Abstract
Compared with a pulverized coal power plant, the integrated gasification combined cycle (IGCC) has several advantages, including, among others better environmental performance and low CO2 capture cost. Hot/warm CO2 removal from syngas has also been a subject of research due to its potentially higher thermal efficiency. In this study, we proposed a generic adsorption based hot/warm CO2 removal process for IGCC power plants. Through analyses of the proposed generic process we have demonstrated that higher temperature of the hot/warm CO2 removal process will results in larger heat of adsorption, which in turns may increase energy consumption of the process. Under most of the operating temperature range, hot/warm CO2 removal process will lead to more electricity loss compared to the baseline Selexol process. However, if the adsorption step takes place at a temperature close to or higher than the highest steam temperature in steam cycle, our analysis indicates that the process may lead to minimal electricity loss. The study also provided some other insights into the pathways for hot/warm CO2 removal process to improve its energy performance through process and sorbent designs.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
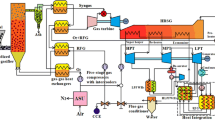
Integrated gasification combined cycle (IGCC) has several advantages compared with a pulverized coal (PC) power plant [1,2,3,4,5,6,7,8,9,10]. It has better environmental performance, less solid waste, lower water consumption, and capability of phased construction. More importantly, it will render a less expensive CO2 sequestration process. Because of these advantages, IGCC is considered as an enabling technology for clean coal processes for future power generation and transportation fuel—as well as for CO2 mitigation. Figure 1 is a schematic diagram of an IGCC power plant with CO2 capture.
Pre-combustion CO2 capture is preferred for IGCC power plants since the gasification systems operate under high pressure, which make CO2 capture equipment much smaller in size compared with the post-combustion configuration. To remove all the carbon in syngas as CO2, water–gas-shift (WGS) reaction is employed to convert CO to CO2 [11]:
WGS reaction is usually carried out at temperatures range from 250 to 450 °C (450 to 800 °F), depending on which WGS catalyst being used. The WGS reaction is slightly exothermic so low temperature favors the forward reaction. After WGS reaction, CO2 partial pressure in the syngas stream is significantly increased up to 10–30 atm.
There are several chemical processes in IGCC power plants as shown in Fig. 1. The first chemical process is the gasification of coal to produce syngas which takes place in the gasifier. The gasification process is carried out at very high temperature and the raw syngas coming out from a gasifier could have temperature as high as 1200 °C. The raw syngas is cooled by the heat recovery steam generator (HRSG), which generates steam for the steam cycle. The second major chemical process is the WGS reaction, which is required only if CO2 capture is requested. The last chemical process is the combustion of the syngas (or H2) in a gas turbine to generate electricity. The desired conditions for the final syngas before combustion turbine are around 260 °C and 20 atm. The operating conditions for these chemical processes are fixed and cannot be changed significantly. Between these chemical processes, there exist the gas cleanup processes including particulates removal, desulfurization process or acid gas removal (AGR) for H2S and AGR for CO2 if pre-combustion CO2 capture is desired. When the gasification is oxygen-blown, then an air separation unit (ASU) will also be required. Usually, the gas cleanup is carried out before the WGS reaction because sulfur in the raw syngas is often poisonous to the catalyst used in WGS. To minimize energy loss, it is desirable that the gas cleanup processes can be carried out between gasification temperature and the WGS shift reaction temperature. Similarly, a desirable CO2 removal temperature is between the WGS reaction temperature and gas turbine inlet temperature. A gas cleanup process operating between gasification temperature and WGS temperature and a CO2 removal process operating at a temperature between WGS and gas turbine inlet temperatures can eliminate extra cooling/heating of the syngas and thus improve the thermal and economic performance of an IGCC process. This is the incentive for the hot/warm gas cleanup and CO2 removal processes. When the WGS reaction temperature and the gas turbine inlet temperature are considered, a CO2 removal process at around 260 °C is the most suitable.
However, an absorption or adsorption process at a higher temperature will be more difficult to achieve. It will result in a higher operating cost in part due to higher energy consumption. Obviously, there is a trade-off between capital cost and operating cost for a hot/warm gas cleanup for CO2 removal.
For desulfurization process, the sulfur content is low (typically few percentage), hence, the operating cost will be relatively insignificant compared to the capital cost. Thus, a hot/warm desulfurization process may have an advantage to reduce capital cost by eliminating cooling/heating of the syngas and increase the thermal efficiency. However, CO2 is a bulk gas, and its concentration in the shifted syngas undergoing the water–gas-shift reaction can reach 40% by volume. The operating cost of a CO2 removal process will be significant compared to its capital cost. Thus, hot/warm gas cleanup is a viable option for desulfurization, but it may not have an economic advantage for CO2 removal.
