Abstract
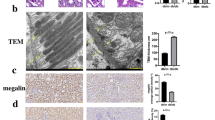
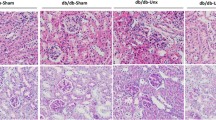
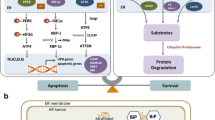
Regulation of the endoplasmic reticulum (ER) stress-response pathway during the course of diabetes specifically in renal tubules is unclear. Since tubule cell dysfunction is critical to progression of diabetic nephropathy, this study analyzed markers of ER stress response and ER chaperones at different stages of diabetes and in different renal tubule subtypes of OVE26 type-1 diabetic mice. ER stress-response-induced chaperones GRP78, GRP94, and protein disulfide isomerase (PDI) were increased in isolated cortical tubules of older diabetic mice, while PDI was decreased in tubules of young diabetic mice. Immunofluorescence staining of kidneys from older mice showed GRP78 and PDI upregulation in all cortical tubule segments, with substantial induction of PDI in distal tubules. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) phosphorylation was increased in cortical tubules of young diabetic mice, with no differences between older diabetic and control mice. Expression of ER stress-induced PERK inhibitor p58IPK was decreased and then increased in all tubule subtypes of young and older mice, respectively. Knockdown of PERK by small interfering RNA (siRNA) increased fibronectin secretion in cultured proximal tubule cells. Tubules of older diabetic mice had significantly more apoptotic cells, and ER stress-induced pro-apoptotic transcription factor C/EBP homologous protein (CHOP) was increased in proximal and distal tubules of diabetic mice and diabetic humans. CHOP induction in OVE26 mice was not altered by severity of proteinuria. Overexpression of CHOP in cultured proximal tubule cells increased expression of fibronectin. These findings demonstrate differential ER stress-response signaling in tubule subtypes of diabetic mice and implicate a role for PERK and CHOP in tubule cell matrix protein production.






Similar content being viewed by others
References
Advani A, Gilbert RE, Thai K, Gow RM, Langham RG, Cox AJ, Connelly KA, Zhang Y, Herzenberg AM, Christensen PK, Pollock CA, Qi W, Tan SM, Parving HH, Kelly DJ (2009) Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol 20:730–741
Asmellash S, Stevens JL, Ichimura T (2005) Modulating the endoplasmic reticulum stress response with trans-4,5-dihydroxy-1,2-dithiane prevents chemically induced renal injury in vivo. Toxicol Sci 88:576–584
Barresi G, Tuccari G, Arena F (1988) Peanut and Lotus tetragonolobus binding sites in human kidney from congenital nephrotic syndrome of Finnish type. Histochemistry 89:117–120
Brezniceanu ML, Liu F, Wei CC, Tran S, Sachetelli S, Zhang SL, Guo DF, Filep JG, Ingelfinger JR, Chan JS (2007) Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 71:912–923
Brezniceanu ML, Lau CJ, Godin N, Chénier I, Duclos A, Ethier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS (2010) Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol 21:943–954
Brosius FC 3rd (2008) New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord 9:245–254
Carlson EC, Audette JL, Klevay LM, Nguyen H, Epstein PN (1997) Ultrastructural and functional analyses of nephropathy in calmodulin-induced diabetic transgenic mice. Anat Rec 247:9–19
Datta R, Shah GN, Rubbelke TS, Waheed A, Rauchman M, Goodman AG, Katze MG, Sly WS (2010) Progressive renal injury from transgenic expression of human carbonic anhydrase IV folding mutants is enhanced by deficiency of p58IPK. Proc Natl Acad Sci U S A 107:6448–6452
Harwood SM, Allen DA, Raftery MJ, Yaqoob MM (2007) High glucose initiates calpain-induced necrosis before apoptosis in LLC-PK1 cells. Kidney Int 71:655–663
Hryciw DH, Lee EM, Pollock CA, Poronnik P (2004) Molecular changes in proximal tubule function in diabetes mellitus. Clin Exp Pharmacol Physiol 31:372–379
Kimura K, Jin H, Ogawa M, Aoe T (2008) Dysfunction of the ER chaperone BiP accelerates the renal tubular injury. Biochem Biophys Res Commun 366:1048–1053
Laurindo FR, Pescatore LA, Fernandes Dde C (2012) Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med 52:1954–1969
Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlöndorff D (2008) Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 19:2225–2236
Liu H, Bowes RC 3rd, van de Water B, Sillence C, Nagelkerke JF, Stevens JL (1997) Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem 272:21751–21759
Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z (2008) Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 370:651–656
Magri CJ, Fava S (2009) The role of tubular injury in diabetic nephropathy. Eur J Intern Med 20:551–555
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077
