Abstract
Electrocatalytic oxygen evolution reaction (OER) is one of the crucial reactions for converting renewable electricity into chemical fuel in the form of hydrogen. To date, there is still a challenge in designing ideal cost-effective OER catalysts with excellent activity and robust durability. The hybridization of transition metal oxides and carbonaceous materials is one of the most effective and promising strategies to develop high-performance electrocatalysts. Herein, this work synthesized hybrids of NiFe2O4 spinel materials with two-dimensional (2D) graphene oxide and one-dimensional (1D) carbon nanotubes using a facile solvothermal approach. Electrocatalytic activities of NiFe2O4 with 2D graphene oxide toward OER were realized to be superior even to the 1D carbon nanotube-based electrocatalyst in terms of overpotential to reach a current density of 10 mA/cm2 as well as Tafel slopes. The NiFe2O4 with 2D graphene oxide hybrid exhibits good stability with an overpotential of 327 mV at a current density of 10 mA/cm2 and a Tafel slope of 103 mV/dec. The high performance of NiFe2O4 with 2D graphene oxide is mainly attributed to its unique morphology, more exposed active sites, and a porous structure with a high surface area. Thus, an approach of hybridizing a metal oxide with a carbonaceous material offers an attractive platform for developing an efficient electrocatalyst for water electrochemistry applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The energy demand of the world is increasing rapidly, and fossil fuels are insufficient to fulfill the future demand. Their extensive use also leaves a harsh impact on the environment and health of other living things. Therefore, these concerns promoted extensive research worldwide on developing clean, hassle-free, and cost-effective sustainable energy technologies [1, 2]. One of the promising methods for energy conversion is the electrochemical water-splitting reaction that includes two half-reactions, namely hydrogen evolution reaction (HER) on the cathode and oxygen evolution reaction (OER) on the anode [3]. This advantageous method is owed to its high efficiency, nontoxicity, and environmental friendliness. Water splitting is an emerging technology in hydrogen production; however, it suffers from intrinsically sluggish kinetics in OER, resulting in the need for a higher potential than the thermodynamic water-splitting potential of 1.23 V to achieve oxygen evolution [4, 5]. Therefore, the design and development of an electrocatalyst with excellent performance are highly desirable. Until now, state-of-the-art electrocatalysts are mainly precious Ru- and Ir-based compounds for OER [6, 7]. Their widespread applications were greatly hindered due to their high cost and scarcity on the earth’s surface. Moreover, their stability is also a crucial drawback for practical applications. In this regard, tremendous scientific efforts have been devoted to investing in stable OER electrocatalysts that are low-cost, sufficient, earth-abundant, and can fulfill electrochemical requirements.
Recently, numerous efforts have been made to design efficient OER electrocatalysts, including doping, alloying, defect/vacancy engineering, and fabrication of hybrids, composites, or heterostructures [8,9,10,11,12]. Among them, preparing hybrid materials composed of earth-abundant transition metal oxides and carbonaceous supports is explored as one of the promising approaches for electrochemical OER applications [13, 14]. Activity enhancement is mainly attributed to the conductivity of carbonaceous materials and their synergistic effect with metallic sites. This approach also helps to overcome the poor conductivity of metal oxides. Apart from their low-cost, carbon materials are stable, biocompatible, environment-friendly, and readily available [15, 16]. Currently, one-dimensional (1D) and two-dimensional (2D) carbon allotropes attract considerable attention in numerous fields [17, 18]. Compared to bulk counterparts, these allotropes have a higher specific surface area, more active edges, tunable electronic structure, and ease of functionalization. 2D graphene, a single layer of graphite, emerges as a very promising material owing to its unusual physical, chemical, electronic, and transport properties [19]. According to the need for specific applications, rich oxygen-containing functionalities that are present in the graphene oxide (GO) are useful for covalent or non-covalent modifications [20,21,22]. Unique properties of 1D carbon nanotubes (CNTs) are mainly attributed to their seamless cylindrical morphology and the presence of highly stable sp2 hybridized carbon–carbon bonds in their outer shells [23]. According to the number of layers rolled in a structure, CNTs are divided into two categories, namely single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). SWCNTs form with a single graphene layer, whereas MWCNTs are shaped with two or more graphene layers with van der Waals forces between adjacent layers. Depending on their chirality and diameter, SWCNTs are either semiconducting, metallic, or semimetallic [24, 25]. Besides the economic benefit of avoiding the use of noble metals, an important benefit of using carbon materials such as graphene and CNTs in the OER application is their flexibility in composition. Graphene or CNTs also act as a superior support for various materials, which is also advantageous for increasing the conductivity of the material [13, 14].
