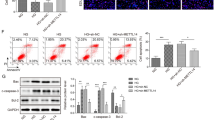
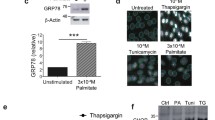
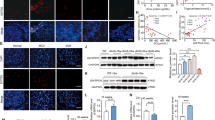
Abstract
Aim
Reduced albumin reabsorption in proximal tubular epithelial cells (PTECs), resulting from decreased megalin plasma membrane (PM) localization due to prolonged endoplasmic reticulum (ER) stress, potentially contributes to albuminuria in early diabetic kidney disease (DKD). To examine this possibility, we investigated the cytoprotective effect of TMBIM6 in promoting diabetic PTEC survival and albumin endocytosis by attenuating ER stress with an IRE1α inhibitor, KIRA6.
Methods and results
Renal TMBIM6 distribution and expression were determined by immunohistochemistry, western blotting, and qPCR, whereas tubular injury was evaluated in db/db mice. High-glucose (HG)-treated HK-2 cells were either treated with KIRA6 or transduced with a lentiviral vector for TMBIM6 overexpression. ER stress was measured by western blotting and ER-Tracker Red staining, whereas apoptosis was determined by performing TUNEL assays. Megalin expression was measured by immunofluorescence, and albumin endocytosis was evaluated after incubating cells with FITC-labeled albumin. Tubular injury and TMBIM6 downregulation occurred in db/db mouse renal cortical tissues. Both KIRA6 treatment and TMBIM6 overexpression inhibited ER stress by decreasing the levels of phosphorylated IRE1α, XBP1s, GRP78, and CHOP, and stabilizing ER expansion in HG-treated HK-2 cells. TUNEL assays performed with KIRA6-treated or TMBIM6-overexpressing cells showed a significant decrease in apoptosis, consistent with the significant downregulation of BAX and upregulation of BCL-2, as measured by immunoblotting. Both KIRA6 and TMBIM6 overexpression promoted megalin PM localization and restored albumin endocytosis in HG-treated HK-2 cells.
Conclusion
TMBIM6 promoted diabetic PTEC survival and albumin endocytosis by negatively regulating the IRE1α branch of ER stress.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
International Diabetes Federation (2019) IDF Diabetes Atlas, 9th edn. Available at: https://wwwdiabetesatlasorg.
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12(12):2032–2045. doi:https://doi.org/10.2215/cjn.11491116
Levey AS, Gansevoort RT, Coresh J et al (2020) Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 75(1):84–104. doi:https://doi.org/10.1053/j.ajkd.2019.06.009
Figueira MF, Castiglione RC, de Lemos Barbosa CM et al (2017) Diabetic rats present higher urinary loss of proteins and lower renal expression of megalin, cubilin, ClC-5, and CFTR. Physiol Rep 5(13). doi:https://doi.org/10.14814/phy2.13335
Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D (2009) Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20(3):489–494. doi:https://doi.org/10.1681/asn.2008050503
Dickson LE, Wagner MC, Sandoval RM, Molitoris BA (2014) The proximal tubule and albuminuria: really! J Am Soc Nephrol 25(3):443–453. doi:https://doi.org/10.1681/asn.2013090950
Zhou L, Liu F, Huang XR et al (2011) Amelioration of albuminuria in ROCK1 knockout mice with streptozotocin-induced diabetic kidney disease. Am J Nephrol 34(5):468–475. doi:https://doi.org/10.1159/000332040
Tojo A, Onozato ML, Kurihara H, Sakai T, Goto A, Fujita T (2003) Angiotensin II blockade restores albumin reabsorption in the proximal tubules of diabetic rats. Hypertens Res 26(5):413–419. doi:https://doi.org/10.1291/hypres.26.413
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529. doi:https://doi.org/10.1038/nrm2199
