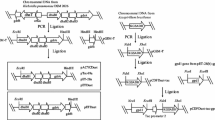
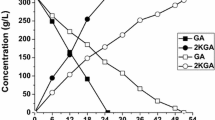
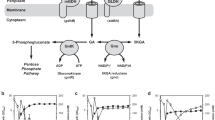
Abstract
1,3-Dihydroxyacetone (DHA), a natural ketose, is widely used in the chemical, cosmetic, and pharmaceutical industries. The current method for DHA production is Gluconobacter oxydans (G. oxydans) fermentation, but the high concentration of glycerol in the fermentation broth inhibits cells growth. To overcome this obstacle, in this study, we overexpressed the glycerol transporter (GlpFp) by the use of promoters PtufB, Pgmr, Pglp1, and Pglp2 in G. oxydans 621H. The results show that the glycerol tolerances of strains overexpressing GlpF were all much better than that of the control strain. The glycerol dehydrogenase gene (Gdh) was overexpressed by the promoters PtufB and Pgdh, which increased the DHA titer by 12.7% compared with that of the control group. When GlpF and Gdh genes were co-overexpressed in G. oxydans 621H, the OD600 value of the engineered strains all increased, but the DHA titers decreased in different degrees, as compared with strains that overexpressed only Gdh. This study provides a reference for future research on DHA production.







Similar content being viewed by others
References
Meybeck A (1977) A spectroseopic study of reaction products of dihydroxyacetone with aminoacids. J Soc Cosmet Chem 28:25–35
Bobin MF, Martii MC, Cotte J (1984) Effect of color adjuvants on the tanning effect of dihydroxyacetone. J Soc Cosmet Chem 35:265–272
Fesq H, Brockow K, Strom K et al (2001) Dihydroxyacetone in a new formulation: a powerful therapeutic option in vitiligo. Dermatology 203(3):241–243. https://doi.org/10.1159/000051757
Stanko RT, Robertson RJ, Spina RJ et al (1990) Enhancement of arm exercise endurance capacity with dihydroxyacetone and pyruvate. J Appl Physiol 68(1):119
Ivy JL (1998) Effect of pyruvate and dihydroxyacetone on metabolism and aerobic endurance capacity. Med Sci Sports Exerc 30(6):837–843
Schlifke AC (1999) Can pyruvate and dihydroxyacetone(DHA) improve athletic performance. Nutr Bytes 5:1–5
Niknahad H, Ghelichkhani E (2002) Antagonism of cyanide poisoning by dihydroxyacetone. Toxicol Lett (Shannon) 132(2):95–100
Cummings TF (2004) The treatment of cyanide poisoning. Occup Med 54(2):82–85
Oh CH, Hong JH (2007) Short synthesis and antiviral evaluation of c-fluoro-branched cyclopropyl nucleosides. Nucleosides, Nucleotides Nucleic Acids 26(4):403–411
Henderson PW, Kadouch DJ, Singh SP et al (2010) A rapidly resorbable hemostatic biomaterial based on dihydroxyacetone. J Biomed Mater Res Part A 93(2):776–782
Hekmat D, Bauer R, Fricke J (2003) Optimization of the microbial synthesis of dihydroxyacetone from glycerol with Gluconobacter oxydans. Bioprocess Biosyst Eng 26(2):109–116
Green SR, Whalen EA, Molokie E (1961) Dihydroxyacetone: production and uses. Biotechnol Bioeng 3(4):351–355
Tanamool V, Hongsachart P, Soemphol W (2018) Bioconversion of biodiesel-derived crude glycerol to 1, 3-dihydroxyacetone by a potential acetic acid bacteria. Sains Malays 47(3):481–488
Dikshit PK, Moholkar VS (2016) Kinetic analysis of dihydroxyacetone production from crude glycerol by immobilized cells of Gluconobacter oxydans MTCC 904. Bioresour Technol 216:948–957
Claret C, Bories A, Soucaille P (1992) Glycerol inhibition of growth and dihydroxyacetone production by Gluconobacter oxydans. Curr Microbiol 25(3):149–155
Dikshit PK, Kharmawlong GJ, Moholkar VS (2018) Investigations in sonication–induced intensification of crude glycerol fermentation to dihydroxyacetone by free and immobilized Gluconobacter oxydans. Biores Technol 256:302–311
Stasiak-Różańska L, Berthold-Pluta A, Dikshit PK (2018) Valorization of waste glycerol to dihydroxyacetone with biocatalysts obtained from Gluconobacter oxydans. Appl Sci 8(12):2517
Bauer R, Katsikis N, Varga S et al (2005) Study of the inhibitory effect of the product dihydroxyacetone on Gluconobacter oxydans in a semi-continuous two-stage repeated-fed-batch process. Bioprocess Biosyst Eng 28(1):37–43
