Abstract
Photocatalytic hydrogen evolution is an attractive field for future environment-friendly energy. However, fast recombination of photogenerated charges severely inhibits hydrogen efficiency. Single-atom cocatalysts such as Pt have emerged as an effective method to enhance the photocatalytic activity by introduction of active sites and boosting charge separation with low-coordination environment. Herein, we demonstrated a new strategy to develop a highly active Pd single atom in carbon-deficient g-C3N4 with a unique coordination. The single-atom Pd–N3 sites constructed by oil bath heating and photoreduction process were confirmed by HADDF-STEM and XPS measurements. Introduction of single-atom Pd greatly improved the separation and transportation of charge carriers, leading to a longer lifespan for consequent reactions. The obtained single-atom Pd loaded on the carbon-deficient g–C3N4 showed excellent photocatalytic activity in hydrogen production with about 24 and 4 times higher activity than that of g–C3N4 and nano-sized Pd on the same support, respectively. This work provides a new insight on the design of single-atom catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photocatalytic hydrogen evolution has been recognized as a promising approach to sustainable clean energy. Although much progress is made in improving photocatalysts’ intrinsic activity, most photocatalysts still suffer from weak photo-absorption, limited active sites, and high charge-carrier recombination [1,2,3,4,5]. Among these adverse factors, the intrinsic nature of charge carriers’ recombination is very critical, which makes limited photoelectrons access the reaction sites to reduce water for hydrogen production even with the presence of hole scavengers [6,7,8,9]. Therefore, modification with a cocatalyst turns out to be an effective alternative, aiming at significantly enhancing catalytic efficiency. Pt has proven to be one of the most promising cocatalysts, but its large-scale applications are limited by its scarcity and high price [1, 10]. Thus, it is necessary to develop alternatives or reduce Pt loading to improve hydrogen production.
Recently, single-atom sites on metal oxide [11, 12], N-doped carbon supports [13, 14], and graphite carbon nitride [15, 16] have been proposed as active sites for various reactions by downsizing metal particles [17]. The finely dispersed isolated metal atoms coordinated by metal or nonmetal atoms can be a promising ideal cocatalyst, which can not only minimize the use of noble metal, but also serve as active sites through exposure to low-coordination environment of metal centers [18, 19]. For example, Chen et al. [20] reported that Ag single atom supported on g-C3N4 in the coordination of Ag–N2 exhibited an H2 production of 1.87 mmol/(g·h). Such performance was as excellent as nano-Pt loaded on g-C3N4. Therefore, desirable hydrogen production performance could be achieved by reasonably regulating the coordination environment of metal cocatalyst.
The specific geometric and electronic structures of the single atom can regulate the catalytic activity of anchored single metal sites, especially for N, which acts as the coordination element. Indeed, the isolated metal–N4 sites with porphyrin units were intensively studied, such as Fe–N4 [21, 22], Ru–N4 [23], Ni–N4 [24], and Cu–N4 [25] sites. Li et al. [26] reported that the isolated bond-shrinking low-valence Cu (+ 1)–N4–C8S2 atomic interface moiety served as an active site for ORR process. Similarly, it has been found that Fe–N4 center presented higher ORR efficiency after its surrounding charges were altered by S doping [27]. Other configurations including metal–N2, metal–N3, and metal–Nx sites are relatively less reported. Chen et al. [20] demonstrated that Ag–N2 sites showed higher activity compared with Ag–N4 sites for hydrogen production. In addition, Pt–N3 single site was reported exhibited photocatalytic N2 fixation ability [18]. Fu et al. [25] also observed that the complex of Cu–N3 and Cu–N4 could boost the hydrogen yield. It is worth mentioning that the construction of Pd–N3 coordination structure in g-C3N4 acting as photocatalytic sites has not yet been reported, which could provide ideal unoccupied N atoms once the carbon in the s-triazine rings was removed.
In this work, we successfully anchored single-atom Pd–N3 sites on carbon-deficient g–C3N4, which showed intriguing photocatalytic H2 evolution activity compared to its counterparts. The isolated sites were confirmed by XPS and HAADF-STEM measurements, and the mechanism was discussed in detail.
