid charge carrier recombination on the photocatalyst surface due to the effect of high Coulomb force [38]. In Step (3), a redox reaction occurs between photogenerated carriers and the molecules on the photocatalyst surface. Holes will mainly participate in the oxidation process of water molecules, while the adsorbed CO2 is reduced by photoexcited electrons to produce fuels and chemicals. The charge carrier recombination in Step (2) should ideally be suppressed; otherwise, this redox process will not occur. Several governing factors affect the efficiency of PCR, such as the quantity of uptake CO2, the concentration of the absorbed CO2 on the surface of photocatalyst materials, and the activated catalytic sites [39].

Reproduced with permission from Ref. [37]. Copyright 2020, John Wiley and Sons, Inc
Schematic illustration of CO2 photoreduction.
In ECR, the CO2RR takes place at the cathode, while water oxidation occurs at the anode. The competing HER also occurs at the cathode with a similar potential [2, 40]. The thermodynamic potentials for the major CO2RR half-reactions and HER in an aqueous electrolyte under standard conditions (1.0 atm, 25 °C, and pH = 7.0) are shown in Fig. 2a. Notably, the reduction process of CO2 involves different electron transfers, ranging from two electrons for CO and formic acid (HCOOH) to 18 electrons for propanol. These differences seem problematic because the reduction potentials of these processes are adjacent to each other, which makes selectivity toward a single product low [1]. Much research has been devoted to understanding fundamental CO2 activation and reduction mechanisms in different electrocatalysts to explore the critical parameters influencing the efficiency and selectivity of ECR products. In general, the first stage of ECR is CO2 adsorption and activation, turning the linear CO2 to interact with electrons on the catalyst surface to generate the carbon dioxide radical anion (CO2·–), occurring at a negative thermodynamic potential (− 1.49 V vs. RHE) [41]. This result is mainly due to the high dissociative energy of the C=O bond (~ 750 kJ/mol), so CO2 is thermodynamically stable. Thus, the CO2RR has sluggish reaction kinetics and poor energy conversion efficiency. In the next step, hydrogenation of CO2– generates *COOH or *OCHO, followed by the formation of *CO or *HCOOH intermediates, respectively, through proton-coupled electron transfer (* represents the binding site of intermediates on the catalyst surface) [42]. The formed *CO can be further desorbed from the surface or undergo a reduction reaction to produce C1 products (hydrocarbons, alcohols, and acids) [43]. Schouten et al. [3] showed the different pathways of *CO dimerization to generate *OC–CO before the hydrogenation process starts. This step will lead to C2+ product formation, where the adsorbed *CO species dimerize before the reduction process to become C2H4 or undergo a reduction reaction to form C1 products, followed by dimerization and a reduction process to create C2+ products [44]. For instance, ethanol production from CO2 involves 12-electron transfer and goes through the following reactions: CO2 → *COOH → *CO → *COCO → *COCOH → *COCHOH → *COCH2OH → *CHOCH2OH → *CH2OCH2OH → CH3CH2OH [45]. The CO2 reaction pathway differs between electrocatalysts; for example, transition metals such as Cu form hydrocarbons, and oxygenates Sn, Pb, and Bi form HCOOH as the primary product, while Ag, Au, and Zn mainly form CO [13, 46]. CO2 reduction pathways also depend on electrolytes, the applied potential, pH, and so on [47]. The CO2 reduction pathways in different metal catalysts and reaction media are shown in Fig. 2b. In this context, the selection of electrocatalysts and suitable conditions for CO2 reduction is crucial for regulating the formation of key intermediates and subsequently producing the targeted C1 or C2+ chemicals.

Reproduced with permission from Ref. [46]. Copyright 2020, John Wiley & Sons, Inc. b Pathways for the CO2RR over metal catalyst surfaces. Reproduced with permission from Ref. [47]. Copyright 2020, Elsevier B.V
a Thermodynamic potentials for the CO2RR and HER in the aqueous electrolyte under standard conditions. The left is the thermodynamic equilibrium, and the colors highlight the overpotential for the specific products.
MXene-Based Catalysts for PCR
MXenes have various intriguing properties, such as tunable functional groups, hydrophilicity, large interlayer spacing, good chemical stability, and outstanding electrical/thermal conductivity [9, 48]. Thus, MXenes are promising noble-metal-free co-catalysts and catalyst supports for efficient PCR [49, 50]. MXenes can boost photocatalytic activity in several ways [38]; they help in charge carrier transfer, serve as robust support, restrict the photocatalyst size, favor reactant adsorption, and act as an electron or hole reservoir [51, 52]. Thus, they have been combined with several photocatalyst materials, such as CdS, Bi2WO6, perovskites, and g-C3N4, to enhance the efficiency of PCR [53].
MXenes as co-catalysts have excellent conductivity to facilitate photocatalytic activity [54] through accelerated charge separation and suppression of carrier recombination [55]. In this case, the photocatalysts absorb visible light, and photogenerated electrons are excited to the conduction band, whereas the holes remain in the valence band. Furthermore, the excited charge carriers are moved to the interface of MXenes mainly due to the higher potential of MXenes. Electrons can transfer to MXenes without recombination and react on the MXene surface to generate CO by reducing CO2. The charge transfer process from the photocatalyst to MXenes enhances the electron–hole pair separation and suppresses charge recombination in photocatalysts, thus enhancing the photoactivity [55]. The rich functional terminations of MXene surfaces also make them potential candidates for combining with other semiconductor materials having a close contact interface and hence generating charge transfer, which enables them to increase the charge carrier separation efficiency [38, 49, 51]. Moreover, the shape and dimension tunability of MXenes also provides a significant advantage; it not only makes them suitable for combining with other materials in the nanocomposite form but also enhances their overall catalytic properties [54].
MXene-Based Heterostructures
Several functional MXenes (including Ti2CO2, Zr2CO2, Hf2CO2, and Sc2CO2) are theoretically promising as co-catalysts, having suitable electronic structures, sufficient active sites, and high carrier mobilities [28]. Thus far, Ti3C2Tx MXene has largely been explored as a photocatalyst support because of its large work function and the relatively low activation energy of CO2 photoreduction [53]. Integrating Ti3C2Tx MXene in nanocomposite form offers excellent photocatalytic properties; for instance, previous results indicate that adding Ti3C2Tx MXene into g-C3N4 improves the surface area in comparison with adding other 2D nanomaterials, such as graphene [54]. Higher electrical conductivity was also found in the low addition of Ti3C2Tx MXene, compared to the addition of other 2D nanomaterials, such as MoS2, which contributed to higher catalytic activity [54]. Several reports have also shown the combination of MXenes with other photocatalyst materials, such as g-C3N4 [53], perovskite materials (Cs3Bi2Br9, FAPbBr3, CsPbBr3, and Cs2AgBiBr6) [56,57,58,59], layered double hydroxides (NiAl, Co–Co, and Co2Al0.95La0.05) [60,61,62], Bi-based photocatalysts (BiOX (X = Cl, Br, I), Bi2XO6 (X = W, Mo), and hybrid Bi2O2SiO3) [50, 63, 64], and metal oxides and metal sulfides (TiO2, CeO2, InVO4, CdS, Cd0.2Zn0.8S, and ZnIn2S4) [9, 49, 53, 65,66,67,68,69].