Transition metal oxides are promising materials as they hold advantages like low prices, abundant resources, and environmental friendliness [26, 27]. These metal oxides exhibit interesting electrochemical and catalytic properties. Among them, earth-abundant, transition metal-based spinel-type oxides have rapidly emerged as promising and highly efficient active materials for potential applications such as supercapacitors, batteries, sensors, and catalysis [28,29,30]. Particularly, NiFe2O4 is one of the most important inverse spinel ferrites where Ni2+ occupies the octahedral site, and Fe3+ occupies the octahedral and tetrahedral sites [31]. The considerable attention toward NiFe2O4 is mainly due to its low-cost, less toxicity, high theoretical capacity, and unique magnetic structure [32, 33]. Due to the presence of multivalent Ni3+/Ni2+and Fe3+/Fe2+ redox couples, NiFe2O4 is one of the suitable catalysts for OER [34]. Recently, Choi et al. [35] showed that the NiFe2O4-x/NiMoO4 nanowire exhibited an overpotential of 326 mV at a high current density of 600 mA/cm2 for OER [35]. In an alkaline solution, the MoS2@NiFe2O4 catalyst showed an overpotential of 290 mV at 10 mA/cm2 and a Tafel slope of 69.2 mV/dec [36]. A sunflower plate-like NiFe2O4/CoNi–S nanosheet heterostructure delivered an overpotential of 219 mV for OER to produce a current density of 10 mA/cm2 [37]. The optimized NiFe2O4/rGO hybrid exhibits a current density of 10 mA/cm2 at an overpotential of only 302 mV with a small Tafel slope of 63 mV/dec [38].
It would be advantageous to utilize more optimally the properties of these low-cost, earth-abundant, and easily synthesizable materials for efficient water splitting applications. With these considerations in mind, this work synthesized NiFe2O4 material hybrids with the 2D GO and 1D CNTs and comparatively studied their OER performance. Electrocatalytic activities of NiFe2O4/SWCNT, NiFe2O4/MWCNT, and NiFe2O4/rGO hybrids were systematically investigated in alkaline conditions using a three-electrode system. This work provides insight into the synthesis mechanism involved in the preparation of the NiFe2O4 as well as OER processes in detail. NiFe2O4/rGO hybrids have shown enhanced OER activity with an overpotential of 327 mV at 10 mA/cm2 (current normalized to the geometric area of the electrode) and a Tafel slope of 58 mV/dec. The resulting graphene oxide-based electrocatalyst delivers superior OER activity compared to CNT-based electrocatalysts. The high performance of NiFe2O4 with the 2D reduced GO is mainly attributed to the unique morphology, more exposed active sites, and porous structure with a high surface area. This work aims to contribute critical insights into the rational designing of other metal oxides and graphene oxide-based electrocatalysts.