Experimental Section
Preparation of Carbon-Deficient g–C3N4
Put 10 g of urea into a 50-mL crucible with a cover. It was then transferred to the muffle furnace and kept at 600 °C for 2 h. The final product was ground into powder and denoted as CN.
Synthesis of Single-Atom Pd Loaded on Carbon-Deficient g-C3N4
0.3 g CN was added into 100 mL of deionized water. Then, 0.5 mL solution of PdCl2 (1 mg/ mL) and NaCl (5 mg/mL) was dissolved into the above suspension after being thoroughly mixed by an ultrasonic cell disruptor for 30 min. The obtained mixture was then constantly stirred at 80 °C for 8 h in an oil bath. After cooling to ambient temperature, the mixture was washed with deionized water several times. The remaining mixture was dispersed with 100 mL deionized water and radiated under 300 W Xe lamp for 1 h. The final product was washed with deionized water and dried in the freezer drier overnight. The final sample was denoted as Pd–CN.
Preparation of Nano-Sized Pd Loaded on Carbon-Deficient g-C3N4
The nano-sized Pd loaded on CN was prepared by a similar route: 4 mL solution of PdCl2 (1 mg/mL) and NaCl (5 mg/mL) was dissolved into suspension of 100 mL deionized water and 0.3 g CN. Then, the mixture was stirred under illumination by the 300 W Xe lamp for 3 h. The final product was washed with deionized water and dried in the freezer drier overnight. The final sample was denoted as Pdnano-CN.
Characterization
Transmission electron microscopy (TEM) observations and energy-dispersive X-ray spectroscopy mapping images were obtained using a Titan G2 60–300 microscope. Aberration-corrected HAADF-STEM characterization was performed on a Cs-corrected Titan G2 60–300 electron microscope (FEI, USA). X-ray diffractions were conducted to identify crystal phases by a D8 Rigaku9000 using a Cu Kα radiation in the 2θ range of 5°–80°. Fourier transform infrared spectra (FTIR) were recorded on a NICOLET iS10 FTIR spectrometer. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Scientific K-Alpha + spectrometer. UV–Vis diffuse reflectance spectra (UV–Vis DRS) were achieved on a UV-8000A system. Photoluminescence (PL) and time-resolved transient PL decay spectroscopic analyses were conducted by an Edinburgh FLS1000 spectrophotometer with an excitation wavelength of 360 nm.
Photocatalytic Hydrogen Evolution
Photocatalytic hydrogen evolution was tested in a typical route: 15 mg of the sample was dispersed in a 100 mL aqueous solution with 5 vol% triethanolamine (TEOA) as the hole scavenger. After bubbled with N2 to remove the oxygen for 30 min, the test was carried out using 300 W Xe light under 5 °C and atmospheric pressure. The amount of H2 was determined by gas chromatography (SHIMADZU GC-2014C). The apparent quantum yield (AQY) for hydrogen evolution was estimated as follows: AQY = 2nH2NA/Nphoton × 100%, where the nH2 refers to the mole amount of produced H2 molecules, NA refers Avogadro constant, and the Nphoton corresponds to the amount of photons. The cycling test was similarly conducted in a typical route: after the first experiment, the light source was turned off and N2 was bubbled into the reactor to remove the hydrogen for 30 min. The experiment was restarted by turning on the light, and the next cycling was conducted similarly.
Results and Discussion
Synthesis and Characterizations of Pd–CN
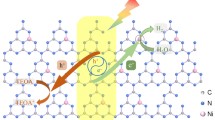
Among different coordination elements, N is the most accessible for N-based materials providing strong anchoring sites for single-atom loading. g-C3N4 is an ideal support since its s-triazine rings are rich in N despite being bonded to carbon atoms. The carbon-deficient g-C3N4 was prepared following a protocol developed by our group previously [28]. Its C/N molar ratio fell from 0.75 (the ideal ratio) to 0.66 according to the XPS spectral analysis, while the ratio for bulk g-C3N4 was 0.72 as confirmed by elemental analysis (Table 1), signifying successful introduction of carbon defects. The carbon-deficient g-C3N4 served as a perfect carrier, owning to its three unoccupied N atoms. After pre-absorption and photo-reduction of Pd2+, the Pd supported on CN can be obtained as illustrated in Fig. 1a. HADDF-TEM images in Fig. 1b reveal uniform dispersion of the Pd element on the surface of CN. Figure 1c confirms the atomically dispersed Pd, which suggests that the single-atom Pd was successfully obtained.
