Abstract
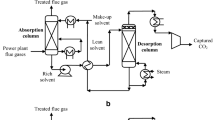
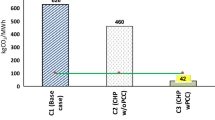
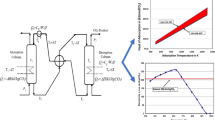
Efficient integration of the carbon capture and storage (CCS) process in power generation plants can help reduce global CO2 emissions. Hydrate technology has emerged as one of the most promising technologies for the separation and sequestration of CO2. This paper compares the process energy consumption of different CO2 capture techniques, gas transportation, and sequestration methods integrated into a pre-combustion power plant. Process modeling of a conceptualized hydrate technology-based CCS process is simulated using a coupled TRNSYS simulation software and engineering equation solver. The performance in efficiency, energy consumption, and potential energy penalty is evaluated and analyzed for three study cases. Furthermore, the energy requirement for transporting and sequestrating CO2 in hydrate as hydrate slurry is evaluated and compared to supercritical transportation and sequestration. The results obtained show that the energy consumption of hydrate-based gas separation amounts to 70 % of the total energy consumption for hydrate-based CO2 capture, transportation, and sequestration.
Similar content being viewed by others
References
IPCC, Climate Change 2022 - Impacts, Adaptation and Vulnerability. Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, H.-O. Pörtner, D. C. Roberts, M. Tignor, E. S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.), Cambridge University Press, New York, USA (2023) https://doi.org/10.1017/9781009325844.
UNFCCC, Aggregate effect of the intended nationally determined contributions: an update, Marrakech Climate Change Conference, Marrakech, Morocco (2016).
IRENA, Perspectives for the Energy Transition: Investment Needs for a Low-Carbon Energy System, IEA Publications, IRENA Publications (2017).
J. Rogelj, M. Den Elzen, N. Höhne, T. Fransen, H. Fekete, H. Winkler, R. Schaeffer, F. Sha, K. Riahi and M. Meinshausen, Paris agreement climate proposals need a boost to keep warming well below 2 °C, Nature, 534(7609) (2016) 631–639, https://doi.org/10.1038/nature18307.
IEA, World Energy Outlook 2019, OECD (2019) https://doi.org/10.1787/caf32f3b-en.
International Energy Agency, Exploring Clean Energy Pathways: The Role of CO2 Storage, IEA Publications (2019).
H. Dashti and X. Lou, Gas hydrate-based CO2 separation process: quantitative assessment of the effectiveness of various chemical additives involved in the process, Z. Sun et al., Energy Technology 2018. TMS 2018. The Minerals, Metals & Materials Series, Springer, Cham (2018) https://doi.org/10.1007/978-3-319-72362-4_1.
N. H. Duc, F. Chauvy and J.-M. Herri, CO2 capture by hydrate crystallization - a potential solution for gas emission of steelmaking industry, Energy Convers. Manag., 48(4) (2007) 1313–1322, https://doi.org/10.1016/J.ENCONMAN.2006.09.024.
D. Yang, L. A. Le, R. J. Martinez, R. P. Currier and D. F. Spencer, Kinetics of CO2 hydrate formation in a continuous flow reactor, Chem. Eng. J., 172(1) (2011) 144–157, https://doi.org/10.1016/j.cej.2011.05.082.
J. Zheng, K. Bhatnagar, M. Khurana, P. Zhang, B.-Y. Zhang and P. Linga, Semiclathrate based CO2 capture from fuel gas mixture at ambient temperature: effect of concentrations of tetra-n-butylammonium fluoride (TBAF) and kinetic additives, Appl. Energy, 217 (2018) 377–389, https://doi.org/10.1016/j.apenergy.2018.02.133.
E. D. Sloan and C. A. Koh, Clathrate Hydrates of Natural Gases, Third Ed., Chemical Industries Series, CRC Press, 752 (2007) 2008, https://doi.org/10.1016/j.fuel.2008.03.028.
P. Babu, P. Linga, R. Kumar and P. Englezos, A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture, Energy, 85 (2015) 261–279, https://doi.org/10.1016/j.energy.2015.03.103.
S. Lee, L. Liang, D. Riestenberg, O. R. West, C. Tsouris and E. Adams, CO2 hydrate composite for ocean carbon sequestration, Environ. Sci. Technol., 37(16) (2003) 3701–3708, https://doi.org/10.1021/es026301l.
S. Oya, M. Aifaa and R. Ohmura, Formation, growth and sintering of CO2 hydrate crystals in liquid water with continuous CO2 supply: implication for subsurface CO2 sequestration, Int. J. Greenh. Gas Control, 63 (2017) 386–391, https://doi.org/10.1016/j.ijggc.2017.06.007.
A. U. Rehman and B. Lal, Gas hydrate-based CO2 capture: a journey from batch to continuous, Energies, 15(21) (2022) 8309, https://doi.org/10.3390/en15218309.
P. Anh and S. Alberto, Chemical promoter performance for CO2 hydrated growth: a molecular perspective, Energy Fuel, 37(8) (2023) 6002–6011.