Experimental Section
Chemicals and Reagents
Pristine graphite flakes, ruthenium (IV) oxide RuO2, and Nafion were purchased from Sigma-Aldrich, India. Nickel nitrate hexahydrate Ni(NO3)2·6H2O and iron (III) nitrate nonahydrate Fe(NO3)2·9H2O were purchased from Himedia and S D Fine Chem Limited, India, respectively. Solvents such as sulfuric acid H2SO4 and nitric acid HNO3 were obtained from S D Fine Chem Limited. Solvents such as sodium nitrate NaNO3, potassium permanganate KMnO4, sulfuric acid H2SO4, and nitric acid HNO3 were obtained from S D Fine Chem Limited, India. High-quality commercial carbon nanotubes (multi-walled and single-walled, > 95%, length 1–10 µm) were purchased from Reinste Nano Ventures Private Limited, India. All dispersions and solutions were prepared in distilled water.
Preparation of the Graphene Oxide and CNTs
GO was first synthesized by the modified Hummers’ method and further used for the synthesis of NiFe2O4/rGO hybrids [39]. CNTs were functionalized by refluxing in a concentrated sulfuric acid/nitric acid mixture (3:1 v/v) for 12 h at 80 °C [40]. Then repeatedly washed with distilled water to maintain a neutral pH and dried at 60 °C for 24 h in a vacuum oven.
Preparation of the NiFe2O4/SWCNT, NiFe2O4/MWCNT, and NiFe2O4/rGO
NiFe2O4/SWCNT, NiFe2O4/MWCNT, and NiFe2O4/rGO hybrids were prepared via a facile hydrothermal approach based on previously reported pieces of literature with modifications [38, 41]. The uniform GO suspension was prepared by adding 40 mg of GO into 60 ml of distilled water, which was then ultrasonicated for 45 min. Nickel nitrate and iron nitrate with a molar ratio of 1:2 as precursors were added into the GO suspension and vigorously stirred for 1 h. The solution pH was adjusted at around 10, slowly adding NH3·H2O. The mixture was then transferred to a Teflon-lined stainless-steel autoclave and maintained at 180 °C for 24 h. After the reaction, the obtained mixture is thoroughly washed with distilled water and ethanol using the centrifugation method. The final product was achieved by a vacuum oven drying at 60 °C for 12 h. A similar methodology was used to synthesize NiFe2O4/SWCNT and NiFe2O4/MWCNT hybrids. For comparison, bare NiFe2O4 was also prepared in a similar procedure without the addition of GO and CNTs.
Characterizations
Crystal structural analyses of all synthesized samples were performed using the Rigaku Ultima IV X-ray diffractometer with Ni filter for Cu Kα radiation (wavelength, λ = 1.541 Å) at a scanning rate of 3°/min. The surface morphology analysis was carried out with the aid of a field emission scanning electron microscope (FESEM, JEOL JSM-7100F, JEOL Ltd., Singapore). Specific surface areas and the porosity of the sample were evaluated based on nitrogen adsorption isotherms via the Brunauer–Emmett–Teller (BET) and Barret-Joyner-Halenda (BJH) measurements. These data were collected with Bellsorp Max, Japan. All electrochemical measurements were performed on the CorrTest-CS350 workstation.
Electrochemical Measurements
Electrochemical measurements were conducted by a three-electrode system in a 1 M KOH electrolyte. The working electrode was prepared as follows: 2 mg of catalyst was added into a mixture of 200 μL isopropanol and 10 μL Nafion. The well ultrasonicated (30 min) slurry was then drop-casted (10 μL) onto the polished glassy carbon electrode (GCE-3 mm in diameter) and dried in a vacuum oven. In a three-electrode glass cell, Ag/AgCl is used as the reference electrode, while Pt wire is used as the counter electrode.
The OER performance of catalysts was analyzed via linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), and chronoamperometry tests. LSV polarization curves were recorded at a scan rate of 5 mV/s. The EIS were recorded in the frequency range of 0.01–100 kHz at an amplitude of 5 mV. The chronoamperometry test was performed at a constant overpotential value for 8.33 h.
Obtained potential values (vs. Ag/AgCl) were converted to the reversible hydrogen electrode (RHE) according to the equation [38]:
where the E0Ag/AgCl value is 0.197 V.
Tafel slopes were derived from the LSV curves using the following equation [38]:
where ɳ is the overpotential, b is the Tafel slope, and j is the current density.