Muruganandan S, Cribb AE (2006) Calpain-induced endoplasmic reticulum stress and cell death following cytotoxic damage to renal cells. Toxicol Sci 94:118–128
Nardai G, Stadler K, Papp E, Korcsmáros T, Jakus J, Csermely P (2005) Diabetic changes in the redox status of the microsomal protein folding machinery. Biochem Biophys Res Commun 334:787–795
Ni M, Zhang Y, Lee AS (2011) Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J 434:181–188
Ohse T, Inagi R, Tanaka T, Ota T, Miyata T, Kojima I, Ingelfinger JR, Ogawa S, Fujita T, Nangaku M (2006) Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 70:1447–1455
Powell DW, Bertram CC, Cummins TD, Barati MT, Zheng S, Epstein PN, Klein JB (2009) Renal tubulointerstitial fibrosis in OVE26 type 1 diabetic mice. Nephron Exp Nephrol 111:e11–e19
Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP (1997) J Lab Clin Med 129:318–329
Rosengren V, Johansson H, Lehtiö J, Fransson L, Sjöholm A, Ortsäter H (2012) Thapsigargin down-regulates protein levels of GRP78/BiP in INS-1E cells. J Cell Biochem 113:1635–1644
Rutkowski DT, Kaufman RJ (2007) That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci 32:469–476
Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4:e374
Rutkowski DT, Kang SW, Goodman AG, Garrison JL, Taunton J, Katze MG, Kaufman RJ, Hegde RS (2007) The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell 18:3681–3691
Sanchez-Niño MD, Benito-Martin A, Ortiz A (2010) New paradigms in cell death in human diabetic nephropathy. Kidney Int 78:737–744
Santos CX, Tanaka LY, Wosniak J, Laurindo FR (2009) Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11:2409–2427
Shi Y, Porter K, Parameswaran N, Bae HK, Pestka JJ (2009) Role of GRP78/BiP degradation and ER stress in deoxynivalenol-induced interleukin-6 upregulation in the macrophage. Toxicol Sci 109:247–255
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13:184–190
Thomas MC, Burns WC, Cooper ME (2005) Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis 12:177–186
Thongboonkerd V, Barati MT, McLeish KR, Benarafa C, Remold-O’Donnell E, Zheng S, Rovin BH, Pierce WM, Epstein PN, Klein JB (2004) Alterations in the renal elastin-elastase system in type 1 diabetic nephropathy identified by proteomic analysis. J Am Soc Nephrol 15:650–662
Vallon V (2011) The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 300:R1009–R1022
Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L, Lauria F, Miji M, Deferrari G, Garibotto G (2007) Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int 72:1262–1272
Wang S, Kaufman RJ (2012) The impact of the unfolded protein response on human disease. J Cell Biol 197:857–867
Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ (2007) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13:351–364
Wu J, Zhang R, Torreggiani M, Ting A, Xiong H, Striker GE, Vlassara H, Zheng F (2010a) Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 176:2163–2176
Wu X, He Y, Jing Y, Li K, Zhang J (2010b) Albumin overload induces apoptosis in renal tubular epithelial cells through a CHOP-dependent pathway. OMICS 14:61–73
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664
Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG (2002) Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci U S A 99:15920–15925
Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN (2004) Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53:3248–3257
Zheng S, Carlson EC, Yang L, Kralik PM, Huang Y, Epstein PN (2008) Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J Am Soc Nephrol 19:2077–2085
Zhuang A, Forbes JM (2014) Stress in the kidney is the road to pERdition: is endoplasmic reticulum stress a pathogenic mediator of diabetic nephropathy? J Endocrinol 222:R97–R111
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995
Acknowledgment
B.D.K. current affiliation: Indiana University Purdue University Indianapolis School of Informatics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work was supported by National Institutes of Health (NIH) grant K01-DK080951 (M.T.B.). J.B.K. was supported by a Department of Energy grant and the Kentucky Research Challenge Trust. M.J.R. was supported by NIH R01-075212, D.W.P. was supported by NIH R21-AI103980, and L.C. was supported by NIH 1R01DK091338.
Additional information
Madhavi J. Rane and Jon B. Klein are equal contributing senior authors.
Rights and permissions
About this article
Cite this article
Barati, M.T., Powell, D.W., Kechavarzi, B.D. et al. Differential expression of endoplasmic reticulum stress-response proteins in different renal tubule subtypes of OVE26 diabetic mice. Cell Stress and Chaperones 21, 155–166 (2016). https://doi.org/10.1007/s12192-015-0648-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0648-2