XRD spectra of Pd–CN in Fig. 2a reveal two characteristic peaks at 27.8° and 13.0°, corresponding to the (002) and (100) crystal plane of graphite phase carbon nitride, respectively. No changes in the position and shape of these two peaks were observed with the addition of single-atom Pd compared to its counterparts, CN and Pdnano–CN, indicating that the triazine ring structure remained the same with CN after introduction of Pd. This is also confirmed by FTIR spectral measurement.
XPS was conducted to explore more information about surface composition of CN, Pd–CN, and Pdnano–CN. Four elements including C, N, O, and Pd were found in the Pd-loaded samples (Fig. 3), which are in accordance with the results of HADDF-STEM. The binding energy peaks at 285 eV and 288 eV obtained from Pd–CN can be assigned to the adventitious carbon and C-N3 in typical aromatic C3N4 heterocycles, respectively. The N 1s peaks in Fig. 3b can be resolved into three peaks centered at 398, 400, and 401 eV, which are related to C=N–C, N–C3, and −NHx (x = 1, 2), respectively. O originated from the N–C–O corresponding to lattice oxygen in the bulk located at 532 eV [29]. Noticeably, two different forms of Pd can be seen in the Pd–CN, namely Pd+ and Pd2+ located between 336.8 and 342.0 eV and between 338.0 and 343.2 eV, respectively [30, 31]. However, metallic Pd0 can only be observed for Pdnano–CN, which is located at 335.2 eV and 340.5 eV (Fig. 3d). This also proves that Pd was introduced in the form of single atom instead of nanoparticle. Furthermore, the peaks of Pd2+ significantly shifted to lower binding energy, compared with the Pd precursor (338.4 eV and 343.7 eV) [15]. In addition, Pd2+ peaks approximated to the shapes and positions of Pd–N coordination (337.2 eV and 342.5 eV) as reported previously [32], suggesting bonding of the single-atom Pd with nearby N atoms. HADDF-STEM and XPS results demonstrated that the atomically dispersed Pd was coordinated with carbon vacancy-resulted N (Pd–N3) in CN. Loading of the single atom on the carbon defects instead on the pore is attributed to ligand–metal charge transfer (LMCT) coordination effect. Wang et al. [28] reported similar observation, suggesting that the Pd–N3 sites on C-deficient g-C3N4 were energetically more favorable.
Photocatalytic Activity and Mechanism Analysis
Samples were tested for hydrogen evolution activity in an aqueous sacrificial solution containing triethanolamine (TEOA). The fully optimized results are shown in Fig. 4a. Pd–CN exhibited superior hydrogen evolution in comparison with CN and Pdnano–CN under full arc irradiation. The Pd–CN evolved hydrogen at approximately 2788 μmol/(g·h), which is 4 times faster than Pdnano–CN, indicating that the single-atom Pd has higher activity toward H2 production. Photocatalytic activities of the samples under two different specific wavelengths of light (λ = 420 nm, 380 nm) were also investigated. As seen in Fig. 4b, Pd–CN showed higher apparent quantum efficiency of H2 evolution under ultraviolet irradiation compared to CN and Pdnano–CN. These results proved the excellent activity of Pd–CN for photocatalytic H2 evolution. The long-term photocatalytic stability was tested in three cycles. As seen in Fig. 4c, Pd–CN remained stable under continuous irradiation and its performance did not decrease significantly.
Light absorption in Fig. 5a increased in the range of 500–800 nm as Pd particle grew. As illustrated in Fig. 5b, the band gap remained the same with CN, but the valence band (VB) position decreased significantly with the introduction of Pd atom (Fig. 5c). As a result, conduction band (CB) shifted to a more negative position, which suggests enhanced reduction capability. By contrast, Pdnano–CN showed enhanced visible light absorption and a narrowed band gap. It is worth mentioning that these advantages for Pdnano–CN did not make much contribution to the H2 production. Otherwise, it should have performed better than Pd–CN, which also suggests that the single atom is more advantageous.