In particular, many reports have shown MXenes combined with 2D g-C3N4 as efficient photocatalyst materials. g-C3N4 has excellent physical and chemical stability, low cost, nontoxicity, and a suitable energy band position for photocatalyst CO2 reduction [70]. However, g-C3N4 has low CO2 adsorption and fast recombination of photoinduced electron–hole pairs, limiting its photocatalytic performance; thus, MXenes solve this problem with their high conductivity. In combining MXenes with perovskites, photocatalyst enhancement can also be obtained because MXenes have good electron transfer properties that can facilitate the charge separation and transport in perovskite [59]. In most cases, perovskite materials, such as Cs2AgBiBr6, suffer from a large exciton binding energy, which diminishes the charge separation and limits photocatalytic reactions [59]. Nevertheless, by exploiting the outstanding optical properties of perovskite, combining MXene with perovskite could induce large light absorption for the photocatalytic reaction [59]. MXenes can also improve the photocatalytic activity of metal oxides, such as CeO2, wherein the noble-metal-free co-catalyst of MXenes could couple with CeO2 to generate a built-in electric field and induce a Schottky junction, as illustrated in Fig. 3 [49]. This strategy is effective in accelerating electron transfer to separate photogenerated carriers. This strategy may also apply to TiO2 because TiO2 has low light usage ability and rapid electron–hole recombination. Thus, MXenes loaded in TiO2 as co-catalyst could boost the photocatalyst reaction [9]. In the case of Bi-based compounds as photocatalysts, a co-catalyst is also important in increasing reactivity. The co-catalyst of MXenes could significantly enhance the PCR performance of the [Bi2O2]2+ layer containing the Bi2O2SiO3 photocatalyst [50]. Moreover, the distinctive 2D structure of MXenes supports the strong construction of 2D–2D MXene/Bi-based semiconductor-layered heterojunctions having a strong interface contact [50]. Similar to previous cases, although LDH is a typical 2D-layered photocatalyst, it suffers from poor charge mobility, aggregated layers, and rapid charge recombination, leading to low photocatalytic activity [60]. Thus, because of great conductivity and an interfacial Schottky junction, LDH as an n-type semiconductor could be combined with MXenes, leading to enhancement of photocatalyst activity [60].

Reproduced with permission from Ref. [49]. Copyright 2019, Elsevier
Enhancement mechanism of the photocatalytic CO2 reduction activity of CeO2/Ti3C2-MXene with a built-in electric field-induced Schottky junction.
MXene-Based Z- and S-Scheme Photocatalysts
Modification of the shape and morphology of MXenes and tandem materials could lead to more efficient photocatalysts by maximizing surface areas as well as the number of adsorption sites [51, 71]. MXene-based nanocomposite photocatalysts can be modified into core–shell heterostructures, having an intimate interaction between MXenes and the semiconductor materials [60]. A tandem semiconductor material, such as Co–Co LDH nanosheets, can also be assembled vertically on layered MXenes to accelerate the separation of photogenerated charge carriers [61]. As MXenes can be modified easily into 0D, 1D, and 3D forms instead of their original 2D form [20, 25], the design of MXene-based nanocomposites for photocatalyst applications relates to not only a conventional layered combination but also various forms and designs. For instance, 2D/2D/0D TiO2/C3N4/Ti3C2 MXene nanocomposites with an S-scheme photocatalyst have excellent CO2 reduction activity [53]. In this case, 0D Ti3C2, as well as the trapped electrons from g-C3N4, could induce photogenerated carrier separation [53]. Conversely, most semiconductor photocatalysts that can be formed in various low-dimensional forms, such as perovskite formamidinium lead bromide (FAPbBr3) quantum dots, can also be combined with 2D layered MXenes to form a hybrid 0D/2D architecture [57]. Some rare design of hydrangea-like morphology of InVO4/Ti3C2Tx has also been reported, exhibiting excellent photocatalyst activity due to enhanced specific surface areas and a multiple photon scattering cross section [67]. The heterostructure scheme is also important in determining the efficiency of MXene nanocomposite-based photocatalysts. For instance, Z-scheme heterojunctions can avoid charge carrier recombination, wherein the co-catalyst Ti3C2 can serve as an electron sink to achieve a quick shift of photoinduced electrons and supply many active sites for photocatalytic reactions [63]. In contrast, based on semiconductors with narrow bandgaps, constructing S-scheme heterojunctions can extend the light absorption range and achieve the requirements of a higher conduction band and lower valence band levels [38]. Here the S-scheme heterojunction not only helps in charge transfer but also has a strong redox potential, which is important in photocatalyst activity. The Z- and S-scheme CO2 photoreduction mechanisms of MXene-semiconductor nanocomposites are illustrated in Fig. 4.
MXene-Based Ternary Nanocomposites
In addition, MXene-based nanocomposite photocatalysts can also involve more than two material combinations, for instance, TiO2/C3N4/Ti3C2 [53], meso-TiO2@ZnIn2S4/Ti3C2 [69], TiO2/g-C3N4/Ti3C2 [72], CdS/Ti3C2/g-C3N4 [73], g-C3N4/Bt/Ti3C2 [74], and BiOIO3/g-C3N4/Ti3C2 [63]. In these nanocomposites, each material makes a contribution, such as TiO2 as photocatalyst [69], ZnIn2S4 as co-catalyst [69], C3N4 as a provider of trapped electrons [53], BiOIO3[63], and Bt as a mediator in Schottky junctions to provide new electron transfer channels [74]. In the case of triple combination material nanocomposites, MXenes could serve as excellent additional support. For example, although TiO2/C3N4 composites show enhanced photocatalytic activity compared to their pristine form (TiO2 and C3N4 alone), defect formation is a major problem because of limited contact between TiO2 and C3N4, which can degenerate the charge carrier migration, and bulky or thick C3N4 having light shielding and a long charge transfer distance, lowering the efficiency of photocatalyst activity [53]. This situation requires a sufficient and unified contact as well as a distinct morphological characteristic supporting the photocatalyst reaction mechanisms [53]. Inhibiting the recombination of photoexcited electrons and holes driven by a strong Coulombic interaction is also a challenge to be solved, where MXenes could be the answer [53]. Moreover, doping has also been explored to obtain high-efficiency MXene-based photocatalysts. The doping could be incorporated in semiconductor sites as well as in MXenes; for example, boron doping in g-C3N4 sites could increase visible light absorption and achieve a lifted valence band for excellent photocatalytic activity in g-C3N4/MXene-based nanocomposites [75]. Gold atoms could also be used as doping for the recovery of oxygen vacancies toward photocatalytic aerobic oxidation in MXene-based photocatalysts [76]. In addition, Qu et al. [77] found that, in general, the important key to boosting the efficiency of CO2 conversion using Ti3C2Tx MXenes is introducing N-doping and metal vacancies to significantly reduce the energy barrier for the intermediates (e.g., *COOH, and *CO). We have summarized recent experimental works on photocatalytic CO2 reduction over MXene-based catalysts and co-catalysts in Table 1.
MXene-Based Catalysts for ECR
Similar to general catalytic reactions, in electrocatalytic reactions, electrocatalysts provide favorable reaction sites that ease the substrate adsorption process and promote alternative reaction pathways, thereby increasing the reaction rate and minimizing energy use, i.e., lowering the overpotential. While a large surface area and an extensive metal-terminated surface make MXenes promising materials for CO2 adsorption [82], their inherent metallic character is considered beneficial for electrochemical reaction processes; therefore, numerous studies using experimental and theoretical methods have explored applying MXenes for the ECR. Theoretical methods have considerable power for simulating various catalytic reaction processes for predicting plausible reaction mechanisms of electrochemical CO2 reduction.
