Abstract
Metal–organic frameworks (MOFs) and layered double hydroxides (LDHs) have been considered to be one of the most promising and worthy hot spot materials to develop advanced catalysts for efficient hydrogen evolution due to their prominent characteristics, including unique structures, environmentally friendly nature, high redox activities, and homogeneously effective utilization of transition metal atoms. In this work, the delicate S-scheme heterojunction photocatalyst, CoAl LDH@Ni-MOF-74, was rationally designed and successfully constructed by coupling Ni-MOF-74 with CoAl LDH based on their peculiar structure, excellent electronic properties, and opposite surface potential for enhancing hydrogen generation activity under visible light irradiation. The CoAl LDH nanolayers evenly and dispersedly load on the surface of Ni-MOF-74. The CoAl LDH@Ni-MOF-74 exhibited higher photocatalytic hydrogen evolution activity compared with Ni-MOF-74 and CoAl LDH alone, mainly because the formation of the CoAl LDH@Ni-MOF-74 S-scheme heterojunction accelerated the recombination of several electrons (from conduction band (CB) of Ni-MOF-74) and holes (from valence band (VB) of CoAl LDH) and prevented the recombination of other electrons (from CB of CoAl LDH) and holes (from VB of Ni-MOF-74).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Given the rapid growth of the world’s population and the increasing pursuit for a high quality of life, over-consumption of energy has resulted in the possibility that the rapidly growing energy demand will trigger an energy crisis. At the same time, a series of potential huge problems, such as the derived environmental pollution and ecological destruction, continuous increases in the global greenhouse gas emissions, the increased area of forest destruction, and the accumulating production of domestic waste, also exist. Thus, the catastrophic environmental pollution caused by the consumption and depletion of fossil fuels has been recognized as a major challenge in the near future [1]. The development of sustainable clean energy has become the most critical issue to solve the above problems [2, 3]. For instance, electrochemical synthesis of hydrogen peroxide (H2O2) provides a clean and safe technology for large-scale H2O2 production [4]. A novel method for synthesizing p-benzoquinone by direct catalytic oxidation of benzene with hydrogen peroxide over copper-doped TS-1 was reported [5]. Hydrogen bonding-assisted synthesis of silica/oxidized mesocarbon microbeads encapsulated in amorphous carbon (SiO2/O’MCMB/C) was used as stable anode for optimized/enhanced lithium storage [6]. Pd-Mg(Al)-LDH/γ-Al2O3 and Pd-Mg(Al)Zr-LDH/γ-Al2O3 precursors were synthesized for 2-ethylanthraquinone hydrogenation [7]. SBA-15 silica was synthesized for hydrogenation of alkylanthraquinone by adding normal paraffin and alkyl benzene as swelling agents and using a low-temperature gelation procedure [8]. Solar energy contains huge amounts of energy, and most of the energy required by humans comes directly or indirectly from the sun [9]. However, thus far, the human conversion and utilization efficiency of solar energy remain at extremely low levels [10, 11]. Light-induced water-splitting produces hydrogen and can effectively convert solar energy into hydrogen energy. Encouragingly, H2 has the advantages of high calorific value, environmental friendliness, and high conversion efficiency [12, 13]. H2 is an efficient and clean energy source and can alleviate and solve environmental and energy problems at the same time.
Layered double hydroxides (LDHs) are two-dimensional clay materials with many advantages, such as environmentally friendly nature, high redox activities, and homogeneously effective utilization of transition metal atoms; they have been considered as the most promising candidates to replace TiO2 and other semiconductor photocatalysts [14,15,16]. Importantly, the easy tunability of cations in their host layers and exchangeability of anions without altering the structure also endow LDHs with intriguing electrochemical/electronic properties [14]; thus, LDHs have important applications in catalysis, anion exchanger, or sensor [17,18,19]. The formula of CoAl LDH is [Co6Al2CO3(OH)6·4H2O] containing divalent metal Co ions and trivalent metal Al ions. Yan’s group [20] reported a novel sandwich-like composite with ultrathin CoAl LDH nanoplates electrostatically assembled on both sides of two-dimensional polypyrrole/graphene substrate, exhibiting excellent gravimetric specific capacitance. Li et al. [21] reported a flexible asymmetric supercapacitor based on a CoAl LDH electrode and a reduced graphene oxide electrode, and it delivered a specific capacitance of 616.9 F/g at a current density of 1 A/g. Yang et al. [22] designed and synthesized novel nanosheet structures of CoAl LDH-polyaniline (PANI) nanocomposite thin films; the CoAl LDH-PANI exhibited significantly improved specific capacitance and cyclic stability. Thus far, the application of CoAl LDH in photocatalytic water-splitting for hydrogen evolution is lacking.