Above argument is generally valid. The capital cost of a separation process is more significant for a low concentration contaminant removal and the operating cost is more significant for a high concentration substance.
In the following sections, we will analyze the energy consumption of a typical CO2 separation process to remove CO2 from the shifted syngas and then discuss the possibility of a hot/warm CO2 removal process.
2 A generic process for CO2 removal from shifted syngas
Many separation processes are available for CO2 removal from shifted syngas [12,13,14,15,16,17]. Absorption, adsorption, cryogenic (low temperature distillation) and membrane processes are all possible options. However, since our focus is on the hot/warm CO2 removal, we may be able to eliminate some of the processes. Cryogenic process performs at a low temperature. Absorption process uses a solvent (liquid) and it would be very difficult to find a solvent which can operate at the hot/warm temperatures unless molten salts are used as the absorption solution. Thus, only adsorption process and membrane process are possible options. Membrane processes, on the other hand, does not use steam but only the electricity and hence will not impact the steam cycle or gas turbine cycle and will not be analyzed here.
2.1 The generic adsorption process
Consider a conceptual adsorption process (see Fig. 2 for schematic process flow diagram) with the following reversible reaction:
Here, M is the active component of the sorbent, N is the reaction product and ΔH is the heat of adsorption (reaction) in the unit of kJ/kgCO2. The minus sign before ΔH means that heat has to be removed.
Since our interest is in hot/warm CO2 capture processes, the adsorption (reaction (2)) will take place at a high temperature (hot/warm temperature), it will require strong chemical interaction between M and CO2, that is, a very high ΔH. It is reasonable to assume that the adsorption process is chemical in nature and a temperature swing adsorption/desorption cycle will be the most appropriate process.
Using the operating parameters shown in Fig. 2 and a CO2 recovery rate of 90%, the heat effect of the generic adsorption/desorption process can be easily estimated.
2.2 Heat effects of the generic adsorption process
To evaluate the energy consumption of the adsorption/desorption cycle in Fig. 2, the heat effects of the process have to be calculated. Figure 3 depicts the adsorption and desorption cycles at temperatures T1 and T2 respectively. In step ① by following reaction (2), the sorbent (M) capture CO2 to form compound (N) and the heat produced in the adsorption column (or adsorption bed) is Q1 (unit: kJ/kgCO2) and it has a temperature of T1:
In step ②, the used sorbent must be heated to desorption temperature T2 and heat needed for this process is Q2 in unit of kJ/kgCO2 and can be calculated:
Here, we assume the heat capacity (cp) is independent of temperature. W is the working capacity of product compound N. The heat required in this step is the sensible heat and the total heat (Q2) is distributed within the range of T1 to T2.
In step ③, the heat required for desorption N back to M and CO2 [reverse reaction (2), Q3 in kJ/kgCO2] is the same as in step ①, if we also assume that ΔH is independent of temperature, which is:
It should be pointed out that in above cycle, the heat of adsorption ΔH and heat capacity Ccp are usually a function of temperature. For simplification of the analysis, we assume that these two parameters are independent of temperature. This assumption should hold well within a narrow range of temperature.
In step ④, the regenerated sorbent must be cooled to T1 from T2 and the required heat Q4 (unit: kJ/kgCO2) can be calculated as follows:
Again, here we assume Ccp is constant within this temperature range. Heats in step ④ and ② can be integrated with each other since both of them are sensible heat. However, a ΔT is needed between these two streams, that is, the regenerated sorbent (at temperature T2) can only heat the used (CO2 rich) sorbent to T2 − ΔT. Thus, the net heat (Qs unit: kJ/kgCO2) required to heat the used sorbent is:
The heat generated in step ① can be integrated into the steam cycle to recover electricity and the heat consumed in step ③ should be extracted from the steam cycle.
Since we are dealing with an adsorption process, heat transfer from solid to steam or from steam to solid could not be done directly or the sorbent will be wet and need to be dried. Instead, it should use solid/gas/steam and steam/gas/solid configurations. The ΔT required for gas/liquid heat exchangers will be higher than liquid/liquid. In this analysis, we assume a ΔT of 50 °C for the heat exchangers.
The steam to provide Q3 at T2 in step ③ will require a temperature of T2 + ΔT and the steam to provide Qs to heat the used sorbent from T2 − ΔT to T2 will also have a temperature T2 + ΔT.
The steam to be recovered from Q1 at T1 will have a temperature of T1 − ΔT. Also, if we try to recover the heat to cool the regenerated sorbent from T1 + ΔT to T1, the steam recovered will have a temperature of T1 − ΔT as well.
To summarize, the heat consumed (QT) in the cycle step ② and ③ is:
And the heat recovered (QR) in step ① and ④ is:
QT and QR are the same in quantity, but they will have different capability (or containing different exergy) to generate electricity. As a result, the generic adsorption cycle consumes electricity and will reduce the thermal efficiency of the IGCC power plant.