Results and Discussion
NiFe2O4/SWCNT, NiFe2O4/MWCNT, and NiFe2O4/rGO hybrids were prepared via a facile solvothermal approach as illustrated in Fig. 1. The chemical exfoliation of GO and functionalization of the CNTs generate a large number of oxygen-containing groups on the surface. Large oxygen-containing functional groups on the surfaces of the GO or CNTs attract metallic ions (Ni2+ and Fe3+) through electrostatic interaction [42]. These metallic ions were then crystallized and finally formed NiFe2O4 materials. During the formation of NiFe2O4/rGO hybrids, GO is simultaneously reduced to the reduced rGO. The proposed mechanism for the formation of NiFe2O4 is suggested as follows [42]:
For convenience, NiFe2O4, NiFe2O4/SWCNT, NiFe2O4/MWCNT, and NiFe2O4/rGO hybrids are herein termed as NFO, NFO/SWCNT, NFO/MWCNT, and NFO/rGO hybrids, respectively.
The successful formation and phase structure of the synthesized hybrids were revealed by XRD analysis. Figure 2a shows XRD patterns of as-made GO, rGO, MWCNT, and SWCNT materials, which are in good agreement with previously reported studies [18, 38, 43]. Figure 2b reveals the XRD patterns of NFO, NFO/rGO, NFO/MWCNT, and NFO/SWCNT hybrids. Characteristic peaks at two theta angles of 18.43°, 30.25°, 35.65°, 37.29°, 43.33°, 53.73°, 57.32°, 63.04°, 71.51°, 74.48°, 75.61°, and 79.56° belong to the (111), (220), (311), (222), (400), (422), (511), (440), (620), (533), (622), and (444) crystal planes, respectively. These diffraction peaks could all be indexed to cubic-phased NiFe2O4 materials, which are in good agreement with JCPDS No. 86-2267 (Fig. 2b). Diffraction peaks in NFO/SWCNT, NFO/MWCNT, and NFO/rGO hybrids were identical and consistent with those of NFO, indicating virtually no effect of rGO or CNT substrate on the crystalline structure of NFO. Besides, no extra diffraction peaks were detected, manifesting the high purity of as-prepared materials.
The morphology of the prepared materials was studied using the FESEM technique. Figure S1 shows the low and high-resolution FESEM images of NFO nanocrystals. The NFO/SWCNT imaged by FESEM represented in Fig. 3a, b shows that SWCNT ultrathin structures are present on the NFO crystals. SWCNTs connect the crystals and form an electrically conductive network. The FESEM image of the NFO/MWCNT indicates that the nanocrystals of NFO blended with MWCNTs (Fig. 3c, d). FESEM images of NFO/rGO are shown in Fig. 3e, f, revealing that NFO crystals are densely anchored on the wrinkled rGO nanosheets. Such a wrinkled structure increases the surface area of nanosheets and decreases the π–π stacking phase of their interlayer [44].
Nitrogen sorption measurements were carried out to investigate the pore structure and size distribution of NFO/SWCNT, NFO/MWCNT, and NFO/rGO hybrids. The BET-specific surface areas of NFO/SWCNT, NFO/MWCNT, and NFO/rGO hybrids are measured to be 95.34 m2/g, 101.77 m2/g, and 109.62 m2/g, respectively. The nitrogen adsorption/desorption isotherm of the synthesized material depicts a typical type IV isotherm and reveals the mesoporous structure’s character (Fig. 4a). Based on the BJH model (Fig. 4b), the pore diameter of NFO/SWCNT, NFO/MWCNT, and NFO/rGO hybrids are measured to be 7.89 nm, 8.72 nm, and 7.47 nm, respectively. The high surface area might be derived from the good dispersion of the NFO material on the rGO substrate. This larger specific surface area favors the extensive exposure of active sites and is advantageous for the electrocatalyst-electrolyte interface.