Theory-Guided CO2 Reduction Mechanism
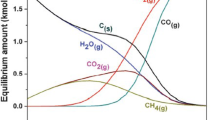
Li et al. [34] initiated the first computational study of MXenes as electrocatalysts for the ECR by investigating the CO2 capturing mechanism and its electrocatalytic reduction process on M3C2-type MXenes. The CO2 molecule is likely to be chemisorbed on the terminated metal atom surface for all metal elements due to spontaneous binding energies (∆G < 0), as shown in Fig. 5a, instead of forming a physisorption interaction. This attribute indicates that M3C2-type MXenes are generally strong CO2 capture materials, despite CO2 capture being thermodynamically unfavorable. The capture of CO2 on MXenes originates from the electron donation of carbides to terminated metals, which increases the metal electronegative charge to interact with the carbon of CO2. The strength of the CO2 chemisorption mode is likely affected by the oxygen affinity of the metal and the distance between each terminated metal. For instance, CO2 chemisorption on Mo3C2 is less spontaneous compared to Cr3C2 because of the lower oxygen affinity of Mo and the longer metal–metal distance on the Mo3C2 terminal layer, which do not permit the O–Mo interaction.

Reproduced with permission from Ref. [34]. Copyright 2017, American Chemical Society
a Proposed path for the interaction of CO2 with M3C2 MXene surfaces. b Side view of the minimum energy path followed for the CO2 conversion mechanism into *CH4 and **H2O catalyzed by Mo3C2.
After the CO2 capturing process, CO2 activation on the MXenes is crucial for allowing the electrocatalytic reduction reaction to proceed. In representative Mo3C2 MXene, the strong interaction between CO2 and Mo3C2 changes the angular structure of CO2 molecules, spontaneously activating the CO2 molecules. Although the first hydrogenation of activated CO2 may occur on the C atom and one of the terminal O atoms of CO2, the formation of *OCHO is more thermodynamically preferred than that of HOCO*, which means that further hydrogenation to the C atom will occur, and CO and HCOOH formation will be minimal. This pathway is different from other classical electrocatalyst materials, such as Cu-based catalysts, where the first hydrogenation mostly occurs on the oxygen of CO2. The first hydrogenation of the C atom causes the breaking of C–M interactions while chemisorption of *OCHO intermediates is formed through the two O atoms of CO2 with the M surface termination of MXenes. In ECR, the first hydrogenation step usually requires energy input to surmount the barrier activation energy or the net nonspontaneous reaction step.
The preference for the *OCHO intermediate as the first hydrogenation intermediate product directs the further hydrogenation to form the OCH2O* intermediate, which is then followed by the spontaneous release of H2O after the fourth hydrogenation step on the consecutive hydrogenation of the O-terminal of the OCH2O* intermediate. The formation of CH3O· as a fifth-reduced radical species also proceeds spontaneously over most of the catalysts examined and is thermodynamically preferred to CH2OH•· radical formation. Although the next hydrogenation step of the CH3O• radical requires the release of its radical for the access of the H+/e− pair and is a path that faces a large energy barrier, the reactive nature of the transition metal carbides provides an alternative path involving the sixth H+/e− pair gain on the CH3O· radical taking place on the CH3 moiety, leading to the release of CH4 and an O atom inserted on the material. In the final step, the highly reactive, chemisorbed O atom on the metal surface undergoes the highly spontaneous hydrogenation reaction, yielding a chemisorbed OH• radical species and releasing the largest amount of energy of all the elementary reactions. Moreover, in contrast to the other electrocatalysts, where the first hydrogenation step is rate-limiting, the release of such chemisorbed OH· radical species as a relatively strongly chemisorbed H2O molecule on M3C2-type MXene is the rate-limiting step. The whole CO2 adsorption, activation, and reduction pathway processes on bare M3C2-type MXene are displayed in Fig. 5b.
Computational Screening of Promising MXenes
Following Li et al. [34], researchers attempted to find the best candidates among the MXene family. Unfortunately, a wide variety of MXenes require laborious experimental work to investigate the suitable MXene-based materials for the electrocatalytic CO2RR, while MXene preparation itself is currently challenging [17, 24, 32, 83, 84]. Within this context, computation-guided discovery is one of the best strategies to minimize experimental work by screening suitable materials and optimizing factors affecting their catalytic performance.
Among the MXene family, M2CTx synthesis is more extensive, with some materials even having no surface group terminal [24, 33, 85]. The reaction mechanisms of M2C-type and M3C2-type MXenes were generally predicted to be similar, and Mo- and Cr-based MXenes were the most promising, with relatively similar rate-limiting step energies [33]. Moreover, Mo2C MXene was a distinctly better electrocatalyst than the earlier reported bulk Mo2C [82]. Although the CO2 adsorption process was likely affected by the electron or charge transfer from MXene to adsorbate, the hydrogenation process mostly originates from the various binding strengths between CO2 molecules and individual MXenes. Too strong binding between the MXene surface and the captured CO2 likely hinders the first hydrogenation process, as its Gibbs free energy was higher for group IV (△G > 0) than that for group V (△G ≈ 0) and group VI (△G < 0) [33]. According to the reaction mechanism, the hydrogenation is mostly spontaneous on the C atom but nonspontaneous on the O atom because of the strong M–O bond.
Surface Termination Effect
In theoretical studies of ECR, an ideal bare MXene surface is the standard model despite most of the successfully synthesized MXenes having –OH, –O, and –F functional groups, depending on etchant materials [24, 86]. Consistent with this model, the study on the effect of surface terminals in MXenes is of great interest, where the effect of –O and –OH terminal species on ECR were reported for M2CTx and M3C2Tx [35, 87]. In general, while the presence of –O and –OH species on the MXenes surface does not tend to enhance ECR, some of these catalysts exhibited even better performance relative to their corresponding bare M2C and M3C2-type MXenes, namely Mo3C2O2, Mo3C2(OH)2, W2CO2, Ti2CO2, Sc2C(OH)2 and Y2C(OH)2. The –O and –OH species facilitate the physisorption of CO2 molecules in M2CO2, M3C2O2, M2C(OH)2, and M3C2(OH)2, which directs to the various favorable reaction pathways. For the first hydrogenation step, HOCO* formation is thermodynamically preferred. The different steps begin from the second hydrogenation, wherein Mo3C2O2, Mo3C2(OH)2, Ti2CO2, and W2CO2 prefer to form HCOOH*, and Sc2C(OH)2 and Y2C(OH)2 generate CO* with the release of H2O instead. The third hydrogenation step of the –O surface also differs from that of –OH species for Mo3C2O2 and Mo3C2(OH)2, in which the catalyst prefers to generate CHO* and H2COOH, respectively. The remaining hydrogenation steps show mostly spontaneous processes until the release of the CH4 molecule.