2.3 Electricity usage of the generic adsorption process
Equations (8) and (9) give the heat used and recovered along with their temperature by the generic adsorption process. To estimate equivalent electricity loss due to the adsorption/desorption cycle, we need the thermal efficiencies of the heats used QT and the heat recovered QR. If the heat extracted at temperature T2 + ΔT has a thermal efficiency of η2 the heat recovered at temperature T1 − ΔT has a thermal efficiency of η1, then the total electricity loss (E in kJ/kgCO2) due to the adsorption cycle will be:
If we know the heat of adsorption ΔH, heat capacity cp of the sorbent, working capacity W of the sorbent and temperature difference ΔT required for heat transfer as well as the thermal efficiency η1 and η2 of the heats, we will be able to calculate the electricity loss (E) by Eq. (10).
In Eq. (10), the heat capacity cp and working capacity W are sorbent specific and not related to operating temperature. On the other hand, the heat of adsorption and thermal efficiency of heats are strongly correlated to the operating temperatures. In the following sections we will demonstrate how to estimate ΔH at different operating temperatures through thermodynamic analysis and the thermal efficiency η1 and η2 through steam cycle analysis.
3 Heat of adsorption of the generic adsorption process
Hot/warm CO2 removal may improve thermal efficiency of an IGCC power plant and reduce the capital cost by eliminating heating and cooling of the syngas. However, a higher temperature will require a higher ΔH and may result in a higher electricity loss. This section will estimate heat of adsorption at different temperatures as required by thermodynamics.
As described in previous section, the adsorption reaction takes place at temperature T1, and the desorption (regeneration) of the CO2 sorbent at temperature T2 and the reaction can be expressed as above reaction (2).
For simplicity of the discussion, we assume that both M and N are crystalline, like an alkali or alkaline oxide to react with CO2 to form a corresponding carbonate, thus the conversion rate of the sorbent or regeneration rate will not impact the equilibrium constant of the reaction (2). The equilibrium constant of reaction (2) is:
For an adsorption reaction to go forward, the partial pressure of CO2 in the syngas should be higher than the equilibrium partial pressure of CO2, which is 1/K.
The typical syngas compositions before and after the WGS reaction from the three types of gasifiers are listed in Table 1. A conversion of 95% is assumed for the WGS reaction (1).
From these data, it can be seen that after WGS reaction the partial pressure of CO2 is about 16 atm, that is, the equilibrium partial pressure of CO2 of the reaction (2) should be less than 16 atm, or to say that the equilibrium constant K should be larger than 0.0625 atm−1. However, considering a 90% CO2 removal, the required equilibrium constant will be larger than 0.625 atm−1.
The relationship between Gibbs free energy and the equilibrium constant of reaction (2) is:
In order for reaction (2) to achieve 90% CO2 removal, it is required that K > 0.63atm−1, or
Expectedly, the entropy change of reaction (2) is dominated by the CO2 molecules. Conversion between solid and solid–solid or solid–liquid usually has a relatively small entropy change. But the conversion between gas phase and solid or liquid has larger entropy change. When CO2 is fixed from a gas phase to a solid phase, the ΔS change is much higher. Table 2 listed heat of formation and Gibbs free energy of formation for many oxides and their carbonates as well as the entropy difference between them [18]. Typically, the entropy difference between oxide and carbonate is in the range of 38 to 63.2 (J/K/mol).
Since the entropy change from oxide to carbonate is in the range from 38.9 to 63.2 J/K/mol, the ΔS of reaction (2) will be in the range: ΔS = (SMxCO3 ~ SMxO) − SCO2 = (38.9–63.2) − 213.6 = − 150.4–174.7 J/K/mol.
Substitute this ΔS into Eq. (13), we have: ΔH < ΔG + TΔS = 0.470RT − (150.4–174.7) T.
Equation (14) gives the required heat of adsorption (in the unit of J/mol CO2) for reaction (2) at different operating temperatures.
Figure 4 shows the heat of adsorption required for a hot/warm adsorption process if the adsorption process can be expressed as reaction (2). It is clear, that for a hot/warm adsorption process to work, its heat of adsorption required will be significant unless the reaction (2) is made invalid.
It should be pointed out, however, that above calculations did not consider the temperature dependence of ΔH, ΔG, ΔS and cP of each compound from the standard temperature to the temperature of interest. As demonstrated in our previous studies [19,20,21], increasing temperature from 300 to 1000 K, the ΔH(T) of reactions for alkali and alkaline oxides capturing CO2 increase less than 20 kJ/mol. When the temperature of interest is high, such error may be large, but it should not be significant to these values of themselves and should not alter our conclusions. In addition, as the temperature increases, some of the solid phases may experience a phase transition from solid phase to liquid phase, in which case the entropy change of reaction (2) will be very different from above estimated range (-150.4–174.7 J/K/mol) and needs to be corrected.