M. Zhang, B. Sun, S. Liu, L. Chen, Y. Gao and Z. Wang, Review on cooperative effect of compound additives on CO2 hydrate formation: recent advances and future directions, Energy Fuels, 37 (2023) 5667–5688, https://doi.org/10.1021/acs.energyfuels.2c04144.
B. Prah and R. Yun, CO2 hydrate slurry transportation in carbon capture and storage, Applied Thermal Engineering (2018) 653–661, https://doi.org/10.1016/j.applthermaleng.2017.09.053.
J. S. Pandey, S. Khan and N. von Solms, Screening of low-dosage methanol as a hydrate promoter, Energies, 15 (2022) 6814, https://doi.org/10.3390/en15186814.
J. Oignet, A. Delahaye, J.-P. Torré, C. Dicharry, H. Hoang, M. P. Clain, V. Osswald, Z. Youssef and L. Fournaison, Rheological study of CO2 hydrate slurry in the presence of sodium dodecyl sulfate in a secondary refrigeration loop, Chem. Eng. Sci., 158 (2017) 294–303, https://doi.org/10.1016/j.ces.2016.10.018.
H. H. Hussain and H. Husin, Review on application of quaternary ammonium salts for gas hydrate inhibition, Applied Sciences, 10(3) (2020) 1011, https://doi.org/10.3390/app10031011.
H. Moradpour, An experimental and modelling investigation of the rheological properties of water/oil/gas hydrate mixtures, Ph.D. Thesis, Heriot-Watt University (2011).
K.-L. Yan, C.-Y. Sun, J. Chen, L.-T. Chen, D.-J. Shen, B. Liu, M.-L. Jia, M. Niu, Y.-N. Lv, N. Li, Z.-Y. Song, S.-S. Niu and G.-J. Chen, Flow characteristics and rheological properties of natural gas hydrate slurry in the presence of anti-agglomerant in a flow loop apparatus, Chem. Eng. Sci., 106 (2014) 99–108, https://doi.org/10.1016/j.ces.2013.11.015.
L. Xiaofang, Z. Jie, Z. Jiangwei, Z. Deyin, L. Yang, Z. Shidong, D. Hui and S. Shangfei, Study on the formation characteristics of CO2 hydrated and the rheological properties of slurry in flow system containing surfactants, ACS Omega, 7(2) (2022) 2444–2457.
B. Prah and R. Yun, Investigations on CO2 hydrate slurry for transportation in carbon capture and storage, J. Mech. Sci. Technol., 33(10) (2019) 5085–5092, https://doi.org/10.1007/s12206-019-0947-0.
F. Qanbari, M. Pooladi-Darvish, S. H. Tabatabaie and S. Gerami, CO2 disposal as hydrate in ocean sediments, J. Nat. Gas Sci. Eng., 8 (2012) 139–149, https://doi.org/10.1016/j.jngse.2011.10.006.
J. J. Rajnauth, A proposed workflow for disposal of CO2 using hydrate technology, SPE Europec/EAGE Annual Conference, Copenhagen, Denmark (2012).
A. C. C. Chow, E. E. E. Adams, P. H. H. Israelsson and C. Tsouris, Carbon dioxide hydrate particles for ocean carbon sequestration, Energy Procedia, 1(1) (2009) 4937–4944, https://doi.org/10.1016/j.egypro.2009.02.325.
IEAGHG, Gas Hydrates for Deep Ocean Storage of CO2, IEAGHG (2004).
Z. W. Ma, P. Zhang, H. S. Bao and S. Deng, Review of fundamental properties of CO2 hydrates and CO2 capture and separation using hydration method, Renewable and Sustainable Energy Reviews, 53 (2016) 1273–1302, https://doi.org/10.1016/j.rser.2015.09.076.
K. M. Sabil and B. Partoon, Recent advances on carbon dioxide capture through a hydrate-based gas separation process, Current Opinion in Green and Sustainable Chemistry, 11 (2018) 22–26, https://doi.org/10.1016/jcogsc.2018.03.006.
F. Casella and P. Colonna, Dynamic modeling of IGCC power plants, Appl. Therm. Eng., 35 (2012) 91–111, https://doi.org/10.1016/J.APPLTHERMALENG.2011.10.011.
Y. Wang, J. Wang, X. Luo, S. Guo, J. Lv and Q. Gao, Dynamic modelling and simulation of IGCC process with texaco gasifier using different coal, Systems Science & Control Engineering, 3(1) (2015) 198–210, https://doi.org/10.1080/21642583.2015.1010046.
D. Bhattacharyya, R. Turton and S. E. Zitney, Steady-state simulation and optimization of an integrated gasification combined cycle power plant with CO2 capture, Ind. Eng. Chem. Res., 50(3) (2011) 1674–1690, https://doi.org/10.1021/ie101502d.
R. Peampermpool, C. Teh, M. Tade, A. Qader and A. Barifcani, More Energy-Efficient CO2 Capture from IGCC GE Flue Gases, C-Journal of Carbon Research, 3(4) (2017) 7, https://doi.org/10.3390/c3010007.