Defective MXenes
Defects on MXenes should also affect the electrocatalytic activity, considering the change in localized electron density on M-terminals [11, 88]. A metal vacancy (VM) or X-vacancy (VX) of M2XO2 affects the interaction between the surface and the fragment-type radical (·COOH, ·CHO, etc.) intermediates in which the VM strengthens and the VX weakens the interaction [88]. Hence, it brings the possibility of tuning the rate-limiting step of ECR by controlling the vacancy site. VZr–Zr2NO2, VHf–Hf2NO2, VW–W2NO2, and VN–Ta2NO2 were listed as the most promising defective M2XO2-type MXenes with very low theoretical overpotential. Substituting the inner M-layer of Mo3C2 with transition metals also enhanced the CO2 adsorption ability and selectivity [89]. Mo2TiC2, Mo2ZrC2, and Mo2HfC2 were the highest among them by having the most electron transfers from MXenes to CO2. Accordingly, compared to groups V and VI, group IV of transition metal (TM)-substituted M3C2 also exhibited remarkable activity in the electrocatalytic process as the only catalysts that spontaneously proceed with the second hydrogenation, as displayed in Fig. 6a, b. Therefore, Mo3C2 (M2(TM)C2) TM-substituted by Ti, Zr, Hf, and Cr exhibits a relatively low limiting potential of 0.350, 0.367, 0.353, and 0.369 V, respectively, compared to the pristine Mo3C2, due to strong localization of lone pair electrons that stabilize and activate the intermediates [11, 89]. These TM-substituted MXenes also exhibited high stability and excellent CH4 product selectivity over CO, HCOOH, and CH3OH. Meanwhile, the single-atom dopants (Cr, Mn, Fe, and Co) on M-terminal Mo2C caused the electron density to be concentrated at the dopant, although this phenomenon was not observed in Ni-, Ru-, or Rh-doped Mo2C because of the similar configuration with the pristine Mo2C [90]. M-doped Mo2C MXenes (M = Cr, Mn, Fe, and Co) have a high ECR activity and selectivity that promote CH4 and CH3OH generation, although CH4 is energetically unfavorable. In particular, Co-doped Mo2C and Fe-doped Mo2C exhibited the most remarkable activity, with a lower overpotential of ~ 0.5 V (the rate-limiting step energy barrier is ca. 0.35 eV) compared to the pristine Mo2C (0.9 V). Moreover, a smaller *OH binding energy of Fe-doped Mo2C could break the inherent linear scaling relationship to trigger high activity for ECR to the C2 products of C2H4 and CH3CH2OH [90].

Reproduced with permission from Ref. [89]. Copyright 2022, Elsevier
a CO2RR volcano plot of Mo3C2- and TM-substituted bimetal MXenes, in which the adsorption free energy of OCHO*, i.e., ΔG(OCHO*), acts as the descriptor of UL. b Adsorption free energy linear relation of ΔG(OCH2O*) (black line) and ΔG(HOCH2O*) (red line) versus ΔG(OCHO*), respectively.
MXene-Based Nanocomposites
The potential use of a single-atom catalyst (SAC)-incorporated MXenes for ECR is promising, particular by anchoring the single-atom TMs. On this topic, CO2 was predicted to be adsorbed on all TM SAC surfaces with a C*O*O configuration, wherein each atom in CO2 is coordinated with the SACs. For the reduction process, however, CH4 selectivity is an issue in the TM SAC-loaded MXene, as the HER could be a strong competitor for the CO2RR because of the high possibility of H* formation in the first hydrogenation process, in addition to possible protonation on a C or O atom of CO2. Baskaran and Jung [91] predicted that Ru-Mo2CS2 has CH4 product selectivity with the lowest rate-limiting step energy barrier of 0.24 eV. All in all, the SAC-anchored MXene catalysts with nonspontaneous CO2 reduction require a large amount of energy for the CO2 adsorption and hydrogenation steps, and the product selectivity varies from CO to HCOOH because the barrier energy for CH4 production is high [91]. The complex structure of MXene hybrids becomes a major challenge in performing a DFT calculation.
The theoretical results suggest that the characteristics of bare and –O or –OH terminated MXenes seemed to make ECR preferable to the HER even in acidic conditions because proton adsorption on the MXene surfaces is thermodynamically unfavorable because of the CO2-philic surfaces [28, 34, 35, 88, 92]. Likewise, the defective MXenes, such as single-metal dopants and metal substitution in the inner layer of M3C2 MXenes, strengthen their CO2 capture abilities and enhance ECR selectivity against the HER according to energy profiles of all possible H* intermediate configurations [89, 90]. Despite the vacancies (VX and VM) offering promising enhancement in ECR, their strong effect on the fragment-type intermediate adsorption caused some of them to have higher selectivity to the HER than ECR [88]. In this case, the metal elements seemed to play an essential role in the selectivity of CO2 adsorption, in which hafnium (Hf) and tantalum (Ta) exhibited remarkable ECR selectivity among them. Interestingly, the anchoring of a single-atom catalyst on the Mo2CS2-type MXenes could generally enhance the ECR selectivity against the HER. However, most noble metals have poor ECR selectivity because of their lower CO2-philicity, while a high ECR selectivity was shown by Fe, Co, Ni, and Ru [91].
Recent Experimental Validations
Armed with valuable suggestions from the theoretical studies, numerous experimental works have been conducted very recently, as listed in Table 2. All the experimental works reported using Mo- and Ti-based MXenes, as they have been predicted to have high activity and selectivity toward ECR [33, 34, 93]. Handoko et al. [94] reported the first direct experimental demonstration of MXenes as electrocatalysts for ECR. As seen in Fig. 7a, b, CO2 reduction on Ti2CTx and Mo2CTx MXenes preferably goes through the *HCOOH pathway to produce formic acid as the main product. The F/Ti ratio in Ti2CTx had a profound effect on electrocatalytic performance, where a low F/Ti ratio exhibited low overpotential and high Faradaic efficiency (FE) [94]. A peak FE of 56% toward formic acid was achieved on Ti2CTx, while a peak partial current density of 2.5 mA/cm2 was attained on Mo2CTx. In a separate study, ECR was also observed on Mo2CTx and Ti3C2Tx MXenes that produced mainly CO [95]. The difference in the products may be due to the different ionic liquids that were used.

Reproduced with permission from Ref. [94]. Copyright 2020, Elsevier
Density functional theory calculations for the CO2RR to formic acid on a Ti2CTx and b Mo2CTx MXenes.
In addition, Cu-loaded MXenes were also tested for ECR as a dopant, single-atom catalyst, or bulk particle. For instance, Ti3C2Tx and Cu-doped Ti3C2Tx can generate HCOOH as a major product and CH3OH as a side product [96]. Introducing a tiny amount of Cu (1.04 wt%) to Ti3C2Tx is enough to enhance the electrocatalytic performance with a high reaction rate, lower potential, and higher FE. This significant enhancement in the ECR performance is ascribed to the combination of the inherent catalytic merits of Cu dopants. Meanwhile, another study reported that incorporating SAC-Cu into Ti3C2Clx enhanced its catalytic activity with a high obtained current density and FE, which is also better than using a single-atom Cu decorated carbon membrane and a single-atom Cu catalyst on graphene [97]. The experimental work in ECR was also evaluated for the ZnO-Fe/Ti3C2 nanocomposite, which exhibited good performance with a high current density (18.75 mA/cm2). Although combining ZnO and Ti3C2 should benefit the CO2 adsorption process and electron transport, Fe helps suppress the HER and direct the HCOO* intermediate formation pathway [98].
In the experimental works, the ECR selectivity over the HER remains a significant, unsolved issue. Therefore, aprotic solvents, such as ionic liquids, were used as the electrolyte for the pristine MXenes to suppress HER activity [94, 95]. Eid et al. [96] found that the ECR selectivity in an aqueous solution was enhanced by atomically doping Cu on Ti3C2Tx, suggesting that Cu can preclude the HER, although the mechanism remains undefined. Similar results were also reported by Zhao et al. [97]: the SAC-Cu/MXene has a high ECR selectivity at a voltage of − 1.4 V vs. RHE based on the FE of the products. This result suggests that appropriate chemical modification of the MXenes may bring new opportunities to enhance the ECR selectivity over the HER.