As a kind of coordination polymer with crystal structure and potential porosity, metal–organic frameworks (MOFs) have rapidly developed into a research field in the past two decades [23, 24]. Emerging MOFs have inherent advantages, including structural diversity, functionality, customizability, and universal applications. In addition, MOFs can immobilize active functional materials and manufacture highly controllable nanostructures as carriers, providing new impetus for energy applications. A high-efficiency Ni-MOF-74/CdS/Co3O4 photocatalyst was designed and synthesized successfully. This photocatalyst exhibits better photocatalytic hydrogen evolution performance compared with single CdS and Co3O4 [25]. The as-synthesized hexagonal microrods of MOF-74-Ni were utilized as a precursor to prepare porous Ni2P/C composite; the as-prepared Ni2P/C exhibited excellent electrocatalytic activity toward hydrogen evolution reaction [26].
In this work, a distinctive hybrid of Ni-based zeolitic imidazolate frameworks (MOF-74) with transition metal-based LDH (CoAl LDH) was designed and synthesized through electrostatic self-assembly means. The CoAl LDH@Ni-MOF-74 composite catalyst exhibited excellent photocatalytic hydrogen evolution performance due to accelerated electron transfer. Ni-MOF-74 and nanosheet CoAl LDH showed characteristic structures and unique performances. The prominent photocatalytic properties of the CoAl LDH@Ni-MOF-74 hybrid depend on the inherent internal structures and unique performances. The internal reaction mechanism of the CoAl LDH@Ni-MOF-74 catalyst for efficient hydrogen evolution will be analyzed in detail next.
Experimental Section
Preparation of Ni-MOF-74
The Ni-MOF-74 was prepared by referring to Refs. [27, 28]. Specifically, 440 mg Ni(NO3)2·6H2O, 120 mg terephthalic acid, and 600 mg polyvinylpyrrolidone (PVP-K30) were added to a 100-mL beaker containing 20 mL purified water, ethanol, and dimethylformamide. Then, the mixture system was stirred for 30 min. Subsequently, the mixture was transferred to a polytetrafluoroethylene (PTFE) autoclave and maintained at 150 °C for 10 h. After natural cooling, the product was obtained by centrifugation, washing, and drying.
Preparation of CoAl LDH
Solution A: A total of 1.5 mmol Na2CO3 and 4 mmol NaOH were dissolved in 15 mL purified water, and the mixture was stirred for 10 min until complete dissolution.
Solution B: A total of 1.5 mmol Co(NO3)2·6H2O and 0.5 mmol Al(NO3)3·9H2O were added to a 100-mL beaker containing 50 mL purified water, and the mixture was stirred for 10 min until complete dissolution.
Then, solution A was dropwise added to solution B. Finally, the as-prepared mixed solution was transferred to the PTFE autoclave and kept at 120 °C for 16 h. After natural cooling, the product was obtained by centrifugation, washing, and drying.
Electrostatic Self-Assembly of CoAl LDH@Ni-MOF-74
Solution A: A total of 1.5 mmol Na2CO3 and 4 mmol NaOH were dissolved in 15 mL purified water, and the mixture was stirred for 10 min until complete dissolution.
Solution C: A total of 1.5 mmol Co(NO3)2·6H2O and 0.5 mmol Al(NO3)3·9H2O were added to a 100-mL beaker containing 50 mL purified water, followed by the addition of x g Ni-MOF-74 (x = 0.05, 0.10, 0.15, 0.20, 0.25 g), and the mixture was stirred for 10 min until complete dissolution.
Solution A was dropwise added to solution C. Finally, the as-prepared mixed solution was transferred to the PTFE autoclave and kept at 120 °C for 16 h. After natural cooling, the product was obtained by centrifugation, washing, and drying. The as-prepared hybrids were named as CoAl@MOF-1, CoAl@MOF-2, CoAl@MOF-3, CoAl@MOF-4, and CoAl@MOF-5.
Characterization of Products
X-ray diffraction (XRD) patterns were recorded on a Rigaku RINT-2000 diffractometer with Cu Kα radiation (λ = 1.5406 Å). The morphology and structure of MOF-74, CoAl LDH, and MOF-74@CoAl LDH were determined with field emission scanning electron microscopy [(FESEM); JSM-6701F JEOL] and transmission electron microscopy (TEM; JEM1200EX JEOL). Ultraviolet (UV)–visible diffuse reflectance spectra were recorded on a UV-2550 spectrophotometer. X-ray photoelectron spectroscopy (XPS) was performed on an ESCALAB 250Xi. The steady-state fluorescence and transient fluorescence were measured on FLUOROMAX-4 fluorescence spectrometer. The average zeta potentials of MOF-74 and CoAl LDH were tested on Litesizer 500. The photoelectrochemical performances were evaluated on a VersaStat4-400 electrochemical workstation. A total of 0.2 mol/L Na2SO4 solution was used as electrolyte, and 300 W xenon lamp was used as light source.
Photocatalytic H2 Evolution Experiments
Photocatalytic H2 evolution experiments were conducted in a sealed system. Typically, 10 mg catalyst and 20 mg eosin Y (EY) were added to a sealed reactor having a definite volume, and 30 mL 15% (V/V) triethanolamine (TEOA) aqueous solution was added to the above quartz reaction bottle. Then, the sample was evenly dispersed under ultrasound treatment, and the air in the sealed reactor was driven away by N2. Subsequently, this closed reaction system was illuminated for 5 h in a nine-channel photocatalytic reaction system (220 V AC/50 HZ) equipped with 5 W LED (PCX50B Discover, Beijing Bofeilai Technologies Co., Ltd, China; current: 0.70 A; lamp power: > 350 mW/cm2) as a light source to simulate solar energy (Fig. 1). The generated H2 was detected on a Tianmei GC7900 gas chromatograph (TCD, 13X column, 220 V/50 Hz, Shanghai Tianmei Scientific Instrument Co., Ltd, China).