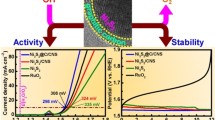
The electrochemical OER performance of all prepared catalysts was evaluated using the three-electrode system in a 1.0 M KOH electrolyte. The alkaline electrolyte was preferred for electrochemical study because oxide dissolution accompanies O2 evolution under acidic conditions [45]. LSV curves were obtained with a scan rate of 5 mV/s using GCE that is modified with the corresponding catalysts as depicted in Fig. 5a. To reach the current density of 10 mA/cm2 based on the geometric electrode area, the NFO/rGO hybrid requires a potential of 327 mV, while that of the pristine NFO is 529 mV. The overpotential value of the NFO/rGO hybrid is significantly lower than those needed for NFO/SWCNT (356 mV) and NFO/MWCNT (501 mV). This indicates the constructive effect of the rGO substrate, which offers unique surface properties and a high surface area with more active sites. For comparison, a benchmark RuO2 catalyst was evaluated under the same conditions. As expected, the state-of-the-art RuO2 catalyst exhibited superior performance at relatively small overpotentials and high current densities. To make a better understanding of the OER performance, Tafel slopes of NFO, NFO/SWCNT, NFO/MWCNT, and NFO/rGO hybrids are also investigated to probe the reaction kinetics. A linear dependency of η versus log(j) was achieved for catalysts, displaying different slopes (Fig. 5b). The Tafel slope of the NFO/rGO hybrid was calculated to be 103 mV/dec, which is much lower than the pristine NFO’s Tafel slope of 165 mV/dec. It is even lower than other CNT-based hybrids such as NFO/SWCNT (158 mV/dec) and NFO/MWCNT (161 mV/dec). The lowest Tafel slope value of the NFO/rGO hybrid indicates superior catalytic activity for the OER. EIS data have carefully evaluated the charge transfer capabilities of all catalysts. Figure 5c presents Nyquist plots of as-prepared materials with the corresponding electrical equivalent circuit diagram in the inset. The radius of the semicircle is related to the charge transfer resistance of the materials, denoted as Rct. The Rs and CPE signify the solution resistance and a constant phase element, respectively. The value of the Rct shows a strong correlation with the electrochemical performance for OER. As presented in Fig. 5c, the Rct of the NFO is greatly decreased with the formation of a hybrid structure. The rGO-based NFO hybrid possesses the smallest Rct compared to the CNT-based hybrid. The smaller Rct of the NFO/rGO hybrid confirms the fast charge transfer for OER. The durability performance of the electrocatalyst is a very crucial factor for practical applications. Long-term stabilities of NFO/SWCNT, NFO/MWCNT, and NFO/rGO hybrid electrocatalysts were tested by chronoamperometry at constant potential values (1.58 V, 1.81 V, 1.55 V (vs. RHE), respectively) in 1.0 M KOH electrolyte. Figure 5d shows the current density vs time curves. All catalysts show good durability for 8.33 h of time, which reveals that the electrocatalysts are very stable.
Overpotentials at 10 mA/cm2 and Tafel slopes of each prepared catalyst were extracted and compared in Fig. 6a, while the mechanism of the oxygen evolution process on the catalyst surface is schematically presented in Fig. 6b [46]. Initially, the adsorption of OH (OHad) and O (Oad) species takes place on the catalyst’s surface. Oad then reacts with OH− species and forms the intermediate OOHad. Additional OH– species react with this OOHad and produce O2 as well as H2O. The outstanding OER electrocatalytic activities of NFO hybrids compared to those of pristine materials can be mainly attributed to (1) the synergistic effect between the two different materials, (2) increased active sites, and (3) improved conductivity and charge transfer capability. Due to the unique morphology, the greater number of exposed active sites, and the porous structure with a high surface area, NFO/rGO hybrids exhibit excellent electrocatalytic activity and remarkable stability. The structural configuration of MWCNTs comprises concentric tubes with one capped end. Due to this, only the outer layer contributes to the electron transport in composites [47, 48]. The existence of extreme cohesive forces between SWCNTs results in the bundling in composites, which was minimized by functionalization. Thus, the SWCNT intermediately contributes toward the active sides for oxygen evolution kinetics under alkaline conditions [49].