Cunard R (2015) Endoplasmic Reticulum Stress in the Diabetic Kidney, the Good, the Bad and the Ugly. J Clin Med 4(4):715–740. doi:https://doi.org/10.3390/jcm4040715
Zhuang A, Forbes JM (2014) Stress in the kidney is the road to pERdition: is endoplasmic reticulum stress a pathogenic mediator of diabetic nephropathy? J Endocrinol 222(3):R97–111. doi:https://doi.org/10.1530/joe-13-0517
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102. doi:https://doi.org/10.1038/nrm3270
Inagi R, Ishimoto Y, Nangaku M (2014) Proteostasis in endoplasmic reticulum–new mechanisms in kidney disease. Nat Rev Nephrol 10(7):369–378. doi:https://doi.org/10.1038/nrneph.2014.67
Huang Y, Sun Y, Cao Y et al (2017) HRD1 prevents apoptosis in renal tubular epithelial cells by mediating eIF2α ubiquitylation and degradation. Cell Death Dis 8(12):3202. doi:https://doi.org/10.1038/s41419-017-0002-y
Mohammed-Ali Z, Lu C, Marway MK et al (2017) Endoplasmic reticulum stress inhibition attenuates hypertensive chronic kidney disease through reduction in proteinuria. Sci Rep 7:41572. doi:https://doi.org/10.1038/srep41572
Lebeaupin C, Blanc M, Vallée D, Keller H, Bailly-Maitre B (2020) BAX inhibitor-1: between stress and survival. Febs j 287(9):1722–1736. doi:https://doi.org/10.1111/febs.15179
Lisbona F, Rojas-Rivera D, Thielen P et al (2009) BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell 33(6):679–691. doi:https://doi.org/10.1016/j.molcel.2009.02.017
Bailly-Maitre B, Belgardt BF, Jordan SD et al (2010) Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem 285(9):6198–6207. doi:https://doi.org/10.1074/jbc.M109.056648
Wang J, Zhu P, Li R, Ren J, Zhang Y, Zhou H(2020) Bax inhibitor 1 preserves mitochondrial homeostasis in acute kidney injury through promoting mitochondrial retention of PHB2. Theranostics. 10(1):384–397. doi:https://doi.org/10.7150/thno.40098
Yadav RK, Lee GH, Lee HY et al (2015) TMBIM6 (transmembrane BAX inhibitor motif containing 6) enhances autophagy and reduces renal dysfunction in a cyclosporine A-induced nephrotoxicity model. Autophagy 11(10):1760–1774. doi:https://doi.org/10.1080/15548627.2015.1082021
Wang X, Zhao L, Ajay AK, Jiao B, Zhang X, Wang C, Gao X, Yuan Z, Liu H, Liu WJ (2019) QiDiTangShen Granules Activate Renal Nutrient-Sensing Associated Autophagy in db/db Mice. Front Physiol 10:1224. doi:https://doi.org/10.3389/fphys.2019.01224
Zhao TT, Zhang HJ, Lu XG et al (2014) Chaihuang-Yishen granule inhibits diabetic kidney disease in rats through blocking TGF-β/Smad3 signaling. PLoS ONE 9(3):e90807. doi:https://doi.org/10.1371/journal.pone.0090807
Marquardt A, Al-Dabet MM, Ghosh S et al (2017) Farnesoid X Receptor Agonism Protects against Diabetic Tubulopathy: Potential Add-On Therapy for Diabetic Nephropathy. J Am Soc Nephrol 28(11):3182–3189. doi:https://doi.org/10.1681/asn.2016101123
Peruchetti DB, Silva-Aguiar RP, Siqueira GM, Dias WB, Caruso-Neves C (2018) High glucose reduces megalin-mediated albumin endocytosis in renal proximal tubule cells through protein kinase B O-GlcNAcylation. J Biol Chem 293(29):11388–11400. doi:https://doi.org/10.1074/jbc.RA117.001337
Durán M, Burballa C, Cantero-Recasens G et al (2021) Novel Dent disease 1 cellular models reveal biological processes underlying ClC-5 loss-of-function. Hum Mol Genet 30(15):1413–1428. doi:https://doi.org/10.1093/hmg/ddab131
Shu S, Wang H, Zhu J et al (2021) Reciprocal regulation between ER stress and autophagy in renal tubular fibrosis and apoptosis. Cell Death Dis 12(11):1016. doi:https://doi.org/10.1038/s41419-021-04274-7