MXene-Based Catalysts for PEC and Photothermal CO2 Reduction
The PEC system can be an alternative that benefits from photocatalytic and electrocatalytic systems to generate more efficient CO2 conversion [99]. For instance, energetic photoelectrons induced by photons may help to alleviate the high overpotential of the electrochemical process. At the same time, the external voltages enable the maintenance of photogenerated electron–hole pairs through efficient carrier separation and increase the mobility of carriers in the catalyst, which results in high electron utilization [99]. A schematic representation of PEC and photothermal catalytic CO2 reduction is depicted in Fig. 8. Recently, MXene-based nanocomposite Ti3C2/g-C3N4 was reported to be an efficient catalyst for CO2 reduction in the PEC system. Ti3C2/g-C3N4, which serves as photocathodes, exhibited narrow bandgaps, rich Ti3+, and abundant pyri-N species that are critical properties for photoelectroreduction of CO2 [100]. Ti3+ benefits recombination suppression of the photogenerated electrons and holes in heterojunctions, increasing the efficiency of PEC, whereas the pyri-N species can generate adsorption of CO2 molecules. The Ti3C2/g-C3N4 catalysts are excellent for coupling CO2 reduction with water splitting to produce chemical fuels [100]. Another MXene used for PEC is TiO2/Ti3CN [99], which was also used as a photocathode in the PEC system. In TiO2/Ti3CN, the Ti3+ and oxygen vacancy play an important role by inducing the generation and transportation of photoelectron–hole pairs [99].

Reproduced with permission from Ref. [6]. Copyright 2016, Royal Society of Chemistry. b Schematic illustration of photothermal catalysis over a semiconductor with external heat input (CB, conduction band; VB, valence band; Eg, bandgap): (I) light absorption, (II) charge separation, and (III) surface reaction. c Schematic illustration of the processes resulting from the LSPR effect over plasmonic metal structures (Ef, Fermi level): (1) local enhancement of the electric field, (2) hot-electron injection, and (3) photothermal effect. Reproduced with permission from Ref. [103]. Copyright 2021, Elsevier B.V
a Photoelectrochemical cell with a photocathode as a working electrode (WE) for CO2 reduction, a counter electrode (CE) for water oxidation, and a reference electrode (RE) immersed in a CO2-containing electrolyte.
Moreover, MXenes can also be used as catalysts in a photothermal catalytic reaction to realize efficient solar energy use [101, 102]. During solar irradiation, the photothermal materials absorb photons from the sunlight, inducing photoexcitation and altering the charge carriers that subsequently generate the so-called light-induced electric field, thus eventually converting the solar energy into heat [103]. MXenes can exhibit an electromagnetic wave absorption capacity and localized surface plasmon resonance (LSPR) effect due to their excellent metallic feature, which makes them a potential material for inducing photothermal conversion [101, 102]. MXenes exhibit an internal light-to-heat conversion efficiency of nearly ∼100% [104]. Further development can be conducted by making an exfoliated MXene into a self-floating thin membrane, serving as a heat barrier to generate light-to-water evaporation with an efficiency of up to 84%, which is comparable to a state-of-art photothermal evaporation system [104]. Wu et al. [101] have recently shown the ability of Nb2C and Ti3C2 MXenes as excellent supports for Ni nanoparticles to achieve efficient photothermal CO2 catalysis. A record CO2 conversion rate of 8.50 mol/(gNi·h) was reached using Nb2C under 36-sun illumination without external heating.
Conclusions and Outlook
Photocatalytic and electrocatalytic CO2 reduction has been nurtured into a promising technology for the sustainable conversion of CO2 into value-added chemicals and fuels to cut the rising CO2 atmospheric concentrations and mitigate their environmental impact. This technology can be economically competitive with the decrease in renewable electricity prices. That being said, the intrinsic competing HER is one of many remaining challenges to the low conversion rate of CO2 in terms of quantum efficiency (QE) for photoreduction and Faradaic efficiency (FE) for electroreduction. MXenes have recently shown excellent CO2 activation and conversion to overcome the sluggish kinetics and low efficiency of CO2 reduction, although multiple challenges must first be solved to realize this technology practically.
In PCR, pristine MXenes cannot absorb solar energy because of their metallic characteristics. Although some modifications have produced MXenes with semiconductor properties, their bandgaps are too small, with inappropriate bandgap alignment to allow the CO2RR to take place. The formation of nanocomposites with other photocatalysts is a major strategy where MXenes can act as co-catalysts. Nonetheless, researchers are having difficulty enhancing the rate of PCR because the thermodynamic stability of CO2 causes the first step of CO2 activation to require high overpotential. Additionally, the achieved QE is far from the industry standard. Finding the best photocatalysts is not an easy task; the conduction band edge of the semiconductor photocatalyst should be over the standard reduction potential of CO2, and the valence band edge potential must allow the photo-oxidation process to occur.
In ECR, the essential indicator of FE should be prioritized for practical applications. However, the increase in FE and current density in multi-carbon products (C2+) is still far from the industry target. The design of an efficient electrocatalyst remains a primary challenge because the various CO2-related intermediates create numerous permutations to protonation sites, complicating C2+ production. From multiple experimental studies, the performance of MXenes-based electrocatalysts in the ECR process is affected by the binding energies of key intermediates on catalyst surfaces and the diffusion behavior of reactants near electrodes. Linear scaling relations between the binding energies of intermediates need to be circumvented to achieve significantly lower overpotential. Therefore, these factors can be prioritized in the CO2RR process to achieve high FE and valuable products.
In PCR and ECR, using single metals or defect engineering has been attempted to enhance CO2 adsorption and reduction over MXene-based catalysts. This approach faces stability challenges under reaction conditions that worsen the performance over time, warranting further research. As the product selectivity from CO2 conversion remains low, researchers need to focus on enhancing selectivity and/or product separation. Porous materials with tunable pore sizes, such as metal/covalent organic frameworks, should play an essential role in product separation and purification. Therefore, designing a catalytic reactor incorporating porous materials is an interesting topic to further advance this emerging field.
In addition to PCR and ECR, PEC and photothermal catalytic CO2 reduction can also be complementary strategies. By combining the photocatalytic and electrocatalytic systems in the PEC process, we may obtain more efficient CO2 reduction. For instance, energetic photoelectrons induced by photons from sunlight may overcome the high overpotential of the electrochemical process. Conversely, the external voltages during the electrochemical process may help suppress photogenerated electron–hole pair recombination. However, several factors need to be solved to obtain an efficient PEC system, such as the requirement of a good match of bandgap with the incident radiation solar spectrum and well-maintained redox processes at both electrodes [105]. As for the photothermal catalytic system, the ability of MXenes to efficiently absorb the near-infrared spectrum from sunlight (that is further converted into heat) is a vital feature in achieving high-efficiency CO2 reduction. MXenes are suitable for photothermal catalysis because of their excellent metallic behavior; however, not every MXene type can be used because some have relatively low conductivity. Ti3C2Tx showed good photo-to-thermal storage efficiencies up to 94.5% under sunlight irradiation because of its excellent metallic behavior, generating a tremendous LSPR effect [106]. Nb2C also exhibited excellent photothermal catalytic ability, yielding a high conversion rate. We believe that an expanding trial on the other types of MXenes and composite-based MXenes to obtain efficient photothermal catalysis will be carried out shortly.
