Abstract
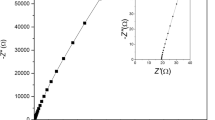
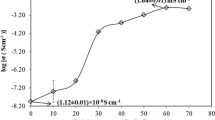
Novel gel polymer electrolytes (GPEs) composed of polyvinyl alcohol (PVA) and sodium thio-cyanate were developed via a solution casting technique. An ionic liquid (IL), 1-ethyl-3-methyl-imidazolium tricyanomethanide ([EMIM][TCM]), was doped into a polymer-salt complex system (PVA + NaSCN) to further enhance the conductivity. IL-doped polymer electrolyte (ILDPE) films were characterized using X-ray diffraction (XRD), polarized optical microscopy (POM), Fourier-transform infrared (FTIR) spectroscopy, and conductivity measurements. XRD was performed to check the degree of crystallinity and amorphicity of the ILDPE films, and the amorphicity of GPEs increased with the increase of the IL content. POM was employed to evaluate the changes in the surface morphology due to the inclusion of salt and IL in the PVA. The compositional nature of the GPE films was examined via FTIR studies. The electrical and electrochemical properties were characterized by cyclic voltammetry and electrochemical impedance spectroscopy. The maximum conductivity for the GPE film was estimated to be 1.10 × 10−5 S/cm for 6% (mass fraction) of IL in the polymer-salt complex. The ionic transference number was approximately 0.97. An electrochemical double-layer capacitor (EDLC) was built from optimized GPE films and reduced graphene oxide-based electrodes. The specific capacitance calculated from the cyclic voltammograms of the EDLC cells was 3 F/g.
摘要
以聚乙烯醇(PVA)和硫氰酸钠为主要原料,采用溶液浇铸造工艺研制了新型凝胶聚合物电 解质(GPEs)。将1-乙基-3-甲基-咪唑三氰基甲烷化物([EMIM][TCM])的离子液体掺入到聚合物-盐配合物体系(PVA + NaSCN),以进一步提高导电性能。利用X射线衍射(XRD)、偏光显微镜术 (POM)、傅里叶变换红外光谱(FTIR)和电导率测量对离子液体掺杂的聚合物电解质薄膜进行了 表征。通过XRD测试了离子液体掺杂聚合物电解质薄膜的结晶度和非结晶性,发现随着离子液体含 量的增加,新型凝胶聚合物电解质的非结晶性增加。POM测评了聚乙烯醇中盐和离子液体的加入对 表面形貌的影响。通过FTIR研究了新型凝胶聚合物电解质薄膜的组成性质。采用循环伏安法和电化 学阻抗谱对其电学和电化学性能进行了表征。聚合物-盐复合物中离子液体的质量分数为6%时,新 型凝胶聚合物电解质薄膜的最大电导率为1.10 × 10−5 S/cm。离子迁移数约为0.97。利用优化的新型 凝胶聚合物电解质薄膜和还原氧化石墨烯基电极制备了电化学双层电容器。由循环伏安法计算的电 化学双层电容器电池的比电容为3 F/g。
Similar content being viewed by others
References
BHARGAV P B, MOHAN VM, SHARMA A K, et al. Structural and electrical properties of pure and NaBr doped poly (vinyl alcohol) (PVA) polymer electrolyte films for solid state battery applications [J]. Ionics, 2007, 13(6): 441–446.
SRIVASTAVA N., CHANDRA A, CHANDRA S. Dense branched growth of (SCN)x and ion transport in the poly(ethyleneoxide) NH4SCN polymer electrolyte [J]. Physical Review B, Condensed Matter, 1995, 52(1): 225–230.
CHOWDARI B V R, CHANDRA S, SINGH S, et al. Solid state ionics: Materials and applications [M]. Varanasi: World Scientific, 1992.
KUMAR Y, HASHMI S A, PANDEY G P. Lithium ion transport and ion-polymer interaction in PEO based polymer electrolyte plasticized with ionic liquid [J]. Solid State Ionics, 2011, 201(1): 73–80.
TUHANIA P, SINGH P K, BHATTACHARYA B, et al. PVDF-HFP and 1-ethyl-3-methylimidazolium thiocyanate-doped polymer electrolyte for efficient su-percapacitors [J]. High Performance Polymers, 2018, 30(8): 911–917.
GUPTA S, SINGH P K, BHATTACHARYA B. Low-viscosity ionic liquid-doped solid polymer electrolytes [J]. High Performance Polymers, 2018, 30(8): 986–992.
DHAPOLA P S, SINGH P K, BHATTACHARYA B, et al. Electrical, thermal, and dielectric studies of ionic liquid-based polymer electrolyte for photoelectro-chemical device [J]. High Performance Polymers, 2018, 30(8): 1002–1008.
SINGH D, KANJILAL D, LAXMI G, et al. Conductivity and dielectric studies of Li3+ -irradiated PVP-based polymer electrolytes [J]. High Performance Polymers, 2018, 30(8): 978–985.