Results and Discussion
Morphology and Structure
Figure 2 shows the morphology and structure of Ni-MOF-74, CoAl LDH, and CoAl LDH@Ni-MOF-74. Figure 2a shows that Ni-MOF-74 had a stereoscopic quadrilateral structure and stratified surface. The typical and regular dodecahedron hexahedron structure displays two large planes of congruence and symmetry and four unequal side planes, which provided good carriers for the loading of CoAl LDH nanosheets. Figure 2b displays the FESEM image of the as-prepared CoAl LDH. CoAl LDH exhibited a typical but inhomogeneous layer structure, and serious aggregation of CoAl LDH nanolayers was observed. However, the serious aggregation phenomenon was completely eliminated with the successful construction of CoAl LDH@Ni-MOF-74 hybrid (Fig. 2c). The FESEM images revealed that the CoAl LDH nanolayers were evenly and dispersedly loaded on the surface of Ni-MOF-74, which strongly reveals the successful assembly of Ni-MOF-74 and CoAl LDH with negative and positive surface potential, respectively. The successful construction of CoAl LDH@Ni-MOF-74 composite was also depicted in the TEM images (Fig. 2d). Both the quadrilateral shape of Ni-MOF-74 and layer structure of CoAl LDH can be observed, and the lattice fringe with d spacing of 0.25 nm was assigned to the (012) lattice planes of CoAl LDH (Fig. 2e). In addition, the mapping image revealed the existence of Ni, Al, and Co and their good distribution in CoAl LDH@Ni-MOF-74 hybrid (Fig. 2f).
XRD Investigation
Figure 3 shows the XRD patterns of all as-synthesized samples. Figure 3a shows that Ni-MOF-74 exhibited typical and sharp diffraction peaks, indicating the very high crystallinity of Ni-MOF-74. The representative diffraction peaks at 2θ = 9.3°, 11.8°, 15.8°, 18.6°, 23.8° corresponded well to the lattice faces of (100), (010), (101), (200), and (020), respectively ([Ni3(OH)2(C8H4O4)2(H2O)4]·2H2O, CCDC No. 638,866, space group: p-1) [25, 29,30,31,32]. Additionally, CoAl LDH unfolded representative diffraction peaks at 2θ = 11.4°, 23.1°, 34.5°, 59.9°, 61.2°, matching well with the corresponding lattice faces of (003), (006), (012), (110), and (0015) (PDF#51-45), respectively, which also coincided well with the published literature [33,34,35,36]. The diffraction intensity of CoAl LDH was lower compared with that of Ni-MOF-74, and this result may be relative to the anions in LDH or large layer spacing. Figure 3b shows the XRD patterns of CoAl@MOF-x samples, which showed that the diffraction peaks of Ni-MOF-74 almost disappeared in the patterns of CoAl@MOF-x hybrids mainly due to not only the low content of Ni-MOF-74 in the composites of CoAl LDH@Ni-MOF-74 but also the successful coverage of CoAl LDH nanosheets on the Ni-MOF-74 surface that obscured its original appearance. This result is shown in Fig. 2c, which expresses the successful construction of CoAl LDH@Ni-MOF-74 hybrids. Moreover, the successful preparation of CoAl LDH@Ni-MOF-74 compounds was embodied by the remarkable decrease in diffraction peaks intensity of CoAl LDH in CoAl@MOF-x hybrids with the increase in Ni-MOF-74 contents. Figure 3c exhibits a result consistent with that in Fig. 3b, which suggests the good structural stability of CoAl LDH@Ni-MOF-74 S-scheme heterojunction catalyst.
XPS Analysis
The chemical composition of elements on CoAl LDH@Ni-MOF-74 hybrid surface was detected by XPS technology (Fig. 4). The survey spectra validate the existence of Al, C, O, Ni, and Co on the surface of CoAl LDH@Ni-MOF-74, which implies the successful synthesis of CoAl LDH@Ni-MOF-74 hybrid. Figure 4b exhibits the high-resolution XPS spectrum of Ni 2p. The band energies at 856.1 and 873.7 eV were ascribed to Ni 2p3/2 and Ni 2p1/2 in Ni-MOF-74, respectively; the band energy at 861.9 eV corresponded to the satellite peak of Ni 2p3/2, and that at 879.9 eV corresponded to the satellite peak of Ni 2p1/2 [37,38,39]. As shown in Fig. 4c, the high-resolution XPS spectrum of Co 2p in CoAl LDH can be divided into six evident peaks with band energies of 781.1, 783.1, 786.6, 796.8, 798.1, and 803.4 eV; the band energy at 781.1 eV was attributed to Co 2p3/2 and that at 796.8 eV to Co 2p1/2 of Co2+; the band energies at 786.6 and 803.4 eV belonged to the corresponding satellite peaks of Co 2p3/2 and Co 2p1/2, respectively, and the band energy at 783.1 eV was indicative of cobalt(III), and that at 798.1 eV corresponded to cobalt(II) [40,41,42,43,44,45,46]. Figure 4d exhibits the high-resolution XPS spectrum of Al 2p in CoAl LDH. The band energies at 68.0 and 74.1 eV were assigned to Al 2p3/2 and Al(OH)3 species, respectively.