Apart from the attempts to enhance CO2RR efficiency, other features of MXenes need to be improved. For instance, the toxicity issue of MXene synthesis should be addressed since they are generally prepared using toxic chemical reagents (such as HF-containing etchants) and complicated fabrication processes. Although MXene-based catalysts can potentially solve the environmental issues of CO2 and convert it into valuable chemicals, the waste from their synthesis process should not contribute to more problematic environmental toxicity. Further research can be directed to developing greener, safer, and more sustainable syntheses of MXenes, such as electrochemical, halogen-based, or molten salt etching. Toxic chemicals related to HF-containing etchants should be avoided, or at least carefully handled, so as not to leak into groundwater resources. Conventional fabrication of MXenes, to the best of our knowledge, still involves solid-state reactions at high temperatures to prepare MAX phases, which are precursor materials of MXenes. Alternative fabrication methods of MAX phases, such as microwave-assisted, physical synthesis methods, and sol–gel chemistry, are promising for reducing the use of toxic chemical reagents and lowering the energy requirement during the synthesis process. These technologies may have the potential to obtain MXenes that are more environmentally benign for practical applications.
References
Su JJ, Liu Y, Song Y et al (2022) Recent development of nanomaterials for carbon dioxide electroreduction. SmartMat 3(1):35–53
Wang BQ, Chen SH, Zhang ZD et al (2022) Low-dimensional material supported single-atom catalysts for electrochemical CO2 reduction. SmartMat 3(1):84–110
Schouten KJP, Kwon Y, van der Ham CJM et al (2011) A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem Sci 2(10):1902
Kuhl KP, Hatsukade T, Cave ER et al (2014) Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J Am Chem Soc 136(40):14107–14113
Kortlever R, Shen J, Schouten KJP et al (2015) Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J Phys Chem Lett 6(20):4073–4082
Chang XX, Wang T, Gong JL (2016) CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 9(7):2177–2196
Shehzad N, Tahir M, Johari K et al (2018) A critical review on TiO2 based photocatalytic CO2 reduction system strategies to improve efficiency. J CO2 Util 26:98–122
Ferreira de Brito J, Corradini PG, Silva AB et al (2021) Reduction of CO2 by photoelectrochemical process using non-oxide two-dimensional nanomaterials—a review. ChemElectroChem 8(22):4305–4320
Low J, Zhang LY, Tong T et al (2018) TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J Catal 361:255–266
Nguyen VH, Nguyen BS, Jin Z et al (2020) Towards artificial photosynthesis: sustainable hydrogen utilization for photocatalytic reduction of CO2 to high-value renewable fuels. Chem Eng J 402:126184
Xiao Y, Zhang WB (2020) High throughput screening of M3C2 MXenes for efficient CO2 reduction conversion into hydrocarbon fuels. Nanoscale 12(14):7660–7673
Ma WC, Xie SJ, Liu TT et al (2020) Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat Catal 3(6):478–487
Zhang WJ, Hu Y, Ma LB et al (2017) Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals. Adv Sci (Weinh) 5(1):1700275
Sa YJ, Lee CW, Lee SY et al (2020) Catalyst-electrolyte interface chemistry for electrochemical CO2 reduction. Chem Soc Rev 49(18):6632–6665
Li YW, Chan SH, Sun Q (2015) Heterogeneous catalytic conversion of CO2: a comprehensive theoretical review. Nanoscale 7(19):8663–8683
Seh ZW, Kibsgaard J, Dickens CF et al (2017) Combining theory and experiment in electrocatalysis insights into materials design. Science 355(6321):eaad4998
Naguib M, Mochalin VN, Barsoum MW et al (2014) 25th anniversary article: MXenes: a new family of two-dimensional materials. Adv Mater 26(7):992–1005
Wang ZJ, Wang F, Hermawan A et al (2021) A facile method for preparation of porous nitrogen-doped Ti3C2Tx MXene for highly responsive acetone detection at high temperature. Funct Mater Lett 14(8):2151043
Alhabeb M, Maleski K, Anasori B et al (2017) Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem Mater 29(18):7633–7644
Hermawan A, Amrillah T, Riapanitra A et al (2021) Prospects and challenges of MXenes as emerging sensing materials for flexible and wearable breath-based biomarker diagnosis. Adv Healthc Mater 10(20):e2100970
Anasori B, Lukatskaya MR, Gogotsi Y (2017) 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater 2:16098
Nemani SK, Zhang B, Wyatt BC et al (2021) High-entropy 2D carbide MXenes: TiVNbMoC3 and TiVCrMoC3. ACS Nano 15(8):12815–12825
Naguib M, Kurtoglu M, Presser V et al (2011) Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater 23(37):4248–4253
Gogotsi Y, Anasori B (2019) The rise of MXenes. ACS Nano 13(8):8491–8494
Amrillah T, Hermawan A, Alviani VN et al (2021) MXenes and their derivatives as nitrogen reduction reaction catalysts: recent progress and perspectives. Mater Today Energy 22:100864
Bai SS, Yang MQ, Jiang JZ et al (2021) Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. Npj 2D Mater Appl 5:78
Zubair M, Ul Hassan MM, Mehran MT et al (2022) 2D MXenes and their heterostructures for HER, OER and overall water splitting: a review. Int J Hydrog Energy 47(5):2794–2818
Chen Y, Liu C, Guo SE et al (2022) CO2 capture and conversion to value-added products promoted by MXene-based materials. Green Energy Environ 7(3):394–410
Handoko AD, Steinmann SN, Seh ZW (2019) Theory-guided materials design: two-dimensional MXenes in electro- and photocatalysis. Nanoscale Horiz 4(4):809–827
Li X, Yu JG, Jaroniec M et al (2019) Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem Rev 119(6):3962–4179
Wang ZJ, Wang F, Hermawan A et al (2022) Surface engineering of Ti3C2Tx MXene by oxygen plasma irradiation as room temperature ethanol sensor. Funct Mater Lett 15(1):2251007
Wang Y, Nian Y, Biswas AN et al (2021) Challenges and opportunities in utilizing MXenes of carbides and nitrides as electrocatalysts. Adv Energy Mater 11(3):2002967
Guo ZL, Li Y, Sa BS et al (2020) M2C-type MXenes: promising catalysts for CO2 capture and reduction. Appl Surf Sci 521:146436
Li N, Chen XZ, Ong WJ et al (2017) Understanding of electrochemical mechanisms for CO2 capture and conversion into hydrocarbon fuels in transition-metal carbides (MXenes). ACS Nano 11(11):10825–10833
Handoko AD, Khoo KH, Tan TL et al (2018) Establishing new scaling relations on two-dimensional MXenes for CO2 electroreduction. J Mater Chem A 6(44):21885–21890