S. Tam, M. Stanton, S. Ghose, G. Deepe, D. Spencer, R. Currier, J. Young, G. Anderson, L. Le and D. Devlin, A high pressure carbon dioxide separation process for IGCC plants, 1st Natl. Conf. Carbon Sequestration (2001).
G. Deppe, S. S. Tam, R. P. Currier, J. S. Young, G. K. Anderson, L. Le and D. F. Spencer, Developments in the simteche process - separation of co2 from coal syngas by formation of hydrates, 2nd Annual Conference on Carbon Ssequestration, Alexandria, Egypt (2013).
B. Castellani, F. Rossi, M. Filipponi and A. Nicolini, Hydrate-based removal of carbon dioxide and hydrogen sulphide from biogas mixtures: experimental investigation and energy evaluations, Biomass and Bioenergy, 70 (2014) 330–338, https://doi.org/10.1016/j.biombioe.2014.08.026.
H. Tajima, A. Yamasaki and F. Kiyono, Energy consumption estimation for greenhouse gas separation processes by clathrate hydrate formation, Energy, 29(11) (2004) 1713–1729.
N. N. Nguyen, V. T. La, C. D. Huynh and A. V. Nguyen, Technical and economic perspectives of hydrate-based carbon dioxide capture, Applied Energy, 307 (2022) 118237, https://doi.org/10.1016/j.apenergy.2021.118237.
TRNSYS 17, A Transient System Simulation Program. Volume 1, Getting Started, 17 ed., Univ. Wisconsin-Madison (2007).
Nexant Laboratory, Simteche Hydrate CO2 Capture Process, Nexant Llc (2006).
L. W. Diamond and N. N. Akinfiev, Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: evaluation of literature data and thermodynamic modelling, Fluid Phase Equilib., 208(1–2) (2003) 265–290, https://doi.org/10.1016/S0378-3812(03)00041-4.
W. Hao, J. Wang, S. Fan and W. Hao, Evaluation and analysis method for natural gas hydrate storage and transportation processes, Energy Convers. Manag., 49 (2008) (10) 2546–2553, https://doi.org/10.1016/J.ENCONMAN.2008.05.016.
S. Marinhas, A. Delahaye, L. Fournaison, D. Dalmazzone, W. Fürst and J.-P. Petitet, Modelling of the available latent heat of a CO2 hydrate slurry in an experimental loop applied to secondary refrigeration, Chem. Eng. Process. Process Intensif., 45(3) (2006) 184–192, https://doi.org/10.1016/j.cep.2005.08.002.
D. L. McCollum and J. M. Ogden, Techno-Economic Models for Carbon Dioxide Compression, Transport, and Storage & Correlations for Estimating Carbon Dioxide Density and Viscosity, Inst. Transp. Stud. California, Davis (2006).
IEAGHG, Pipeline Transmission of CO2 and Energy, IEAGHG (2002) PH4/6.
P. N. Seevam, J. M. Race, M. J. Downie and P. Hopkins, Transporting the next generation of CO2 for carbon, capture and storage: the impact of impurities on supercritical CO2 pipelines, 7th Int. Pipeline Conf., Alberta, Canada (2008) 39–51.
Z. Xue, D. Tanase and J. Watanabe, Estimation of CO2 saturation from time-lapse CO2 well logging in an onshore aquifer, nagaoka, Japan, Explor. Geophys., 37(1) (2006) 19, https://doi.org/10.1071/EG06019.
M. Ramezan, T. J. Skone and G. N. Liljedahl, Carbon Dioxide Capture from Existing Coal-Fired Power Plants Final Report, NETL (2007).
P. Maas, N. Nauels, L. Zhao, P. Markewitz, V. Scherer, M. Modigell, D. Stolten and J.-F. Hake, Energetic and economic evaluation of membrane-based carbon capture routes for power plant processes, Int. J. Greenh. Gas Control, 44 (2016) 124–139, https://doi.org/10.1016/J.IJGGC.2015.11.018.
M. M. J. Knoope, A. Ramírez and A. P. C. Faaij, A state-of-the-art review of techno-economic models predicting the costs of CO2 pipeline transport, Int. J. Greenh. Gas Control, 16 (2013) 241–270, https://doi.org/10.1016/j.ijggc.2013.01.005.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2016R1D1A1B02010075).
Author information
Authors and Affiliations
Corresponding author
Additional information
Rin Yun is a Professor of Department of Mechanical Engineering, Hanbat National University, Daejeon, South Korea. His research interests are utilizing Hydrothermal energy, natural refrigerants, transportation of Captured CO, and gas-hydrate as a secondary fluid.
Rights and permissions
About this article
Cite this article
Prah, B., Anokye, M. & Yun, R. Energy consumption analysis of hydrate-based technology in the carbon capture storage process. J Mech Sci Technol 37, 6727–6737 (2023). https://doi.org/10.1007/s12206-023-1140-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12206-023-1140-z