Optical Property Analysis
Figure 5 shows the UV–Vis diffuse reflectance spectra and UV–Vis absorption spectra of CoAl LDH, Ni-MOF-74, and CoAl@MOF-2 samples. Figure 5a reveals that the introduction of CoAl LDH can greatly improve the absorbance intensity of the hybrid, and this condition is related to the intrinsic gray of CoAl LDH, which induced the black color of CoAl@MOF-2. In addition, the absorbance intensity of Ni-MOF-74 was lower compared with that of CoAl LDH, which implies that the loading of CoAl LDH can greatly enhance the light absorption of the catalyst. Meanwhile, CoAl LDH still exhibited an enhanced light absorption intensity in the EY sensitization system (photocatalytic hydrogen evolution reaction system; Fig. 5b), and the response of CoAl@MOF-2 to light mainly depended on the light absorption of CoAl LDH, which reveals the excellent optical properties of CoAl LDH for efficient photocatalytic hydrogen generation in the constructed EY sensitization reaction system. Evidently, CoAl@MOF-2 showed higher light absorption than Ni-MOF-74, which may suggest that the introduction of CoAl LDH improved the light absorption performance of Ni-MOF-74, thereby enhancing the photocatalytic hydrogen production activity. Figure 5b illustrates that all the catalysts showcased similar absorption curves from 400 to 600 nm, showing the visible light response range of EY dye [45]. The maximum absorption wavelength was observed at 518 nm, which was assigned to the n–π* transition of C=O bond in the EY molecular structure [46, 47].
Photocatalytic H2 Evolution, PL (Photoluminescence) Analysis, and Zeta Potential Test
The hydrogen evolution properties of CoAl LDH, Ni-MOF-74, and CoAl@MOF-2 under visible light irradiation in the reaction environment of pH 10 were compared (Fig. 6a). CoAl LDH and Ni-MOF-74 separately exhibited poor photocatalytic hydrogen generation activity due to the low light-driven separation and transfer efficiency of electron–hole pairs. However, CoAl LDH@Ni-MOF-74 showed more excellent photocatalytic activity than either CoAl LDH or Ni-MOF-74 LDH alone, suggesting the intense interaction between CoAl LDH and Ni-MOF-74 that drove hydrogen evolution. The hydrogen evolution yields of Ni-MOF-74 and CoAl LDH were 64 and 34 µmol, respectively. CoAl@MOF-2 exhibited hydrogen evolution activities that were 3.3 and 6.2 times those of Ni-MOF-74 and CoAl LDH, respectively. The high photocatalytic hydrogen evolution activity of CoAl@MOF-2 was mainly due to the intense interaction between CoAl LDH and Ni-MOF-74, which promoted the rapid separation and migration of charge carriers under visible light excitation. The introduction of CoAl LDH also improved the light absorption of Ni-MOF-74. In addition, the introduction of Ni-MOF-74 greatly inhibited the agglomeration of CoAl LDH nanolayers, which also aided in electron migration during the photocatalytic water-splitting reaction. The matching energy levels of CoAl LDH and Ni-MOF-74 allowed the construction of an S-scheme heterojunction between CoAl LDH and Ni-MOF-74, which substantially improved the separation efficiency of electron–hole pairs in space. Consequently, the coupling of CoAl LDH and Ni-MOF-74 exhibited the higher photocatalytic hydrogen generation efficiency compared with CoAl LDH or Ni-MOF-74 alone. The specific migration process of light-excited electrons between CoAl LDH and Ni-MOF-74 will be discussed in detail in the hydrogen evolution mechanism section. Figure 6b shows the photocatalytic hydrogen production properties of CoAl LDH@Ni-MOF-74 composite catalysts containing different contents of Ni-MOF-74. CoAl LDH@Ni-MOF-74 with 0.10 g Ni-MOF-74 showed an optimum hydrogen generation performance under visible light irradiation, which indicates the highest efficiency of spatial separation and migration of electrons for CoAl@MOF-2 catalyst. The mass ratio of Ni-MOF-74 to CoAl LDH is vital for the formation of S-scheme heterojunction of the most favorable for the spatial transfer of charges. In addition, the stability testing of CoAl@MOF-2 for the photocatalytic evolution activity also was performed (Fig. 6c). The added amount of EY was 5 mg in each cycle to avoid the shielding effect of excessive EY on the photocatalytic activity of hydrogen evolution. CoAl@MOF-2 exhibited good stability for long-term photocatalytic hydrogen evolution, and this result suggests the significant effect of EY on the photocatalytic water-splitting reaction to evolve hydrogen. Figure 6d displays the hydrogen evolution activity of CoAl@MOF-2 in different reaction conditions. Evidently, CoAl@MOF-2 catalyst cannot evolve hydrogen in these conditions (without light, EY, and TEOA), suggesting that CoAl@MOF-2 has strong selectivity for hydrogen evolution systems.