It is interesting to estimate the magnitude of the heat of adsorption for hot/warm CO2 removal. Since WGS reaction and gas turbine inlet temperatures are both at or above 260 °C, we consider a hot/warm CO2 removal process as a CO2 removal process which takes place at a temperature at or above temperature 260 °C. According to Eq. (14), the heat of adsorption at this temperature will be in the range of: ΔH = − (146.5–170.8) * 533.15 = − (78.1–91.1) (kJ/mol CO2). Obviously, such heat of adsorption is still significant (close to or higher than the heat of absorption of MEA). Considering the fact that this temperature may be too low to recover the heat of adsorption economically, such a warm CO2 removal process will be obviously disadvantageous compared to the Selexol process.
4 Thermal efficiency of heats from steam cycle
The maximum thermal efficiency of heat is, of course, the Carnot cycle efficiency. To estimate the thermal efficiency of the heat, either extracted from or returned to the IGCC power plant, we need to know how heat is converted to electricity in a power plant.
To obtain thermal efficiencies (η1, η2) in the Eq. (10), we need to know the steam cycle, where we can extract or inject steam into the cycle. Theoretically, we can extract the required steam from or inject the recovered steam into the steam cycle at any temperatures. However, in some cases, that may not be economical or practical at all. Firstly, very low-quality heat (low temperature) cannot be used to generate electricity economically. Secondly, heat may only be integrated into the steam cycle at certain places or the steam turbines need be heavily modified. Thirdly, the recovered heat is mostly latent heat, which is concentrated at T1. This heat could be used to generate saturated steam with temperature at T1 − ΔT. However, such saturated steam cannot be used in the steam cycle because it will have too much moisture after expansion. The highest moisture allowed is about 10%. Thus, superheated steam must be used to match the exhaust steam conditions at the low pressure (LP) turbine. To answer these questions, the steam cycles should be analyzed in detail. However, detailed analyses of a real-world steam cycle are too complicated to provide a simple and general answer for us. Here, we make some assumptions and conduct a simple theoretical analysis to obtain thermal efficiency for steam at a certain temperature.
Figure 5 shows a typical steam cycle with high pressure (HP), intermediate pressure (IP) and low pressure (LP). The cycle shown in the Fig. 5 has one reheat. The reason for reheating is that saturation temperature of the HP steam is too high and it cannot be directly expanded to IP and LP. In addition, such saturation temperature will generate too much moisture during the expansion and damage the turbine blades.
In the steam temperature-entropy (T-S) diagram as shown in Fig. 6, the point 1 to 2 corresponds to the HP turbine, point 2 to 3 corresponds to reheat and point 3 to 4 is the IP and LP combined.
Steam temperature–entropy (T–S) diagram. Red lines X refers to dryness fraction. Green line refers to volume, while blue line corresponds to pressure. The initial T–S diagram was taken from Ref [22]. under the terms of the Creative Commons Attribution -ShareAlike 3.0 Unpotted (CC BY-SA 3.0) license
Now, we suppose the heat of adsorption can be recovered at point 5. This heat can generate saturated steam with a pressure of 60 bar. However, the saturated steam cannot be injected into the steam cycle. To be injected into the turbine, the conditions (T and P) of the steam should be on the line between points 3 and 4, in this case, the steam should be on point 6. The pressure of the steam at the point 6 is, however, only 6 bar, which is equal to the pressure of the saturated steam at point 7 (they are both on the isobaric line).
The above discussions demonstrate that the steam generated from the recovered heat should be on the path of steam cycle (the line between 3 and 4 in Fig. 5), which is determined by the design of steam turbine. The operating conditions of the steam turbines from different manufacturers are very close, and they all have similar thermal efficiencies.
Using typical operating conditions of a steam turbine, the thermal efficiency of steam on the steam cycle path can be calculated using process simulation software, such as Aspen Plus, CHEMCAD, or Thermoflex. Figure 7 shows the results. For any temperature we can find a pressure from the curve marked IP and LP steam, and for that pressure we can find its thermal efficiency. Figure 6 also displayed the curve for saturated steam, which is obtained when the IP or LP steam is cooled isobarically.
Using Fig. 7 and Eq. (10) we can calculate the electricity consumption of any adsorption processes (this principle can also be used for absorption process at low temperatures). The following section will demonstrate the application of above generic process to several specific adsorptions based on CO2 capture process for IGCC power plants.