Conclusion
In summary, this study has systematically investigated the effects of GO and CNTs on the OER activity by engineering their hybrids with spinel NiFe2O4. NiFe2O4/SWCNT, NiFe2O4/MWCNT, and NiFe2O4/rGO hybrids were prepared via a facile solvothermal approach. The 2D GO substrate provides rich active sites for OER, demonstrating a highly efficient electrocatalytic activity even better than the 1D CNT substrate. The NiFe2O4 with 2D GO hybrid exhibits good stability with an overpotential of 327 mV at a current density of 10 mA/cm2 and a Tafel slope of 103 mV/dec. This high performance of NiFe2O4 with 2D GO is mainly attributed to the unique morphology, more exposed active sites, and porous structure with a high surface area. The current finding would motivate the community to focus more on fabricating hybrids of transition metal oxides and GOs.
References
Wang B, Cui XY, Huang JQ et al (2018) Recent advances in energy chemistry of precious-metal-free catalysts for oxygen electrocatalysis. Chin Chem Lett 29(12):1757–1767
Guo YY, Yuan PF, Zhang JN et al (2018) Co2P-CoN double active centers confined in N-doped carbon nanotube: heterostructural engineering for trifunctional catalysis toward HER, ORR, OER, and Zn-air batteries driven water splitting. Adv Funct Mater 28(51):1805641
Peng X, Pi CR, Zhang XM et al (2019) Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain Energy Fuels 3(2):366–381
Walter MG, Warren EL, McKone JR et al (2010) Solar water splitting cells. Chem Rev 110(11):6446–6473
Man IC, Su HY, Calle-Vallejo F et al (2011) Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3(7):1159–1165
Chen HY, Wang AJ, Zhang L et al (2019) A facile and robust method for synthesis of hierarchically multibranched PtIrCo alloyed nanowires: growth mechanism and efficient electrocatalysis for hydrogen evolution reaction. ACS Appl Energy Mater 2(11):7886–7892
Damjanovic A, Dey A, Bockris JO (1966) Electrode kinetics of oxygen evolution and dissolution on Rh, Ir, and Pt-Rh alloy electrodes. J Electrochem Soc 113(7):739
Wang MS, Fu WY, Du L et al (2020) Surface engineering by doping manganese into cobalt phosphide towards highly efficient bifunctional HER and OER electrocatalysis. Appl Surf Sci 515:146059
Liu J, Jia E, Wang L et al (2019) Tuning the electronic structure of LaNiO3 through alloying with strontium to enhance oxygen evolution activity. Adv Sci 6(19):1901073
Cai Z, Bi YM, Hu EY et al (2018) Single-crystalline ultrathin Co3O4 nanosheets with massive vacancy defects for enhanced electrocatalysis. Adv Energy Mater 8(3):1701694
Choi Y, Kim D, Lin LW et al (2021) CuFeN/CNT composite derived from kinetically modulated urchin-shaped MOF for highly efficient OER catalysis. Electrochimica Acta 389:138637
Wang J, Wei XQ, Wang XY et al (2021) Plasmonic Au nanoparticle@Ti3C2Tx heterostructures for improved oxygen evolution performance. Inorg Chem 60(8):5890–5897
Abd-Elrahim AG, Chun DM (2020) Fabrication of efficient nanostructured Co3O4-graphene bifunctional catalysts: oxygen evolution, hydrogen evolution, and H2O2 sensing. Ceram Int 46(15):23479–23498
Shen J, Gao J, Ji LD et al (2019) Three-dimensional interlinked Co3O4-CNTs hybrids as novel oxygen electrocatalyst. Appl Surf Sci 497:143818
Kong XK, Liu QC, Chen DB et al (2017) Identifying the active sites on N-doped graphene toward oxygen evolution reaction. ChemCatChem 9(5):846–852