SIYAHJANI S, ONER S, SINGH P K, et al. Highly efficient supercapacitor using single-walled carbon nan-otube electrodes and ionic liquid incorporated solid gel electrolyte [J]. High Performance Polymers, 2018, 30(8): 971–977.
TAO R Y, FUJINAMI T. Application of mix-salts composed of lithium borate and lithium aluminate in PEO-based polymer electrolytes [J]. Journal of Power Sources, 2005, 146(1/2): 407–411.
WANG Y J, PAN Y, CHEN L S. Ion-conducting polymer electrolyte based on poly(ethylene oxide) com-plexed with Li1.3Al0.3Ti1.7(PO4)3 salt [J]. Materials Chemistry and Physics, 2005, 92(2/3): 354–360.
DISSANAYAKE M A K L, JAYATHILAKA P A R D, BOKALAWELA R S P. Ionic conductivity of PEO9: Cu(CF3SO3)2 nano-composite solid polymer electrolyte [J]. Electrochimica Acta, 2005, 50(28): 5602–5605.
LIEW C W, RAMESH S, AROF A K. Investigation of ionic liquid-based poly(vinyl alcohol) proton conductor for electrochemical double-layer capacitor [J]. High Performance Polymers, 2014, 26(6): 632–636.
KADIR M F Z, MAJID S R, AROF A K. Plasticized chitosan-PVA blend polymer electrolyte based proton battery [J]. Electrochimica Acta, 2010, 55(4): 1475–1482.
LIEW C W, RAMESH S, AROF A K. Characterization of ionic liquid added poly(vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors [J]. International Journal of Hydrogen Energy, 2015, 40(1): 852–862.
LIEW C W, RAMESH S, AROF A K. Good prospect of ionic liquid based-poly(vinyl alcohol) polymer electrolytes for supercapacitors with excellent electrical, electrochemical and thermal properties [J]. International Journal of Hydrogen Energy, 2014, 39(6): 2953–2963.
KANBARA T, INAMI M, YAMAMOTO T. New solid-state electric double-layer capacitor using poly(vinyl alcohol)-based polymer solid electrolyte [J]. Journal of Power Sources, 1991, 36(1): 87–93.
EVERY H A, ZHOU F, FORSYTH M, et al. Lithium ion mobility in poly(vinyl alcohol) based polymer electrolytes as determined by 7Li NMR spectroscopy [J]. Electrochimica Acta, 1998, 43(10/11): 1465–1469.
HIRANKUMAR G, SELVASEKARAPANDIAN S, KUWATA N, et al. Thermal, electrical and optical studies on the poly(vinyl alcohol) based polymer electrolytes [J]. Journal of Power Sources, 2005, 144(1): 262–267.
DAMODARAN S, KINSELLA J E. The effects of neutral salts on the stability of macromolecules. A new approach using a protein-ligand binding system [J]. Journal of Biological Chemistry, 1981, 256(7): 3394–3398.
BHARGAV P B, MOHAN V M, SHARMA A K, et al. Structural and electrical properties of pure and NaBr doped poly (vinyl alcohol) (PVA) polymer electrolyte films for solid state battery applications [J]. Ionics, 2007, 13(6): 441–446.
NIK AZIZ N A, IDRIS N K, ISA M I N. Solid polymer electrolytes based on methylcellulose: FT-IR and ionic conductivity studies [J]. International Journal of Polymer Analysis and Characterization, 2010, 15(5): 319–327.
SAMSUDIN A S, KHAIRUL W M, ISA M I N. Characterization on the potential of carboxy methylcel-lulose for application as proton conducting biopolymer electrolytes [J]. Journal of Non-Crystalline Solids, 2012, 358(8): 1104–1112.
SALLEH N S, AZIZ S B, et al. Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide [J]. Ionics, 2016, 22(11): 2157–2167.
IQBAL S, KHATOON H, HUSSAIN PANDIT A, et al. Recent development of carbon based materials for energy storage devices [J]. Materials Science for Energy Technologies, 2019, 2(3): 417–428.
BACH-TOLEDO L, HRYNIEWICZ B M, MARCH-ESI L F, et al. Conducting polymers and composites nanowires for energy devices: A brief review [J]. Materials Science for Energy Technologies, 2020, 3: 78–90.
MAHESHWARI P H. Developing the processing stages of carbon fiber composite paper as efficient materials for energy conversion, storage, and conservation [J]. Materials Science for Energy Technologies, 2019, 2(3): 490–502.
ANJANA P M, BINDHU M R, RAKHI R B. Green synthesized gold nanoparticle dispersed porous carbon composites for electrochemical energy storage [J]. Materials Science for Energy Technologies, 2019, 2(3): 389–395.
EEDULAKANTI S R, GAMPALA A K, VENKATESWARA RAO K, et al. Ultrasonication assisted thermal exfoliation of graphene-tin oxide nanocomposite material for supercapacitor [J]. Materials Science for Energy Technologies, 2019, 2(3): 372–376.
HUANG S J, LEE H K, KANG W H. Proton conducting behavior of a novel composite based on phospho-silicate/poly(vinyl alcohol) [J]. Journal of the Korean Ceramic Society, 2005, 42(2): 77–80.