Chou X, Ma K, Shen Y, Min Z, Wu Q, Sun D (2021) Dual role of inositol-requiring enzyme 1α (IRE-1α) in Cd-induced apoptosis in human renal tubular epithelial cells: Endoplasmic reticulum stress and STAT3 signaling activation. Toxicology 456:152769. doi:https://doi.org/10.1016/j.tox.2021.152769
Chen X, Han Y, Gao P et al (2019) Disulfide-bond A oxidoreductase-like protein protects against ectopic fat deposition and lipid-related kidney damage in diabetic nephropathy. Kidney Int 95(4):880–895. doi:https://doi.org/10.1016/j.kint.2018.10.038
Qu X, Zhai B, Liu Y et al (2022) Pyrroloquinoline quinone ameliorates renal fibrosis in diabetic nephropathy by inhibiting the pyroptosis pathway in C57BL/6 mice and human kidney 2 cells. Biomed Pharmacother 150:112998. doi:https://doi.org/10.1016/j.biopha.2022.112998
Ni L, Yuan C, Wu X(2021) Endoplasmic Reticulum Stress in Diabetic Nephrology: Regulation, Pathological Role, and Therapeutic Potential. Oxid Med Cell Longev. 2021:7277966. doi:https://doi.org/10.1155/2021/7277966
Jiang WJ, Xu CT, Du CL et al (2022) Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics 12(1):324–339. doi:https://doi.org/10.7150/thno.63735
Qiu D, Song S, Wang Y et al (2022) NAD(P)H: quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating Sirt1 in diabetic nephropathy. J Transl Med 20(1):44. doi:https://doi.org/10.1186/s12967-021-03197-3
Ishikawa T, Watanabe N, Nagano M, Kawai-Yamada M, Lam E (2011) Bax inhibitor-1: a highly conserved endoplasmic reticulum-resident cell death suppressor. Cell Death Differ 18(8):1271–1278. doi:https://doi.org/10.1038/cdd.2011.59
Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y (2021) Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol 18(7):499–521. doi:https://doi.org/10.1038/s41569-021-00511-w
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13(3):184–190. doi:https://doi.org/10.1038/ncb0311-184
Wang S, Wang Z, Fan Q et al (2016) Ginkgolide K protects the heart against endoplasmic reticulum stress injury by activating the inositol-requiring enzyme 1α/X box-binding protein-1 pathway. Br J Pharmacol 173(15):2402–2418. doi:https://doi.org/10.1111/bph.13516
Wu L, Wang Q, Guo F et al (2021) Involvement of miR-27a-3p in diabetic nephropathy via affecting renal fibrosis, mitochondrial dysfunction, and endoplasmic reticulum stress. J Cell Physiol 236(2):1454–1468. doi:https://doi.org/10.1002/jcp.29951
Li X, Zhang DQ, Wang X et al (2022) Irisin alleviates high glucose-induced hypertrophy in H9c2 cardiomyoblasts by inhibiting endoplasmic reticulum stress. Peptides 152:170774. doi:https://doi.org/10.1016/j.peptides.2022.170774
Zhang X, Zhou C, Miao L et al(2021) Panax Notoginseng Protects against Diabetes-Associated Endothelial Dysfunction: Comparison between Ethanolic Extract and Total Saponin. Oxid Med Cell Longev. 2021:4722797. doi:https://doi.org/10.1155/2021/4722797
Bailly-Maitre B, Fondevila C, Kaldas F et al (2006) Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A 103(8):2809–2814. doi:https://doi.org/10.1073/pnas.0506854103
Zhang J, Zhang J, Ni H et al (2021) Downregulation of XBP1 protects kidney against ischemia-reperfusion injury via suppressing HRD1-mediated NRF2 ubiquitylation. Cell Death Discov 7(1):44. doi:https://doi.org/10.1038/s41420-021-00425-z
Hetz C, Bernasconi P, Fisher J et al (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312(5773):572–576. doi:https://doi.org/10.1126/science.1123480