Zhang LL, Xiao J, Wang HY et al (2017) Carbon-based electrocatalysts for hydrogen and oxygen evolution reactions. ACS Catal 7(11):7855–7865
Mohan VB, Lau KT, Hui D et al (2018) Graphene-based materials and their composites: a review on production, applications and product limitations. Compos Part B: Eng 142:200–220
Chew SY, Ng SH, Wang JZ et al (2009) Flexible free-standing carbon nanotube films for model lithium-ion batteries. Carbon 47(13):2976–2983
Sattar T (2019) Current review on synthesis, composites and multifunctional properties of graphene. Top Curr Chem 377(2):10
Li QZ, Fan F, Wang Y et al (2013) Enzyme immobilization on carboxyl-functionalized graphene oxide for catalysis in organic solvent. Ind Eng Chem Res 52(19):6343–6348
Liu J, Xue YH, Gao YX et al (2012) Hole and electron extraction layers based on graphene oxide derivatives for high-performance bulk heterojunction solar cells. Adv Mater 24(17):2228–2233
Cakici M, Kakarla RR, Alonso-Marroquin F (2017) Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chem Eng J 309:151–158
Munir KS, Wen CE, Li YC (2019) Carbon nanotubes and graphene as nanoreinforcements in metallic biomaterials: a review. Adv Biosyst 3(3):e1800212
Cao Q, Rogers JA (2009) Ultrathin films of single-walled carbon nanotubes for electronics and sensors: a review of fundamental and applied aspects. Adv Mater 21(1):29–53
Gao R, Dai QB, Du F et al (2019) C60-adsorbed single-walled carbon nanotubes as metal-free, pH-universal, and multifunctional catalysts for oxygen reduction, oxygen evolution, and hydrogen evolution. J Am Chem Soc 141(29):11658–11666
Matsumoto Y, Sato E (1986) Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater Chem Phys 14(5):397–426
Samal R, Kandasamy M, Chakraborty B et al (2021) Experimental and theoretical realization of an advanced bifunctional 2D δ-MnO2 electrode for supercapacitor and oxygen evolution reaction via defect engineering. Int J Hydrog Energy 46(55):28028–28042
Zhang CY, Bhoyate S, Zhao C et al (2019) Electrodeposited nanostructured CoFe2O4 for overall water splitting and supercapacitor applications. Catalysts 9(2):176
Fu Z, Liu S, Mai Z et al (2020) Heterostructure and oxygen vacancies promote NiFe2O4 /Ni3S4 toward oxygen evolution reaction and Zn-air batteries. Chem Asian J 15(21):3568–3574
Karuppasamy K, Sharma B, Vikraman D et al (2021) Switchable p-n gas response for 3D-hierarchical NiFe2O4 porous microspheres for highly selective and sensitive toluene gas sensors. J Alloy Compd 886:161281
Cherian CT, Sundaramurthy J, Reddy MV et al (2013) Morphologically robust NiFe2O4 nanofibers as high capacity Li-ion battery anode material. ACS Appl Mater Interfaces 5(20):9957–9963
Bandgar SB, Vadiyar MM, Ling YC et al (2018) Metal precursor dependent synthesis of NiFe2O4 thin films for high-performance flexible symmetric supercapacitor. ACS Appl Energy Mater 1(2):638–648
Taha TA, Azab AA, Sebak MA (2019) Glycerol-assisted sol-gel synthesis, optical, and magnetic properties of NiFe2O4 nanoparticles. J Mol Struct 1181:14–18
Naik KM, Sampath S (2018) Two-step oxygen reduction on spinel NiFe2O4 catalyst: rechargeable, aqueous solution- and gel-based, Zn-air batteries. Electrochim Acta 292:268–275
Choi J, Kim D, Zheng WR et al (2021) Interface engineered NiFe2O4−x/NiMoO4 nanowire arrays for electrochemical oxygen evolution. Appl Catal B: Environ 286:119857