5 Applications of the generic adsorption process
There are many research projects aimed to develop hot/warm CO2 removal from syngas of an IGCC power plant and many sorbents have been developed [7,8,9, 23]. In this study, we will use our generic process to analyze several sorbents, and to find out the minimum energy consumption of these sorbents. The sorbents to be investigated includes Toshiba’s Li4SiO4 sorbent [24,25,26,27,28], National Energy Technology Laboratory (NETL)’s Mg (OH)2 sorbent[19, 29,30,31] and novel Sodium-Based Sorbent [20, 31,32,33,34,35].
5.1 The Toshiba sorbent
The physical and chemical properties of the Toshiba Li4SiO4 sorbent and its operating conditions at the adsorption/desorption steps are listed in Table 3. In the same table, we also listed the typical flow rate of CO2 in an IGCC power plant with 520 MWe net output.
The adsorption/desorption temperatures of the Toshiba sorbent are 500 and 900 °C respectively [28, 36]. This sorbent does not operate between WGS reaction and GT inlet temperatures, thus is not suitable for IGCC process. But the energy requirement calculations will be still valid for the process.
The adsorption temperature of the Toshiba sorbent is 500 °C, assuming a ΔT of 50 °C for heat transfer, the recovered steam will have a temperature of 450 °C. According to Fig. 6, the thermal efficiency η1 of steam will be 0.27. The desorption temperature is 900 °C, and the steam required for desorption have a temperature of 950 °C. This temperature is above the steam cycle, and its thermal efficiency η2 has a maximum around 0.42 (Fig. 6 only covers up to IP). Using these numbers and the physical and chemical properties listed in Table 1, we can calculate electricity loss (E) by Eq. (10):
The pressure of the desorbed CO2 is 49 atm, so it must be compressed to 150 atm. The compression work to compress from 49 to 150 atm is about 40 kJ/kgCO2. Thus, the total electricity consumption for the Toshiba sorbent will be around 345.74 kJ/kgCO2, which is 85% higher than the Selexol process (187 kJ/kgCO2) [37,38,39]. The adsorption process may be able to improve the plant thermal efficiency about 1 percent, which is about 90 kJ/kgCO2. Clearly, the Toshiba sorbent has higher electricity loss than the Selexol process. Considering that the former is the minimum required and the latter is real process data, the Selexol process should be favorable.
5.2 The Mg (OH) 2 sorbent
Mg (OH)2 is a sorbent developed at NETL [29, 30]. The chemical process of this sorbent is as follows: [19]
The chemical and physical properties of the sorbent and its operating conditions of the process are listed in Table 4. The capacity listed is the maximum theoretical values.
In this process the first reaction consumes steam at temperature 827 K with heat of reaction 116.9 kJ/mol-CO2, and the following two reactions generate heat at temperatures 505 K and 539 K respectively. The heats of reaction are 37.8 and 79.1 kJ/mol-CO2 respectively. Since these two temperatures are so close, we can lump them together to use same thermal efficiency. The total electricity loss can be calculated in a similar way with Eq. (10):
The pressure of the desorbed CO2 has a pressure of 46.6 atm, and to compress this CO2 to 150 atm will require energy of 40 kJ/mol-CO2. Thus, the total electricity loss will be 421.3 kJ/kgCO2, which is more than double that of the Selexol’s electricity loss.
5.3 The novel sodium-based sorbents
Another solid sorbent targeted for IGCC warm CO2 removal is the sodium-based regenerable sorbent. The physical and chemical properties of the sorbent and the operating conditions of the process are listed in Table 5.
Based on these data the electricity loss (E) due to heat of adsorption and sensible heat can be calculated the same way in the previous examples by following Eq. (10):
This result indicated that the heat of adsorption plays a major role in determining the electricity loss. Sensible heat, on the other hand, is less significant (the heat capacity data used maybe not correct for the second and third sorbents, but the results should be similar). To reduce the electricity loss, the heat of adsorption must be reduced. Unfortunately, as it has been shown in the previous sections, the heat of adsorption for the hot/warm CO2 removal is a function of operating temperature and hard to be reduced.
6 Insights into the generic adsorption process
From the generic adsorption cycle analysis (Eq. (10)), we obtained that the total electricity consumption (E) for a hot/warm adsorption process is equal to (ΔH + Cp)/W × ΔT) × (η2 − η1). The variables in the first parentheses are related to the sorbent properties and the process design, and in the second parentheses (η2 − η1) are related to the steam cycle. There are several ways to minimize the electricity loss through process and sorbent designs.