a Comparison of H2 evolution activity of CoAl LDH, Ni-MOF-74, and CoAl@MOF-2 samples; b comparison of H2 evolution activity of CoAl@MOF-x samples; c stability testing of CoAl@MOF-2; d hydrogen evolution of CoAl@MOF-2 in different reaction conditions; e steady-state fluorescence spectrum of EY, CoAl LDH, Ni-MOF-74, and CoAl@MOF-2 samples; f zeta potential of CoAl LDH and Ni-MOF-74
The steady-state fluorescence spectra of CoAl LDH, Ni-MOF-74, and CoAl LDH@Ni-MOF-74 were measured to further understand the accelerated separation and transfer of charge carriers in CoAl LDH@Ni-MOF-74 heterojunction catalyst (Fig. 6e). EY displayed a maximal fluorescence emission intensity, which indicates the most serious recombination of electron–hole pairs. However, the fluorescence emission intensity was lower compared with that of EY when CoAl LDH, Ni-MOF-74, or CoAl LDH@Ni-MOF-74 samples were introduced, which manifested the intense interaction between EY and catalyst, resulting in the fluorescence quenching of light-excited EY. However, the fluorescence emission intensity was the lowest when CoAl LDH@Ni-MOF-74 heterojunction catalyst was added to the EY reaction system, which suggests that the rational design and successful construction of the S-scheme heterojunction accelerated charge migration and greatly inhibited the recombination of electron–hole pairs. Consequently, the hydrogen evolution activity was efficiently improved.
Figure 6f shows the mean zeta potential of CoAl LDH and Ni-MOF-74. CoAl LDH exhibited a mean zeta potential of about 21.0 mV, and Ni-MOF-74 presented a mean zeta potential about − 14.7 mV. This pair of opposite surface potentials can strengthen the coupling force between CoAl LDH and Ni-MOF-74. Ni-MOF-74, with a negative surface zeta potential and larger size compared with CoAl LDH, can enhance the proton absorption of CoAl LDH@Ni-MOF-74, which is conducive to promoting the efficiency of hydrogen evolution under visible light.
Photoelectrochemical Measures
The photocurrent–time response curves of CoAl LDH@FTO, Ni-MOF-74@FTO, and CoAl@MOF-2@FTO electrodes were determined to further gain insights into the important effect of the constructed S-scheme heterojunction and the band alignment between CoAl LDH and Ni-MOF-74 during charge transfer and the superiority of CoAl LDH@Ni-MOF-74 heterojunction photomaterial for efficient hydrogen production reaction. As shown in Fig. 7a, the CoAl@MOF-2@FTO electrode exhibited a stronger photocurrent intensity compared with the CoAl LDH@FTO and Ni-MOF-74@FTO electrodes, which indicates that the architecture of the S-scheme CoAl LDH@Ni-MOF-74 heterojunction catalyst can enhance the separation and migration of charge carriers. This condition implies the excellent hydrogen evolution properties of CoAl LDH@Ni-MOF-74 under visible light irradiation, consistent with the results of the steady-state fluorescence and hydrogen evolution kinetics.
The same result was obtained in linear sweep voltammetry (LSV) measures (Fig. 7b). The three electrodes displayed cathodic currents in the interval from − 0.7 to − 0.3 V versus the reversible hydrogen electrode, and this result was ascribed to the H2 evolution reaction [47, 48]. The CoAl LDH@FTO electrode expressed the smallest cathodic current response compared with Ni-MOF-74@FTO and CoAl@MOF-2@FTO electrodes. Thus, CoAl LDH can be considered as a prominent carrier to construct advanced catalytic materials. However, the CoAl@MOF-2@FTO electrode exhibited the largest cathodic current response, which indicates that the construction of the S-scheme heterojunction by coupling CoAl LDH and Ni-MOF-74 efficiently enhanced the photocatalytic water-splitting activity.
Figure 7c, d shows the Mott–Schottky plots of Ni-MOF-74 and CoAl LDH. The positive slopes of the E–C−2 plots reveal that Ni-MOF-74 and CoAl LDH are n-type semiconductor. The flat-band potentials (Efb) of Ni-MOF-74 and CoAl LDH were − 0.49 and − 0.66 V, respectively [49]. The conduction band (CB) potential (ECB) for n-type semiconductor was more negative at approximately 0.1 or 0.2 V. The ECB of Ni-MOF-74 and CoAl LDH was − 0.69 and − 0.86 V versus SCE; that is, the ECB of Ni-MOF-74 and CoAl LDH was − 0.45 and − 0.62 V versus normal hydrogen electrode (NHE) (ENHE = ESCE + 0.241 V). The band gaps (Eg) of 2.37 and 2.40 eV for Ni-MOF-74 and CoAl LDH were obtained in Refs. [34, 37, 50], respectively. The valence-band (VB) potentials (EVB) of Ni-MOF-74 and CoAl LDH were 1.92 and 1.78 eV, respectively (EVB = ECB + Eg).