Karpuraranjith M, Chen YF, Wang B et al (2021) Hierarchical ultrathin layered MoS2@NiFe2O4 nanohybrids as a bifunctional catalyst for highly efficient oxygen evolution and organic pollutant degradation. J Colloid Interface Sci 592:385–396
Shi YL, Feng XJ, Guan HY et al (2021) Porous sunflower plate-like NiFe2O4/CoNi-S heterostructure as efficient electrocatalyst for overall water splitting. Int J Hydrog Energy 46(12):8557–8566
Shinde P, Rout CS, Late D et al (2021) Optimized performance of nickel in crystal-layered arrangement of NiFe2O4/rGO hybrid for high-performance oxygen evolution reaction. Int J Hydrog Energy 46(2):2617–2629
Eda G, Fanchini G, Chhowalla M (2008) Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat Nanotechnol 3(5):270–274
Hirsch A, Vostrowsky O (2005) Functionalization of carbon nanotubes. In: Schlüter AD (ed) Functional molecular nanostructures. Topics in current chemistry, vol 245. Springer, Berlin, Heidelberg, pp 193–237
Sahoo S, Sahoo PK, Manna S et al (2020) A novel low cost nonenzymatic hydrogen peroxide sensor based on CoFe2O4/CNTs nanocomposite modified electrode. J Electroanal Chem 876:114504
Zhang YL, Wang XX, Cao MS (2018) Confinedly implanted NiFe2O4-rGO: cluster tailoring and highly tunable electromagnetic properties for selective-frequency microwave absorption. Nano Res 11(3):1426–1436
Samal R, Bhat M, Kapse S et al (2021) Enhanced energy storage performance and theoretical studies of 3D cuboidal manganese diselenides embedded with multiwalled carbon nanotubes. J Colloid Interface Sci 598:500–510
Sakthinathan S, Keyan AK, Rajakumaran R et al (2021) Synthesis of N-rGO-MWCNT/CuCrO2 catalyst for the bifunctional application of hydrogen evolution reaction and electrochemical detection of bisphenol-A. Catalysts 11(3):301
Surendranath Y, Kanan MW, Nocera DG (2010) Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J Am Chem Soc 132(46):16501–16509
Ji SY, Li TT, Gao ZD et al (2018) Boosting the oxygen evolution reaction performance of CoS2 microspheres by subtle ionic liquid modification. Chem Commun 54(63):8765–8768
Chayad FA, Jabur AR, Jalal NM (2015) Effect of MWCNT addition on improving the electrical conductivity and activation energy of electrospun nylon films. Karbala Int J Mod Sci 1(4):187–193
Norkhairunnisa M, Azizan A, Mariatti M et al (2012) Thermal stability and electrical behavior of polydimethylsiloxane nanocomposites with carbon nanotubes and carbon black fillers. J Compos Mater 46(8):903–910
O’Connell MJ, Boul P, Ericson LM et al (2001) Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem Phys Lett 342(3–4):265–271
Acknowledgements
The authors would like to acknowledge the financial support from the SERB Early Career Research Project (No. ECR/2017/001850), Department of Science and Technology (Nos. DST/NM/NT/2019/205(G), DST/TDT/SHRI-34/2018), Karnataka Science and Technology Promotion Society (KSTePS/VGST-RGS-F/2018-19/GRD NO. 829/315), start-up grant, Jain University (11 (39)/17/013/2017SG), Nanomission (SR/NM/NS-20/2014) for the characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shinde, P.V., Samal, R. & Rout, C.S. Comparative Electrocatalytic Oxygen Evolution Reaction Studies of Spinel NiFe2O4 and Its Nanocarbon Hybrids. Trans. Tianjin Univ. 28, 80–88 (2022). https://doi.org/10.1007/s12209-021-00310-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-021-00310-x