6.1 Minimizing electricity loss through raising adsorption temperature
Both η1 and η2 are functions of temperature. The values of η1 and η2 increase as the temperature increases. However, there is a maximum value for η1 and η2 when the temperature reaches the highest steam temperature, which is around 600 °C (1100 °F) for a subcritical PC plant and 650 °C (1200 °F) for a supercritical PC plant. After η2 reaches its maximum η1 − η2 will gradually decrease as the temperature increases further. Figure 8 displays the relationship between electricity losses of an adsorption/desorption cycle and its operating temperatures. Both the adsorption and desorption steps take places at temperatures higher than the steam cycle temperature of 600 °C for subcritical PC and 650 °C for supercritical PC. Under such circumstance, the η1 and η2 will both reach the same maximum value and the electricity consumption for such an adsorption/desorption cycle will virtually be zero!
The above analysis reveals that the higher the temperature of the hot/warm CO2 removal process is, the larger the required heat of adsorption will be, but if the most or entire of the adsorption/desorption cycle takes place at temperature above the highest steam temperature in a steam cycle, the process may lead to minimal electricity loss.
6.2 Minimizing heat of adsorption through process or sorbent designs
From above analysis, we may notice that if we can find a chemical reaction, which has very little entropy change, then this reaction will be less sensitive to temperature change. For example, the following reaction will have much smaller entropy change:
Alkali or alkaline hydroxide can satisfy such reaction:
Unfortunately, the reverse reaction does not take place as desired, rather, it reacts the following way:
since Mg(OH)2 is much less stable than MgCO3. If similar hydroxide compounds and carbonate can be found, then a low ΔH could be possible to achieve even at high temperature.
Another alternative is to find a product N, which is in liquid phase. When N is a liquid, the reaction (2) will have a smaller entropy change and will be less sensitive to temperature. As a result, ΔH as expressed by Eq. (14) could be smaller. This is probably the case with the Toshiba sorbent, Li4SiO4, which forms a liquid phase product, Li2CO3, and its heat of reaction is actually out of the range shown in Fig. 4.
6.3 High pressure desorption for CO 2 regeneration
Most of the adsorption based processes don’t use high pressure CO2 regeneration. It is possible, however, to reduce overall energy consumption through elevating the regeneration pressure. The potential regeneration pressure of reaction (2) can be estimated using following equation:
We assume that all CO2 desorbed in the desorption column of Fig. 2 is at P2 = 150 atm, so that no further compression will be required.
It should be noted, however, that the CO2 partial pressure P1 in Eq. (22) will actually change from 16 atm in the inlet to 1.6 atm in the outlet of the adsorption column. With P2 = 150 atm, P1 changes from 16 atm to 1.6 atm, and with some mathematical manipulations, we have:
Equation (22) gives the relationship between heat of adsorption and its adsorption and desorption temperatures. For the carbonate/oxide based adsorption CO2 capture cycle ΔH is given by Eq. (14). Using an average ΔH = − (146.5 + 170.8)/2 = − 158.7 * T1 in above equation, we obtained T2 = 1.18524 * T1.
Assuming T1 = 533 K and ΔT1 = ΔT2 = 50 K, we will have:
Recovered steam at: TR = T1 − ΔT = 483 K.
Extracted steam at: T = T2 + ΔT = 1.18524*T1 + 50 = 631.73 + 50 = 681.73 (K).
The corresponding thermal efficiencies for these two temperatures are η1 = 0.23 and η2 = 0.35 respectively. Ignoring the sensible heat we have:
Electricity loss (E) = (η2 − η1)*ΔH = 0.12 * 158.7 * 533 = 230.69 (kJ/kgCO2).
Hence, even under this real best case scenario, the hot/warm CO2 removal is still ~ 10% higher compared to 206.8 kJ/kgCO2 for Selexol.
7 Conclusions and perspectives
In this work we have conducted simplified process and thermodynamic analyses for a sorbent based hot/warm CO2 capture process for an IGCC power plant. The following conclusions can be made from these analyses.
-
1.
A typical adsorption based process will require a higher heat of adsorption when it is operated at a hot/warm temperature and accordingly, the electricity consumption of the process will also increase. Electricity loss for a hot/warm CO2 capture process, for the most operating temperature range, is higher than the current baseline, the Selexol based absorption process.
-
2.
A desired process that may be advantageous is that if the entire adsorption/desorption cycle is outside of the steam cycle. To be more precise, the adsorption temperature must be close to or above the highest steam temperature in a steam cycle.
-
3.