Hoang VC, Bui TS, Nguyen HTD et al (2021) Solar-driven conversion of carbon dioxide over nanostructured metal-based catalysts in alternative approaches: fundamental mechanisms and recent progress. Environ Res 202:111781
Wang JJ, Lin S, Tian N et al (2021) Nanostructured metal sulfides: classification, modification strategy, and solar-driven CO2 reduction application. Adv Funct Mater 31(9):2008008
Liu XJ, Chen TQ, Xue YH et al (2022) Nanoarchitectonics of MXene/semiconductor heterojunctions toward artificial photosynthesis via photocatalytic CO2 reduction. Coord Chem Rev 459:214440
He YQ, Li CG, Chen XB et al (2020) Critical aspects of metal-organic framework-based materials for solar-driven CO2 reduction into valuable fuels. Glob Chall 5(2):2000082
Mohamed AGA, Huang YY, Xie JF et al (2020) Metal-free sites with multidimensional structure modifications for selective electrochemical CO2 reduction. Nano Today 33:100891
Yang Y, Yin LC, Gong Y et al (2018) An unusual strong visible-light absorption band in red anatase TiO2 photocatalyst induced by atomic hydrogen-occupied oxygen vacancies. Adv Mater 30(6):1704479
Wang L, Nitopi SA, Bertheussen E et al (2018) Electrochemical carbon monoxide reduction on polycrystalline copper: effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. ACS Catal 8(8):7445–7454
Vidal AB, Feria L, Evans J et al (2012) CO2 activation and methanol synthesis on novel Au/TiC and Cu/TiC catalysts. J Phys Chem Lett 3(16):2275–2280
Nguyen TN, Guo JX, Sachindran A et al (2021) Electrochemical CO2 reduction to ethanol: from mechanistic understanding to catalyst design. J Mater Chem A 9(21):12474–12494
Fan Q, Zhang ML, Jia MW et al (2018) Electrochemical CO2 reduction to C2+ species: heterogeneous electrocatalysts, reaction pathways, and optimization strategies. Mater Today Energy 10:280–301
Li MH, Wang HF, Luo W et al (2020) Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction. Adv Mater 32(34):e2001848
Zhang RZ, Wu BY, Li Q et al (2020) Design strategies and mechanism studies of CO2 electroreduction catalysts based on coordination chemistry. Coord Chem Rev 422:213436
Im JK, Sohn EJ, Kim S et al (2021) Review of MXene-based nanocomposites for photocatalysis. Chemosphere 270:129478
Shen JY, Shen J, Zhang WJ et al (2019) Built-in electric field induced CeO2/Ti3C2-MXene Schottky-junction for coupled photocatalytic tetracycline degradation and CO2 reduction. Ceram Int 45(18):24146–24153
Wang K, Wang QP, Zhang KJ et al (2022) Selective solar-driven CO2 reduction mediated by 2D/2D Bi2O2SiO3/MXene nanosheets heterojunction. J Mater Sci Technol 124:202–208
Li X, Bai Y, Shi X et al (2021) Mesoporous g-C3N4/MXene (Ti3C2Tx) heterojunction as a 2D electronic charge transfer for efficient photocatalytic CO2 reduction. Appl Surf Sci 546:149111
Hermawan A, Hasegawa T, Asakura Y et al (2021) Enhanced visible-light-induced photocatalytic NOx degradation over (Ti, C)-BiOBr/Ti3C2Tx MXene nanocomposites: role of Ti and C doping. Sep Purif Technol 270:118815
He F, Zhu BC, Cheng B et al (2020) 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl Catal B Environ 272:119006
Tahir M, Sherryna A, Mansoor R et al (2022) Titanium carbide MXene nanostructures as catalysts and cocatalysts for photocatalytic fuel production: a review. ACS Appl Nano Mater 5(1):18–54
Sun YL, Meng X, Dall’Agnese Y et al (2019) 2D MXenes as co-catalysts in photocatalysis: synthetic methods. Nanomicro Lett 11(1):79
Li QL, Song T, Zhang YP et al (2021) Boosting photocatalytic activity and stability of lead-free Cs3Bi2Br9 perovskite nanocrystals via in situ growth on monolayer 2D Ti3C2Tx MXene for C–H bond oxidation. ACS Appl Mater Interfaces 13(23):27323–27333
Que MD, Zhao Y, Yang YW et al (2021) Anchoring of formamidinium lead bromide quantum dots on Ti3C2 nanosheets for efficient photocatalytic reduction of CO2. ACS Appl Mater Interfaces 13(5):6180–6187
Zhang YY, Chen W, Zhou M et al (2021) Efficient photocatalytic CO2 reduction by the construction of Ti3C2/CsPbBr3 QD composites. ACS Appl Energy Mater 4(9):9154–9165
Zhang ZP, Wang BZ, Zhao HB et al (2022) Self-assembled lead-free double perovskite-MXene heterostructure with efficient charge separation for photocatalytic CO2 reduction. Appl Catal B Environ 312:121358
Zhao S, Pan D, Liang Q et al (2021) Ultrathin NiAl-Layered double hydroxides grown on 2D Ti3C2Tx MXene to construct core–shell heterostructures for enhanced photocatalytic CO2 Reduction. J Phys Chem C 125:10207–10218
Chen WY, Han B, Xie YL et al (2020) Ultrathin Co-Co LDHs nanosheets assembled vertically on MXene: 3D nanoarrays for boosted visible-light-driven CO2 reduction. Chem Eng J 391:123519
Ali Khan A, Tahir M (2022) Constructing S-scheme heterojunction of CoAlLa-LDH/g-C3N4 through monolayer Ti3C2-MXene to promote photocatalytic CO2 re-forming of methane to solar fuels. ACS Appl Energy Mater 5(1):784–806
Hong LF, Guo RT, Yuan Y et al (2022) 2D Ti3C2 decorated Z-scheme BiOIO3/g-C3N4 heterojunction for the enhanced photocatalytic CO2 reduction activity under visible light. Colloids Surf A Physicochem Eng Aspects 639:128358
Cao SW, Shen BJ, Tong T et al (2018) 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv Funct Mater 28(21):1800136
Tahir M, Tahir B (2021) In-situ growth of TiO2 imbedded Ti(3)C(2)TA nanosheets to construct PCN/Ti3C2TA MXenes 2D/3D heterojunction for efficient solar driven photocatalytic CO2 reduction towards CO and CH4 production. J Colloid Interface Sci 591:20–37
Song QJ, Shen BJ, Yu JG et al (2021) A 3D hierarchical Ti3C2Tx/TiO2 heterojunction for enhanced photocatalytic CO2 reduction. ChemNanoMat 7(8):910–915
Li L, Yang Y, Yang LQ et al (2021) 3D hydrangea-like InVO4/Ti3C2Tx hierarchical heterosystem collaborating with 2D/2D interface interaction for enhanced photocatalytic CO2 reduction. ChemNanoMat 7(7):815–823
Saeed A, Chen W, Shah AH et al (2021) Enhancement of photocatalytic CO2 reduction for novel Cd0.2Zn0.8S@Ti3C2 (MXenes) nanocomposites. J CO2 Util 47:101501
Wang K, Li X, Wang N et al (2021) Z-scheme core–shell meso-TiO2@ZnIn2S4/Ti3C2 MXene enhances visible light-driven CO2-to-CH4 selectivity. Ind Eng Chem Res 60:8720–8732
Yang C, Tan QY, Li Q et al (2020) 2D/2D Ti3C2 MXene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: dual effects of urea. Appl Catal B Environ 268:118738
Hu JM, Ding J, Zhong Q (2021) Ultrathin 2D Ti3C2 MXene Co-catalyst anchored on porous g-C3N4 for enhanced photocatalytic CO2 reduction under visible-light irradiation. J Colloid Interface Sci 582(Pt B):647–657
Khan AA, Tahir M, Bafaqeer A (2020) Constructing a stable 2D layered Ti3C2 MXene cocatalyst-assisted TiO2/g-C3N4/Ti3C2 heterojunction for tailoring photocatalytic bireforming of methane under visible light. Energy Fuels 34(8):9810–9828