The charge transfer ability of Pd–CN was demonstrated by combining the analysis of steady-state and time-resolved photoluminescence (TRPL) spectroscopy with the CN benchmark as a reference. The steady-state PL spectra showed that the intrinsic emission peak of CN was greatly weakened by introduction of Pd, implying a lower charge recombination rate in Pd–CN and Pdnano–CN (Fig. 6a). A customized time-correlated single-photon counting apparatus was employed in the collection of the TRPL spectra (Fig. 6b) to gain a deep insight into the charge-transfer dynamics, where a biexponential fitting is applied to analyze the luminescence decay curves due to their complexity. Obviously, in contrast to CN, the lifetime of charge carriers was prolonged for Pd–CN (7.70 ns), whereas that for Pdnano–CN (7.01 ns) was shortened, elucidating that single-atom Pd loaded on CN can enhance the charge transfer and separation. However, shorter lifetime was observed in Pdnano–CN, which means that nano-Pd can transfer to be combination centers rather than active sites. As a result, the photocatalytic activity is dropped with the nano-sized Pd loading compared to single-atom Pd loading.
Conclusion
In summary, a strategy based on deficient carbon structure was applied to develop single-atom Pd–N3 sites. The HADDF-STEM and XPS measurements supported that the single-atom Pd was successfully loaded on the carbon-deficient g–C3N4 in the form of isolated Pd–N3 sites. The addition of single-atom Pd improved the photogenerated charge carriers’ separation and transfer, leading to remarkably improved performance of photocatalytic H2 evolution compared to its counterparts, the addition of loading nano-sized Pd and pristine g–C3N4.
References
Cao YJ, Wang DH, Lin Y et al (2018) Single Pt atom with highly vacant d-orbital for accelerating photocatalytic H2 evolution. ACS Appl Energy Mater 1(11):6082–6088
Ran JR, Ma TY, Gao GP et al (2015) Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ Sci 8(12):3708–3717
Zhang JH, Wei MJ, Wei ZW et al (2020) Ultrathin graphitic carbon nitride nanosheets for photocatalytic hydrogen evolution. ACS Appl Nano Mater 3(2):1010–1018
Gao JF, Zhang FD, Xue HQ et al (2021) In-situ synthesis of novel ternary CdS/PdAg/g-C3N4 hybrid photocatalyst with significantly enhanced hydrogen production activity and catalytic mechanism exploration. Appl Catal B: Environ 281:119509
Kim D, Yong K (2021) Boron doping induced charge transfer switching of a C3N4/ZnO photocatalyst from Z-scheme to type II to enhance photocatalytic hydrogen production. Appl Catal B: Environ 282:119538
Chen ZW, Bu YY, Wang L et al (2020) Single-sites Rh-phosphide modified carbon nitride photocatalyst for boosting hydrogen evolution under visible light. Appl Catal B: Environ 274:119117
Wang X, Zhang YW, Si HN et al (2020) Single-atom vacancy defect to trigger high-efficiency hydrogen evolution of MoS2. J Am Chem Soc 142(9):4298–4308
Wang YY, Zhao S, Zhang YW et al (2018) Facile synthesis of self-assembled g-C3N4 with abundant nitrogen defects for photocatalytic hydrogen evolution. ACS Sustain Chem Eng 6(8):10200–10210
Qiu CH, Bai S, Cao WJ et al (2020) Tunable syngas synthesis from photocatalytic CO2 reduction under visible-light irradiation by interfacial engineering. Trans Tianjin Univ 26(5):352–361
Li XG, Bi WT, Zhang L et al (2016) Single-atom Pt as Co-catalyst for enhanced photocatalytic H2 evolution. Adv Mater 28(12):2427–2431
Xin P, Zhao Y, Qin RX et al (2016) Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352(6287):797–801
Lee BH, Park S, Kim M et al (2019) Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat Mater 18(6):620–626
Lu PL, Yang Y, Yao JN et al (2019) Facile synthesis of single-nickel-atomic dispersed N-doped carbon framework for efficient electrochemical CO2 reduction. Appl Catal B 241:113–119