Mechanism Analysis of Hydrogen Evolution Reaction
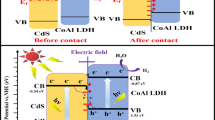
Based on the internal migration mechanism of S-scheme heterojunction photocatalyst [51, 52], the electron migration process of the CoAl LDH@Ni-MOF-74 S-scheme heterojunction catalyst for efficient photocatalytic hydrogen evolution was depicted. CoAl LDH had higher CB and VB positions and smaller work function relative to Ni-MOF-74 (Fig. 8). When both semiconductors were in close contact, the electrons in CoAl LDH spontaneously diffused to Ni-MOF-74, creating electron depletion and accumulation layers near the interface in CoAl LDH and Ni-MOF-74. The Ni-MOF-74 is negatively charged and CoAl LDH positively charged. Concurrently, an internal electric field directing from CoAl LDH to Ni-MOF-74 was formed (Fig. 8). This internal electric field accelerated the transfer of photogenerated electrons from Ni-MOF-74 to CoAl LDH, instead of the other way round. When Ni-MOF-74 and CoAl LDH came into contact, their Fermi energy should be aligned to the same level. This phenomenon led to upward and downward shifts in the Fermi levels of Ni-MOF-74 and CoAl LDH, respectively. Band bending promoted the recombination of photogenerated electrons in the CB of Ni-MOF-74 and holes in the VB of CoAl LDH at the interface region, which can be understood by considering how water flows downhill. The photogenerated electrons in the CB of Ni-MOF-74 and holes in the VB of CoAl LDH recombined at the interface under the Coulombic attraction between holes and electrons. In summary, these factors, namely the internal electric field, band bending, and Coulombic attraction, acted as driving forces for the recombination of electrons in the CB of Ni-MOF-74 and holes in the VB of CoAl LDH. Consequently, the useless electrons and holes were eliminated through recombination, whereas the powerful photogenerated electrons in the CB of CoAl LDH and the holes in the VB of Ni-MOF-74 were preserved to engage in photocatalytic reactions; that is, the aggregated holes on the VB of Ni-MOF-74 were consumed by the sacrificial reagent TEOA immediately. Simultaneously, the ground-state EY molecule was excited, and the excited-state EY (EY−·) was formed. The charges from EY−· mainly transferred to the CB of CoAl LDH due to the strong competition between the energy levels of CoAl LDH and Ni-MOF-74. The reduction potential of CB of CoAl LDH was stronger than that of Ni-MOF-74. Finally, the electrons on the CB of CoAl LDH combined with H+ to produce H2.
Conclusions
The delicate S-scheme heterojunction photocatalyst, that is, CoAl LDH@Ni-MOF-74, was rationally designed and successfully constructed by coupling Ni-MOF-74 with CoAl LDH based on their excellent characteristic for accelerating the spatial separation and migration of electrons and holes and promoting hydrogen evolution reaction under visible light irradiation. CoAl LDH nanolayers were successfully loaded onto the surface of Ni-MOF-74 by electrostatic self-assembly in the hydrothermal system. The introduction of CoAl LDH greatly improved the light absorption performance of CoAl LDH@Ni-MOF-74 S-scheme heterojunction photocatalyst. CoAl LDH@Ni-MOF-74 also showed a low steady-state fluorescence emission intensity and high light-driven photocurrent response, which indicates that the architecture of CoAl LDH@Ni-MOF-74 S-scheme heterojunction photocatalyst efficiently inhibits the recombination of electron–hole pairs and greatly boosts the photocatalytic hydrogen evolution reaction.