A novel potential sorbent should utilize the following reaction:
$$M + CO_{2} \Rightarrow N + H_{2} O$$
Such a reaction will greatly reduce the entropy change of the reaction and thus significantly reduces its required heat of adsorption, which in turn will considerably improve the energy performance and thus the process economics.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AGR:
-
Acid gas removal
- ASU:
-
Air separation unit
- c p :
-
Sorbent specific heat capacity (kJ/K/kg-sorbent)
- E:
-
Total electricity loss (kJ/kgCO2)
- ΔH:
-
Heat of adsorption (kJ/kgCO2)
- HP:
-
High pressure (atm)
- HRSG:
-
Heat recovery steam generator
- IP:
-
Intermediate pressure (atm)
- IGCC:
-
Integrated gasification combined cycle
- LP:
-
Low pressure (atm)
- η1 :
-
Thermal efficiency at T1
- η2 :
-
Thermal efficiency at T2
- P1 :
-
CO2 partial pressure at adsorption (atm = 1.01325 bar)
- P2 :
-
CO2 partial pressure at desorption (atm)
- T1 :
-
CO2 adsorption temperature (K)
- T2 :
-
Regeneration temperature (K)
- W:
-
Adsorption (working) capacity (kgCO2/kg-sorbent)
- WGS:
-
Water–gas-shift
References
Zhu XC, Shi YX, Cai NS. Integrated gasification combined cycle with carbon dioxide capture by elevated temperature pressure swing adsorption. Appl Energy. 2016;176:196–208.
Vakharia V, Ramasubramanian K, Winston Ho WS. An experimental and modeling study of CO2-selective membranes for IGCC syngas purification. J Membr Sci. 2015;488:56–66.
Rubin ES, Davison JE, Herzog HJ. The cost of CO2 capture and storage. Int J Greenhouse Gas Control. 2015;40:378–400.
Prins M, Van den Berg R, Van Holthoon E, Van Dorst E, Geuzebroek F. Technological Developments in IGCC for carbon capture. Chem Eng Technol. 2012;35(3):413–9.
Mondal MK, Balsora HK, Varshney P. Progress and trends in CO2 capture/separation technologies: a review. Energy. 2012;46(1):431–41.
Kanniche M, Bouallou C. CO2 capture study in advanced integrated gasification combined cycle. Appl Therm Eng. 2007;27(16):2693–702.
Aaron D, Tsouris C. Separation of CO2 from flue gas: a review. Sep Sci Technol. 2005;40(1–3):321–48.
Huaman RNE, Jun TX. Energy related CO2 emissions and the progress on CCS projects: a review. Renew Sustain Energy Rev. 2014;31:368–85.
Li BY, Duan Y, Luebke D, Morreale B. Advances in CO2 capture technology: a patent review. Appl Energy. 2013;102:1439–47.
Bui M, Adjiman CS, Bardow A, Anthony EJ, Boston A, Brown S, Fennell PS, Fuss S, Galindo A, Hackett LA, et al. Carbon capture and storage (CCS): the way forward. Energy Environ Sci. 2018;11(5):1062–176.
Park SW, Sung DH, Choi BS, Lee JW, Kumazawa H. Carbonation kinetics of potassium carbonate by carbon dioxide. J Ind Eng Chem. 2006;12(4):522–30.
Jie X, Chau J, Obuskovic G, Sirkar KK. Enhanced pressure swing membrane absorption process for CO2 removal from shifted syngas with dendrimer-ionic liquid mixtures as absorbent. Ind Eng Chem Res. 2014;53(8):3305–20.
Chau J, Jie X, Sirkar KK. Polyamidoamine-facilitated poly(ethylene glycol)/ionic liquid based pressure swing membrane absorption process for CO2 removal from shifted syngas. Chem Eng J. 2016;305:212–20.
Wang Y, Lang X, Fan S. Hydrate capture CO2 from shifted synthesis gas, flue gas and sour natural gas or biogas. J Energy Chem. 2013;22(1):39–47.
Wibowo H, Susanto H, Grisdanurak N, Hantoko D, Yoshikawa K, Qun H, Yan M. Recent developments of deep eutectic solvent as absorbent for CO2 removal from syngas produced from gasification: current status, challenges, and further research. J Environ Chem Eng. 2021;9(4): 105439.
Berstad D, Anantharaman R, Nekså P. Low-temperature CO2 capture technologies—applications and potential. Int J Refrig. 2013;36(5):1403–16.
Huang W, Jiang X, He G, Ruan X, Chen B, Nizamani AK, Li X, Wu X, Xiao W. A novel process of H2/CO2 membrane separation of shifted syngas coupled with gasoil hydrogenation. Processes. 2020;8(5):590.
HSC Chemistry software 61, Pori: Outotec Research Oy; 2006. http://www.outoteccom/hsc.
Duan Y, Sorescu DC. CO2 capture properties of alkaline earth metal oxides and hydroxides: a combined density functional theory and lattice phonon dynamics study. J Chem Phys. 2010;133(7): 074508.
Duan Y, Zhang B, Sorescu DC, Johnson JK. CO2 capture properties of M-C-O-H (M=Li, Na, K) systems: a combined density functional theory and lattice phonon dynamics study. J Solid State Chem. 2011;184(2):304–11.