Zhang RR, Jin JY, Jia LM et al (2022) Fabrication of CdS/Ti3C2/g-C3N4NS Z-scheme composites with enhanced visible light-driven photocatalytic activity. Environ Sci Pollut Res Int 29(11):16371–16382
Tahir M, Tahir B (2020) 2D/2D/2D O-C3N4/Bt/Ti3C2Tx heterojunction with novel MXene/clay multi-electron mediator for stimulating photo-induced CO2 reforming to CO and CH4. Chem Eng J 400:125868
Wang H, Tang Q, Wu Z (2021) Construction of few-layer Ti3C2 MXene and boron-doped g-C3N4 for enhanced photocatalytic CO2 reduction. ACS Sustain Chem Eng 45(18):24656–24663
Yu KF, Wang SM, Li Q et al (2022) Au atoms doped in Ti3C2Tx MXene: benefiting recovery of oxygen vacancies towards photocatalytic aerobic oxidation. Nano Res 15(4):2862–2869
Qu D, Peng XY, Mi YY et al (2020) Nitrogen doping and titanium vacancies synergistically promote CO2 fixation in seawater. Nanoscale 12(33):17191–17195
Tang QJ, Sun ZX, Deng S et al (2020) Decorating g-C3N4 with alkalinized Ti3C2 MXene for promoted photocatalytic CO2 reduction performance. J Colloid Interface Sci 564:406–417
Ye MH, Wang X, Liu EZ et al (2018) Boosting the photocatalytic activity of P25 for carbon dioxide reduction by using a surface-alkalinized titanium carbide MXene as cocatalyst. Chemsuschem 11(10):1606–1611
Zeng ZP, Yan YB, Chen J et al (2019) Boosting the photocatalytic ability of Cu2O nanowires for CO2 conversion by MXene quantum dots. Adv Funct Mater 29(2):1806500
Pan AZ, Ma XQ, Huang SY et al (2019) CsPbBr3 perovskite nanocrystal grown on MXene nanosheets for enhanced photoelectric detection and photocatalytic Co2 reduction. J Phys Chem Lett 10(21):6590–6597
Kim S, Zhang YJ, Bergstrom H et al (2016) Understanding the low-overpotential production of CH4 from CO2 on Mo2C catalysts. ACS Catal 6(3):2003–2013
Liu JP, Peng WC, Li Y et al (2020) 2D MXene-based materials for electrocatalysis. Trans Tianjin Univ 26(3):149–171
Papadopoulou KA, Chroneos A, Parfitt D et al (2020) A perspective on MXenes: their synthesis, properties, and recent applications. J Appl Phys 128(17):170902
Xu C, Wang LB, Liu ZB et al (2015) Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat Mater 14(11):1135–1141
Jiang XT, Kuklin AV, Baev A et al (2020) Two-dimensional MXenes: from morphological to optical, electric, and magnetic properties and applications. Phys Rep 848:1–58
Chen HT, Handoko AD, Xiao JW et al (2019) Catalytic effect on CO2 electroreduction by hydroxyl-terminated two-dimensional MXenes. ACS Appl Mater Interfaces 11(40):36571–36579
Chen HT, Handoko AD, Wang TS et al (2020) Defect-enhanced CO2 reduction catalytic performance in O-terminated MXenes. Chemsuschem 13(21):5690–5698
Li Y, Chen YP, Guo ZL et al (2022) Breaking the linear scaling relations in MXene catalysts for efficient CO2 reduction. Chem Eng J 429:132171
Zhang Y, Cao Z (2021) Tuning the activity of molybdenum carbide MXenes for CO2 electroreduction by embedding the single transition-metal atom. J Phys Chem C 125(24):13331–13342
Baskaran S, Jung J (2022) Mo2CS2-MXene supported single-atom catalysts for efficient and selective CO2 electrochemical reduction. Appl Surf Sci 592:153339
Handoko AD, Fredrickson KD, Anasori B et al (2018) Tuning the basal plane functionalization of two-dimensional metal carbides (MXenes) to control hydrogen evolution activity. ACS Appl Energy Mater 1(1):173–180
Lim KRG, Handoko AD, Nemani SK et al (2020) Rational design of two-dimensional transition metal carbide/nitride (MXene) hybrids and nanocomposites for catalytic energy storage and conversion. ACS Nano 14(9):10834–10864
Handoko AD, Chen HT, Lum Y et al (2020) Two-dimensional titanium and molybdenum carbide MXenes as electrocatalysts for CO2 reduction. iScience 23(6):101181
Attanayake NH, Banjade HR, Thenuwara AC et al (2021) Electrocatalytic CO2 reduction on earth abundant 2D Mo2C and Ti3C2 MXenes. Chem Commun 57(13):1675–1678
Eid K, Lu QQ, Abdel-Azeim S et al (2022) Highly exfoliated Ti3C2Tx MXene nanosheets atomically doped with Cu for efficient electrochemical CO2 reduction: an experimental and theoretical study. J Mater Chem A 10(4):1965–1975
Zhao Q, Zhang C, Hu RM et al (2021) Selective etching quaternary MAX phase toward single atom copper immobilized MXene (Ti3C2Clx) for efficient CO2 electroreduction to methanol. ACS Nano 15(3):4927–4936
Kannan K, Sliem MH, Abdullah AM et al (2020) Fabrication of ZnO-Fe-MXene based nanocomposites for efficient CO2 reduction. Catalysts 10(5):549
Xu YJ, Wang F, Zhao D et al (2022) Two-dimensional TiO2/MXene Ti3CN heterojunction for highly efficient photoelectrocatalytic CO2 reduction. SSRN J. https://doi.org/10.2139/ssrn.4076641
Xu YJ, Wang S, Yang J et al (2018) Highly efficient photoelectrocatalytic reduction of CO2 on the Ti3C2/g-C3N4 heterojunction with rich Ti3+ and pyri-N species. J Mater Chem A 6(31):15213–15220
Wu ZY, Li CR, Li Z et al (2021) Niobium and titanium carbides (MXenes) as superior photothermal supports for CO2 photocatalysis. ACS Nano 15(3):5696–5705
Xu DX, Li ZD, Li LS et al (2020) Insights into the photothermal conversion of 2D MXene nanomaterials: synthesis, mechanism, and applications. Adv Funct Mater 30(47):2000712
Zhang F, Li YH, Qi MY et al (2021) Photothermal catalytic CO2 reduction over nanomaterials. Chem Catal 1(2):272–297
Li RY, Zhang LB, Shi L et al (2017) MXene Ti3C2: an effective 2D light-to-heat conversion material. ACS Nano 11(4):3752–3759
Decker F, Cattarin S (2009) Photoelectrochemical cells | overview. Encycl Electrochem Power Sources. https://doi.org/10.1016/B978-044452745-5.00035-6
Fan XQ, Liu L, Jin X et al (2019) MXene Ti3C2Tx for phase change composite with superior photothermal storage capability. J Mater Chem A 7(23):14319–14327
Acknowledgements
T.A. gratefully acknowledges the internal financial support of Universitas Airlangga through Riset Mandat Muda No. 399/UN3.14/PT2020. V.P. and A.H. acknowledge the support of the National Research and Innovation Agency, Republic of Indonesia. Z.W.S. acknowledges the support of the Singapore National Research Foundation (NRF-NRFF2017-04) and the Agency for Science, Technology and Research (Central Research Fund Award).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amrillah, T., Supandi, A.R., Puspasari, V. et al. MXene-Based Photocatalysts and Electrocatalysts for CO2 Conversion to Chemicals. Trans. Tianjin Univ. 28, 307–322 (2022). https://doi.org/10.1007/s12209-022-00328-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-022-00328-9