KRUMOVA M, LOPEZ D, BENAVENTE R, et al. Effect of crosslinking on the mechanical and thermal properties of poly(vinyl alcohol) [J]. Polymer, 2000, 41(26): 9265–9272.
BENAVENTE E, SANTA ANA M A, MENDIZABAL F, et al. Intercalation chemistry of molybdenum disulfide [J]. Coordination Chemistry Reviews, 2002, 224(1/2): 87–109.
HODGE R M, EDWARD G H, SIMON G P. Water absorption and states of water in semicrystalline poly(vinyl alcohol) films [J]. Polymer, 1996, 37(8): 1371–1376.
SAAID F, RODI I, WINIE T. Effect of temperature on the transport property of PVdF-HFP-MPII-PC/DME gel polymer electrolytes [J]. AIP Conference Proceedings, 2017, 1877(1): 020006.
MISHRA R, RAO K J. Electrical conductivity studies of poly(ethyleneoxide)-poly(vinylalcohol) blends [J]. Solid State Ionics, 1998, 106(1/2): 113–127.
SINGH R, SINGH P K, TOMAR S K, et al. Synthesis, characterization, and dye-sensitized solar cell fabrication using solid biopolymer electrolyte membranes [J]. High Performance Polymers, 2016, 28(1): 47–54.
AZIZ S B, HAMSAN M H, ABDULLAH R M, et al. A promising polymer blend electrolytes based on chitosan: Methyl cellulose for EDLC application with high specific capacitance and energy density [J]. Molecules, 2019, 24(13): 2503.
SAMPATHKUMAR L, CHRISTOPHER SELVIN P, SELVASEKARAPANDIAN S, et al. Synthesis and characterization of biopolymer electrolyte based on tamarind seed polysaccharide, lithium perchlorate and ethylene carbonate for electrochemical applications [J]. Ionics, 2019, 25(3): 1067–1082.
PRATAP R, SINGH B, CHANDRA S. Polymeric rechargeable solid-state proton battery [J]. Journal of Power Sources, 2006, 161(1): 702–706.
XU G H, ZHENG C, ZHANG Q, et al. Binder-free activated carbon/carbon nanotube paper electrodes for use in supercapacitors [J]. Nano Research, 2011, 4(9): 870–881.
DIFABIOA, GIORGIA, MASTRAGOSTINOM, et al. Carbon-poly(3-methylthiophene) hybrid super-capacitors [J]. Journal of the Electrochemical Society, 2001, 148(8): A845.
HARSOJO, DOLOKSARIBU M, PRIHANDOKO B, et al. The effect of reduced graphene oxide on the activated carbon metal oxide supercapacitor [J]. Materials Today: Proceedings, 2019, 13: 181–186.
ZHANG L L, ZHAO X, STOLLER M D, et al. Highly conductive and porous activated reduced graphene oxide films for high-power supercapacitors [J]. Nano Letters, 2012, 12(4): 1806–1812.
PALEO A J, STAITI P, BRIGANDÌ A, et al. Supercapacitors based on AC/MnO2 deposited onto dip-coated carbon nanofiber cotton fabric electrodes [J]. Energy Storage Materials, 2018, 12: 204–215.
SHEN H J, ZHANG Y, SONG X L, et al. Facile hydrothermal synthesis of Actiniaria-shaped α-MnO2/activated carbon and its electrochemical performances of supercapacitor [J]. Journal of Alloys and Compounds, 2019, 770: 926–933.
WINTERSGILL M C, FONTANELLA J J, GREEN-BAUM S G, et al. D.s.c., electrical conductivity, and n.m.r. studies of salt precipitation effects in PPO complexes [J]. British Polymer Journal, 1988, 20(3): 195–198.
GREENBAUM S G, PAK Y S, WINTERSGILL M C, et al. NMR, DSC, DMA, and high pressure electrical conductivity studies in PPO complexed with sodium perchlorate [J]. Journal of the Electrochemical Society, 1988, 135(1): 235–238.
HU T, CHU X F, GAO F, et al. Acetone sensing properties of reduced graphene oxide-CdFe2O4 composites prepared by hydrothermal method [J]. Materials Science in Semiconductor Processing, 2015, 34: 146–153.
FAUTEUX D, LUPIEN M D, ROBITAILLE C D. Phase diagram, conductivity, and transference number of PEO-NaI electrolytes [J]. Journal of the Electrochemical Society, 1987, 134(11): 2761–2767.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item
the Early Career Research Award Scheme of Science and Engineering Research Board, Department of Science and Technology, Government of India (No. ECR/20216/001871)
Rights and permissions
About this article
Cite this article
Azemtsop, M.T., Mehra, R.M., Kumar, Y. et al. Physical Characterization of Ionic Liquid-Modified Polyvinyl Alcohol and Sodium Thiocyanate Polymer Electrolytes for Electrochemical Double-Layer Capacitor Application. J. Shanghai Jiaotong Univ. (Sci.) 28, 161–171 (2023). https://doi.org/10.1007/s12204-021-2397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12204-021-2397-y