Duan Y, Luebke D, Pennline HW. Efficient theoretical screening of solid sorbents for CO2 capture applications. Int J Clean Coal Energy. 2012;1(1):1–11.
A temperature-versus-entropy diagram for steam. https://en.wikipedia.org/wiki/Steam#/media/File:TS-Wasserdampf_engl.png
Chi JL, Zhao LF, Wang B, Li Z, Xiao YH, Duan Y. Thermodynamic performance assessment and comparison of IGCC with solid cycling process for CO2 capture at high and medium temperatures. Int J Hydrogen Energy. 2014;39:6479–91.
Essaki K, Nakagawa K, Kato M, Uemoto H. CO2 absorption by lithium silicate at room temperature. J Chem Eng Jpn. 2004;37(6):772–7.
Kato M, Nakagawa K, Essaki K, Maezawa Y, Takeda S, Kogo R, Hagiwara Y. Novel CO2 absorbents using lithium-containing oxide. Int J Appl Ceram Tec. 2005;2(6):467–75.
Nakagawa K, Ohashi T. A novel method of CO2 capture from high temperature gases. J Electrochem Soc. 1998;145(4):1344–6.
Nakagawa K, Ohashi T. A reversible change between lithium zirconate and zirconia in molten carbonate. Electrochemistry. 1999;67(6):618–21.
Duan Y, Parlinski K. Density functional theory study of the structural, electronic, lattice dynamical, and thermodynamic properties of Li4SiO4 and its capability for CO2 capture. Phys Rev B. 2011;84(10): 104113.
Siriwardane RV, Stevens RW. Novel regenerable magnesium hydroxide sorbents for CO2 capture at warm gas temperatures. Ind Eng Chem Res. 2009;48(4):2135–41.
Shimozori M, Katayama K, Hoshino T, Ushida H, Yamamoto R, Fukada S. Water vapor concentration dependence and temperature dependence of Li mass loss from Li2TiO3 with excess Li and Li4SiO4. Fusion Eng Des. 2015;98–99:1808–11.
Siriwardane RV, Robinson C, Shen M, Simonyi T. Novel regenerable sodium-based sorbents for CO2 capture at warm gas temperatures. Energy Fuels. 2007;21(4):2088–97.
Guzman-Velderrain V, Delgado-Vigil D, Collins-Martinez V, Ortiz AL. Synthesis, characterization and evaluation of SodiumDoped lithium zirconate as a high temperature CO2 absorbent. J New Mater Electrochem Syst. 2008;11(2):131–6.
Zandi N, Sadeghi H, Habibi M, Jalali I, Zare M. Blanket simulation and tritium breeding ratio calculation for ITER reactor. J Fusion Energy. 2015;34(6):1365–8.
Rodriguez MT, Pfeiffer H. Sodium metasilicate (Na2SiO3): a thermo-kinetic analysis of its CO2 chemical sorption. Thermochim Acta. 2008;473(1–2):92–5.
Duan Y. Electronic structural and lattice thermodynamic properties of MAlO2 and M5AlO4 (M = Li, Na, K) sorbents for CO2 capture applications. Discov Chem Eng. 2023;3(1):6.
Seggiani M, Puccini M, Vitolo S. High-temperature and low concentration CO2 sorption on Li4SiO4 based sorbents: study of the used silica and doping method effects. Int J Greenhouse Gas Control. 2011;5(4):741–8.
Ashrafi O, Bashiri H, Esmaeili A, Sapoundjiev H, Navarri P. Ejector integration for the cost effective design of the Selexol (TM) process. Energy. 2018;162:380–92.
Kapetaki Z, Brandani P, Brandani S, Ahn H. Process simulation of a dual-stage Selexol process for 95% carbon capture efficiency at an integrated gasification combined cycle power plant. Int J Greenhouse Gas Control. 2015;39:17–26.
Chen WH, Chen SM, Hung CI. Carbon dioxide capture by single droplet using selexol, rectisol and water as absorbents: a theoretical approach. Appl Energy. 2013;111:731–41.
Acknowledgements
This project was funded by the United States Department of Energy, National Energy Technology Laboratory under the Point Sources Carbon capture Field Work Proposal (FWP) #1025010. This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Funding
This study was supported by National Energy Technology Laboratory (Grant No. FWP #1025010).
Author information
Authors and Affiliations
Contributions
SC: Conceptualization, resources, investigation, formal analysis, writing—original draft. PW: investigation, visualization, writing—review & editing. DH: resources, writing—review & editing. JC: writing—review & editing. YD: conceptualization, visualization, resources, writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S., Wang, P., Hopkinson, D.P. et al. Analyses of hot/warm CO2 removal processes for IGCC power plants. Discov Chem Eng 3, 19 (2023). https://doi.org/10.1007/s43938-023-00034-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-023-00034-5