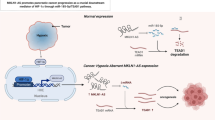
Abstract
The tumor-suppressing role of miR-455-3p has been reported in lung cancer, but the working mechanism remains to be fully elucidated. This study aims to explore the possible mechanism of miR-455-3p in regulating epithelial–mesenchymal transition (EMT) progression and angiogenesis in non-small cell lung cancer (NSCLC) cells.
The expressions of miR-455-3p, HSF1, GLS1, and EMT-related proteins (E-cadherin, N-cadherin, vimentin, and Snail-1) in both NSCLC tissues and cell lines were determined by RT-qPCR and western blot. After cell transfection, cell proliferation and angiogenesis ability on NSCLC cells were assessed by MTT and tube formation assay. The binding of miR-455-3p with HSF1 was measured by luciferase reporter gene assay, while the interaction between HSF1 and GLS1 was determined by co-immunoprecipitation assay (Co-IP).
HSF1 was highly expressed in NSCLC tissues and cells. Inhibition of HSF1 expression or overexpression of miR-455-3p in NSCLC cells can suppress cell proliferation, angiogenesis ability, and EMT progression. miR-455-3p was found to negatively regulate HSF1 expression. Co-transfection of miR-455-3p overexpression and HSF1 inhibition in NSCLC cells showed that miR-455-3p can partially counteract the effect of HSF1 in NSCLC cells. HSF1 can interact with GLS1 and elevate the expression of GLS1. GLS1 can partially abolish the suppressive effect of miR-455-3p in NSCLC cells.
miR-455-3p can bind HSF1 to suppress the GLS1 in NSCLC cells, therefore suppressing EMT progression and angiogenesis of NSCLC cells.






Similar content being viewed by others
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
16 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12192-022-01259-z
References
Burja B et al (2019) Olive leaf extract attenuates inflammatory activation and DNA damage in human arterial endothelial cells. Front Cardiovasc Med 6(56.
Cao J et al (2019) Expression of GLS1 in intrahepatic cholangiocarcinoma and its clinical significance. Mol Med Rep 20(2):1915–1924
Carpenter RL, Gokmen-Polar Y (2019) HSF1 as a cancer biomarker and therapeutic target. Curr Cancer Drug Targets 19(7):515–524
Cui H et al (2019) Inhibition of glutaminase 1 attenuates experimental pulmonary fibrosis. Am J Respir Cell Mol Biol 61(4):492–500
Duma N, Santana-Davila R, Molina JR (2019) Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 94(8):1623–1640
Hensley CT, Wasti AT, DeBerardinis RJ (2013) Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 123(9):3678–3684
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553(7689):446–454
Jiang S et al (2015) Multifaceted roles of HSF1 in cancer. Tumour Biol 36(7):4923–4931
Jonna S, Subramaniam DS (2019) Molecular diagnostics and targeted therapies in non-small cell lung cancer (NSCLC): an update. Discov Med 27(148):167–170
Lee JS, Kang JH, Lee SH, Lee CH, Son J, Kim SY (2016) Glutaminase 1 inhibition reduces thymidine synthesis in NSCLC. Biochem Biophys Res Commun 477(3):374–382
Li G, Song Y, Zhang Y, Wang H, Xie J (2016a) miR-34b targets HSF1 to suppress cell survival in acute myeloid leukemia. Oncol Res 24(2):109–116
Li J et al (2018) Heat shock factor 1 epigenetically stimulates glutaminase-1-dependent mTOR activation to promote colorectal carcinogenesis. Mol Ther 26(7):1828–1839
Li YJ, Ping C, Tang J, Zhang W (2016b) MicroRNA-455 suppresses non-small cell lung cancer through targeting ZEB1. Cell Biol Int 40(6):621–628
Liang W et al (2018) MicroRNA-644a promotes apoptosis of hepatocellular carcinoma cells by downregulating the expression of heat shock factor 1. Cell Commun Signal 16(1):30
Ren L et al (2020) Glutaminase-1 (GLS1) inhibition limits metastatic progression in osteosarcoma. Cancer Metab 8(4.
Song P et al (2020) Beta-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol Cancer 19(1):129
Wan T, Shao J, Hu B, Liu G, Luo P, Zhou Y (2018) Prognostic role of HSF1 overexpression in solid tumors: a pooled analysis of 3,159 patients. Onco Targets Ther 11(383–393.
Wang G, Cao P, Fan Y (1874) Tan K (2020) Emerging roles of HSF1 in cancer: cellular and molecular episodes. Biochim Biophys Acta Rev Cancer 1:188390
Wei S et al (2019) miR-455–3p alleviates hepatic stellate cell activation and liver fibrosis by suppressing HSF1 expression. Mol Ther Nucleic Acids 16(758–769.
Workman P (2020) Reflections and outlook on targeting HSP90, HSP70 and HSF1 in cancer: a personal perspective. Adv Exp Med Biol 1243(163–179.
Yun HH, Baek JY, Seo G, Kim YS, Ko JH, Lee JH (2018) Effect of BIS depletion on HSF1-dependent transcriptional activation in A549 non-small cell lung cancer cells. Korean J Physiol Pharmacol 22(4):457–465
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the affiliation has been updated
Rights and permissions
About this article
Cite this article
Meng, C., Liu, K., Cai, X. et al. Mechanism of miR-455–3 in suppressing epithelial–mesenchymal transition and angiogenesis of non-small cell lung cancer cells. Cell Stress and Chaperones 27, 107–117 (2022). https://doi.org/10.1007/s12192-022-01254-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-022-01254-4