References
Zhao CX, Chen ZP, Shi R et al (2020) Recent advances in conjugated polymers for visible-light-driven water splitting. Adv Mater 32(28):1907296
Yang XF, Tian L, Zhao XL et al (2019) Interfacial optimization of g-C3N4-based Z-scheme heterojunction toward synergistic enhancement of solar-driven photocatalytic oxygen evolution. Appl Catal B Environ 244:240–249
Zhang LJ, Hao XQ, Li YB et al (2020) Performance of WO3/g-C3N4 heterojunction composite boosting with NiS for photocatalytic hydrogen evolution. Appl Surf Sci 499:143862
Zhang BS, Xu WW, Lu ZY et al (2020) Recent progress on carbonaceous material engineering for electrochemical hydrogen peroxide generation. Trans Tianjin Univ 26(3):188–196
Li CX, Zhang QJ, Zeng AW (2019) A novel method for synthesizing p-benzoquinone by direct catalytic oxidation of benzene with hydrogen peroxide over copper-doped TS-1. Trans Tianjin Univ 25(5):517–526
Cao ZJ, Liu HT, Huang WL et al (2020) Hydrogen bonding-assisted synthesis of silica/oxidized mesocarbon microbeads encapsulated in amorphous carbon as stable anode for optimized/enhanced lithium storage. Trans Tianjin Univ 26(1):13–21
Wang YH, Gao KG, Ye CL et al (2019) Highly dispersed Pd nanoparticles supported on Zr-doped MgAl mixed metal oxides for 2-ethylanthraquinone hydrogenation. Trans Tianjin Univ 25(6):576–585
Wang N, Ma QQ, Yuan EX et al (2019) Hydrogenation of alkylanthraquinone over pore-expanded and channel-shortened Pd/SBA-15. Trans Tianjin Univ 25(6):595–602
Zhang LJ, Hao XQ, Li JK et al (2020) Unique synergistic effects of ZIF-9(Co)-derived cobalt phosphide and CeVO4 heterojunction for efficient hydrogen evolution. Chin J Catal 41(1):82–94
Xiao R, Zhao CX, Zou ZY et al (2020) In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl Catal B Environ 268:118382
Xiang QJ, Yu JG, Jaroniec M (2011) Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J Phys Chem C 115(15):7355–7363
Zheng M, Cao XH, Ding Y et al (2018) Boosting photocatalytic water oxidation achieved by BiVO4 coupled with iron-containing polyoxometalate: analysis the true catalyst. J Catal 363:109–116
Li YB, Jin ZL, Zhang LJ et al (2019) Controllable design of Zn–Ni–P on g-C3N4 for efficient photocatalytic hydrogen production. Chin J Catal 40(3):390–402
Li XJ, Du DF, Zhang Y et al (2017) Layered double hydroxides toward high-performance supercapacitors. J Mater Chem A 5(30):15460–15485
Zhao YF, Jia XD, Waterhouse GIN et al (2016) Layered double hydroxide nanostructured photocatalysts for renewable energy production. Adv Energy Mater 6(6):1501974
Sun YF, Gao S, Xie Y (2014) Atomically-thick two-dimensional crystals: electronic structure regulation and energy device construction. Chem Soc Rev 43(2):530–546
Liang HF, Meng F, Cabán-Acevedo M et al (2015) Hydrothermal continuous flow synthesis and exfoliation of NiCo layered double hydroxide nanosheets for enhanced oxygen evolution catalysis. Nano Lett 15(2):1421–1427
Lv L, Sun PD, Gu ZY et al (2009) Removal of chloride ion from aqueous solution by ZnAl–NO3 layered double hydroxides as anion-exchanger. J Hazard Mater 161(2–3):1444–1449
D’Souza OJ, Mascarenhas RJ, Thomas T et al (2013) Electrochemical determination of l-tryptophan based on a multiwall carbon nanotube/Mg–Al layered double hydroxide modified carbon paste electrode as a sensor. J Electroanal Chem 704:220–226
Zhang Y, Du DF, Li XJ et al (2017) Electrostatic self-assembly of sandwich-like CoAl-LDH/polypyrrole/graphene nanocomposites with enhanced capacitive performance. ACS Appl Mater Interfaces 9(37):31699–31709
Li SS, Cheng PP, Luo JX et al (2017) High-performance flexible asymmetric supercapacitor based on CoAl-LDH and rGO electrodes. Nano Micro Lett 9:31
Yang GS, Takei T, Yanagida S et al (2019) Enhanced supercapacitor performance based on CoAl layered double hydroxide-polyaniline hybrid electrodes manufactured using hydrothermal-electrodeposition technology. Molecules 24(5):976
Férey G (2008) Hybrid porous solids: past, present, future. Chem Soc Rev 37(1):191–214
Zhao D, Timmons DJ, Yuan DQ et al (2011) Tuning the topology and functionality of metal–organic frameworks by ligand design. Acc Chem Res 44(2):123–133
Zhang YK, Wang GR, Ma W et al (2018) CdS p–n heterojunction co-boosting with Co3O4 and Ni-MOF-74 for photocatalytic hydrogen evolution. Dalton Trans 47(32):11176–11189
He SQ, He SY, Bo X et al (2018) Porous Ni2P/C microrods derived from microwave-prepared MOF-74-Ni and its electrocatalysis for hydrogen evolution reaction. Mater Lett 231:94–97
Wang MM, Lin MT, Li JT et al (2017) Metal–organic framework derived carbon-confined Ni2P nanocrystals supported on graphene for an efficient oxygen evolution reaction. Chem Commun 53(59):8372–8375
Sun DR, Sun FX, Deng XY et al (2015) Mixed-metal strategy on metal–organic frameworks (MOFs) for functionalities expansion: Co substitution induces aerobic oxidation of cyclohexene over inactive Ni-MOF-74. Inorg Chem 54(17):8639–8643