Claret C, Bories A, Soucaille P (1993) Inhibitory effect of dihydroxyacetone on Gluconobacter oxydans: kinetic aspects and expression by mathematical equations. J Ind Microbiol 11(2):105–112
Dikshit PK, Moholkar VS (2018) Batch and repeated-batch fermentation for 1,3-dihydroxyacetone production from waste glycerol using free, immobilized and resting Gluconobacter oxydans cells. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0307-9
Gätgens C, Degner U, Bringer-Meyer S et al (2007) Biotransformation of glycerol to dihydroxyacetone by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 76(3):553–559
Habe H, Fukuoka T, Morita T et al (2010) Disruption of the membrane-bound alcohol dehydrogenase-encoding gene improved glycerol use and dihydroxyacetone productivity in Gluconobacter oxydans. Biosci Biotechnol Biochem 74(7):1391–1395
Calmes R, Deal SJ (1972) Glycerol transport by Nocardia asteroides. Can J Microbiol 18(11):1703–1708
Richey DP, Lin EC (1972) Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol 112(2):784–790
Saheb SA (1972) Perméation du glycérol et sporulation chez Bacillus subtilis. Can J Microbiol 18(8):1307–1313
Stasiak-Różańska L, Błażejak S, Gientka I (2014) Effect of glycerol and dihydroxyacetone concentrations in the culture medium on the growth of acetic acid bacteria Gluconobacter oxydans ATCC 621. Eur Food Res Technol 239(3):453–461
Claret C, Salmon JM, Romieu C et al (1994) Physiology of Gluconobacter oxydans during dihydroxyacetone production from glycerol. Appl Microbiol Biotechnol 41(3):359–365
Hedfalk K, Bill RM, Hohmann S et al (2000) Overexpression and purification of the glycerol transport facilitators, fps1p and GlpF, in Saccharomyces Cerevisiae and Escherichia coli. Mol Biol Physiol Water Solut Transp. https://doi.org/10.1007/978-1-4615-1203-5_4
Wei DZ, Ma XY, Zheng Y et al (2007) Gene engineering bacteria for producing dihydroxy acetone, constructing method, and application: CN, 101092604A. 2007-12-26 (in Chinese)
Saito Y, Ishii Y, Hayashi H et al (1997) Cloning of genes coding for L-sorbose and L-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-L-gulonate, a precursor of L-ascorbic acid, in a recombinant G. oxydans strain. Appl Environ Microbiol 63(2):454–460
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166(4):557–580
Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41(3):459–472
Yang XP, Wei LJ, Lin JP et al (2008) Membrane-bound pyrroloquinoline quinone-dependent dehydrogenase in Gluconobacter oxydans M5, responsible for production of 6-(2-hydroxyethyl) amino-6-deoxy-L-sorbose. Appl Environ Microbiol 74(16):5250–5253
Kovach ME, Elzer PH, Steven Hill D et al (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166(1):175–176
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci 76(4):1648–1652
Xue CY, Zhang XM, Yu ZR et al (2013) Up-regulated spinosad pathway coupling with the increased concentration of acetyl-CoA and malonyl-CoA contributed to the increase of spinosad in the presence of exogenous fatty acid. Biochem Eng J 81:47–53
Sugisawa T, Hoshino T (2002) Purification and properties of membrane-bound D-sorbitol dehydrogenase from Gluconobacter suboxydans IFO 3255. Biosci Biotechnol Biochem 66(1):57–64
Li MH, Wu J, Liu X et al (2010) Enhanced production of dihydroxyacetone from glycerol by overexpression of glycerol dehydrogenase in an alcohol dehydrogenase-deficient mutant of Gluconobacter oxydans. Bioresour Technol 101(21):8294–8299
Acknowledgements
This work was supported by the Major Research Plan of Tianjin (16YFXTSF00460).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, J., Yang, X. & Lu, W. Research of 1,3-Dihydroxyacetone Production by Overexpressing Glycerol Transporter and Glycerol Dehydrogenase. Trans. Tianjin Univ. 25, 549–558 (2019). https://doi.org/10.1007/s12209-019-00207-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-019-00207-w