Geng ZG, Liu Y, Kong XD et al (2018) Achieving a record-high yield rate of 120.9 μgNH3 mgcat.-1 h-1 for N2 electrochemical reduction over Ru single-atom catalysts. Adv Mater. 30(40):1803498
Cao SW, Li H, Tong et al (2018) Photocatalysis: single-atom engineering of directional charge transfer channels and active sites for photocatalytic hydrogen evolution. Adv Funct Mater 28(32):1870224
Chen ZP, Vorobyeva E, Mitchell S et al (2018) Single-atom heterogeneous catalysts based on distinct carbon nitride scaffolds. National Science Review 5:642–652
Yang XF, Wang AQ, Qiao BT et al (2013) Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc Chem Res 46(8):1740–1748
Liu KP, Zhao XT, Ren GQ et al (2020) Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts. Nat Commun 11:1263
Dong CY, Lian C, Hu SC et al (2018) Size-dependent activity and selectivity of carbon dioxide photocatalytic reduction over platinum nanoparticles. Nat Commun 9:1252
Jiang XH, Zhang LS, Liu HY et al (2020) Silver single atom in carbon nitride catalyst for highly efficient photocatalytic hydrogen evolution. Angew Chem Int Ed Engl 59:1–6
Zhang CH, Yang SZ, Wu JJ et al (2018) Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene. Adv Energy Mater 8(19):1703487
Wan X, Liu XF, Li YC et al (2019) Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat Catal 2(3):259–268
Hu XL, Luo G, Zhao QN et al (2020) Ru single atoms on N-doped carbon by spatial confinement and ionic substitution strategies for high-performance Li–O2 batteries. J Am Chem Soc 142(39):16776–16786
Zhao SY, Chen GX, Zhou GM et al (2020) A universal seeding strategy to synthesize single atom catalysts on 2D materials for electrocatalytic applications. Adv Funct Mater 30(6):1906157
Xiao XD, Gao YT, Zhang LP et al (2020) A promoted charge separation/transfer system from Cu single atoms and C3N4 layers for efficient photocatalysis. Adv Mater 32(33):2003082
Jiang ZL, Sun WM, Shang HS et al (2019) Atomic interface effect of a single atom copper catalyst for enhanced oxygen reduction reactions. Energy Environ Sci 12(12):3508–3514
Li QH, Chen WX, Xiao H et al (2018) Fe isolated single atoms on S, N codoped carbon by copolymer pyrolysis strategy for highly efficient oxygen reduction reaction. Adv Mater 30(25):1800588
Liu GM, Huang Y, Lv H et al (2021) Confining single-atom Pd on g-C3N4 with carbon vacancies towards enhanced photocatalytic NO conversion. Appl Catal B Environ 84:119683
Martin DJ, Qiu KP, Andrew S et al (2014) Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew Chem Int Ed 53(35):9240–9245
Sharma P, Sasson Y (2019) Sustainable visible light assisted in situ hydrogenation via a magnesium–water system catalyzed by a Pd-g-C3N4 photocatalyst. Green Chem 21(2):261–268
Xu XL, Luo JJ, Li LP et al (2018) Unprecedented catalytic performance in amine syntheses via Pd/g-C3N4 catalyst-assisted transfer hydrogenation. Green Chem 20(9):2038–2046
Arrigo R, Schuster ME, Xie ZL et al (2015) Nature of the N-Pd interaction in nitrogen-doped carbon nanotube catalysts. ACS Catal 5(5):2740–2753
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21976116, 21473248); Guangdong Science and Technology Program (No. 2018A050506025); Guangzhou Science and Technology Program (Nos. 202002030406, 201804010181); High Level Talents Introduction Project of "Pearl River Talent Plan" in Guangdong Province (No. 2019CX01L308); the Support Scheme of Guangzhou for Leading Talents in Innovation and Entrepreneurship Funding (No. 2016015); and the Key Deployment Projects of Chinese Academy of Sciences (No. ZDRW_CN_2020_1).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, G., Lv, H., Zeng, Y. et al. Single-Atom Pd–N3 Sites on Carbon-Deficient g-C3N4 for Photocatalytic H2 Evolution. Trans. Tianjin Univ. 27, 139–146 (2021). https://doi.org/10.1007/s12209-020-00279-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-020-00279-z