Jiao Y, Pei J, Yan CS et al (2016) Layered nickel metal–organic framework for high performance alkaline battery-supercapacitor hybrid devices. J Mater Chem A 4(34):13344–13351
Jiang XT, Zhang LJ, Liu SX et al (2018) Ultrathin metal-organic framework: an emerging broadband nonlinear optical material for ultrafast photonics. Adv Opt Mater 6:1800561
Li GC, Liu PF, Liu R et al (2016) MOF-derived hierarchical double-shelled NiO/ZnO hollow spheres for high-performance. Dalton Trans 45:13311–13316
Zhang LJ, Zhang YY, Huang SL et al (2018) Co3O4/Ni-based MOFs on carbon cloth for flexible alkaline battery-supercapacitor hybrid devices and near-infrared photocatalytic hydrogen evolution. Electrochim Acta 281:189–197
Jo WK, Tonda S (2019) Novel CoAl-LDH/g-C3N4/RGO ternary heterojunction with notable 2D/2D/2D configuration for highly efficient visible-light-induced photocatalytic elimination of dye and antibiotic pollutants. J Hazard Mater 368:778–787
Li YB, Wang GR, Wang YB et al (2020) Phosphating 2D CoAl LDH anchored on 3D self-assembled NiTiO3 hollow rods for efficient hydrogen evolution. Catal Sci Technol 10(9):2931–2947
Wang YN, Dou LG, Zhang H (2017) Nanosheet array-like palladium-catalysts pdx/rGO@CoAl-LDH via lattice atomic-confined in situ reduction for highly efficient heck coupling reaction. ACS Appl Mater Interfaces 9(44):38784–38795
Liu ZP, Ma RZ, Osada M et al (2006) Synthesis, anion exchange, and delamination of Co–Al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J Am Chem Soc 128(14):4872–4880
Li M, Li JK, Jin ZL (2020) 0D/2D spatial structure of CdxZn1–xS/Ni-MOF-74 for efficient photocatalytic hydrogen evolution. Dalton Trans 49(16):5143–5156
Xu JX, Qi YH, Wang WB et al (2019) Montmorillonite-hybridized g-C3N4 composite modified by NiCoP cocatalyst for efficient visible-light-driven photocatalytic hydrogen evolution by dye-sensitization. Int J Hydrog Energy 44(8):4114–4122
Ye P, Liu XL, Iocozzia J et al (2017) A highly stable non-noble metal Ni2P co-catalyst for increased H2 generation by g-C3N4 under visible light irradiation. J Mater Chem A 5(18):8493–8498
Kumar S, Isaacs MA, Trofimovaite R et al (2017) P25@CoAl layered double hydroxide heterojunction nanocomposites for CO2 photocatalytic reduction. Appl Catal B 209:394–404
Zeng HX, Zhang HJ, Deng L et al (2020) Peroxymonosulfate-assisted photocatalytic degradation of sulfadiazine using self-assembled multi-layered CoAl-LDH/g-C3N4 heterostructures: performance, mechanism and eco-toxicity evaluation. J Water Process Eng 33:101084
Jiang SD, Song L, Zeng WR et al (2015) Self-assembly fabrication of hollow mesoporous silica@Co–Al layered double hydroxide@graphene and application in toxic effluents elimination. ACS Appl Mater Interfaces 7(16):8506–8514
Li HY, Hao XQ, Liu Y et al (2020) ZnxCd1−xS nanoparticles dispersed on CoAl-layered double hydroxide in 2D heterostructure for enhanced photocatalytic hydrogen evolution. J Colloid Interface Sci 572:62–73
Wang KF, Zhang L, Su Y et al (2018) Photoreduction of carbon dioxide of atmospheric concentration to methane with water over CoAl-layered double hydroxide nanosheets. J Mater Chem A 6(18):8366–8373
Li Z, Wu YQ, Lu GX (2016) Highly efficient hydrogen evolution over Co(OH)2 nanoparticles modified g-C3N4 co-sensitized by Eosin Y and Rose Bengal under Visible Light Irradiation. Appl Catal B Environ 188:56–64
Li YB, Jin ZL, Zhao TS (2020) Performance of ZIF-67–derived fold polyhedrons for enhanced photocatalytic hydrogen evolution. Chem Eng J 382:123051
Guo SE, Deng ZP, Li MX et al (2016) Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew Chem Int Ed 55(5):1830–1834
Hou YD, Laursen AB, Zhang JS et al (2013) Layered nanojunctions for hydrogen-evolution catalysis. Angew Chem Int Ed 52:3621–3625
Tan L, Xu S-M, Wang ZL et al (2019) Highly selective photoreduction of CO2 with suppressing H2 evolution over monolayer layered double hydroxide under irradiation above 600 nm. Angew Chem Int Ed 58:11860–11867
Li HY, Li J, Xu CC et al (2017) Hierarchically porous MoS2/CoAl-LDH/HCF with synergistic adsorption-photocatalytic performance under visible light irradiation. J Alloy Compd 698:852–862
Fu JW, Xu QL, Low J et al (2019) Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl Catal B Environ 243:556–565
Xu QL, Zhang LY, Cheng B et al (2020) S-Scheme heterojunction photocatalyst. Chem 6:1543–1559
Acknowledgements
This work was financially supported by the Natural Science Foundation of the Ningxia Hui Autonomous Region (No. 2020AAC02026).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, Z., Li, Y. & Ma, Q. CoAl LDH@Ni-MOF-74 S-Scheme Heterojunction for Efficient Hydrogen Evolution. Trans. Tianjin Univ. 27, 127–138 (2021). https://doi.org/10.1007/s12209-020-00269-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-020-00269-1