Rong J, Chen L, Toth JI, Tcherpakov M, Petroski MD, Reed JC (2011) Bifunctional apoptosis regulator (BAR), an endoplasmic reticulum (ER)-associated E3 ubiquitin ligase, modulates BI-1 protein stability and function in ER Stress. J Biol Chem 286(2):1453–1463. doi:https://doi.org/10.1074/jbc.M110.175232
Isbat M, Zeba N, Kim SR, Hong CB (2009) A BAX inhibitor-1 gene in Capsicum annuum is induced under various abiotic stresses and endows multi-tolerance in transgenic tobacco. J Plant Physiol 166(15):1685–1693. doi:https://doi.org/10.1016/j.jplph.2009.04.017
Feldman HC, Tong M, Wang L et al (2016) Structural and Functional Analysis of the Allosteric Inhibition of IRE1α with ATP-Competitive Ligands. ACS Chem Biol 11(8):2195–2205. doi:https://doi.org/10.1021/acschembio.5b00940
Ghosh R, Wang L, Wang ES et al (2014) Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158(3):534–548. doi:https://doi.org/10.1016/j.cell.2014.07.002
Gekle M (2005) Renal tubule albumin transport. Annu Rev Physiol 67:573–594. doi:https://doi.org/10.1146/annurev.physiol.67.031103.154845
Teixeira DE, Peruchetti DB, Souza MC, das Graças Henriques MG, Pinheiro AAS, Caruso-Neves C (2020) A high salt diet induces tubular damage associated with a pro-inflammatory and pro-fibrotic response in a hypertension-independent manner. Biochim Biophys Acta Mol Basis Dis 1866(11):165907. doi:https://doi.org/10.1016/j.bbadis.2020.165907
D’Amico G, Bazzi C (2003) Pathophysiology of proteinuria. Kidney Int 63(3):809–825. doi:https://doi.org/10.1046/j.1523-1755.2003.00840.x
Gekle M, Mildenberger S, Freudinger R, Silbernagl S (1998) Long-term protein exposure reduces albumin binding and uptake in proximal tubule-derived opossum kidney cells. J Am Soc Nephrol 9(6):960–968. doi:https://doi.org/10.1681/asn.V96960
Caruso-Neves C, Pinheiro AA, Cai H, Souza-Menezes J, Guggino WB (2006) PKB and megalin determine the survival or death of renal proximal tubule cells. Proc Natl Acad Sci U S A 103(49):18810–18815. doi:https://doi.org/10.1073/pnas.0605029103
Osoro EK, Du X, Liang D et al (2021) Induction of PDCD4 by albumin in proximal tubule epithelial cells potentiates proteinuria-induced dysfunctional autophagy by negatively targeting Atg5. Biochem Cell Biol 99(5):617–628. doi:https://doi.org/10.1139/bcb-2021-0028
Li X, Zou T, Wang S et al (2021) Mechanism and restoration strategy of lysosomal abnormalities induced by urinary protein overload in proximal tubule epithelial cells. Dev Dyn 250(7):943–954. doi:https://doi.org/10.1002/dvdy.297
Morigi M, Macconi D, Zoja C et al (2002) Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J Am Soc Nephrol 13(5):1179–1189
Kawanami D, Matoba K, Takeda Y et al (2017) SGLT2 Inhibitors as a Therapeutic Option for Diabetic Nephropathy. Int J Mol Sci 18(5). doi:https://doi.org/10.3390/ijms18051083
Abdel-Rafei MK, Thabet NM, Rashed LA, Moustafa EM (2021) Canagliflozin, a SGLT-2 inhibitor, relieves ER stress, modulates autophagy and induces apoptosis in irradiated HepG2 cells: Signal transduction between PI3K/AKT/GSK-3β/mTOR and Wnt/β-catenin pathways; in vitro. J Cancer Res Ther 17(6):1404–1418. doi:https://doi.org/10.4103/jcrt.JCRT_963_19
Gravotta D, Perez Bay A, Jonker CTH et al (2019) Clathrin and clathrin adaptor AP-1 control apical trafficking of megalin in the biosynthetic and recycling routes. Mol Biol Cell 30(14):1716–1728. doi:https://doi.org/10.1091/mbc.E18-12-0811
Chen J, Wu H, Tang X, Chen L (2022) 4-Phenylbutyrate protects against rifampin-induced liver injury via regulating MRP2 ubiquitination through inhibiting endoplasmic reticulum stress. Bioengineered 13(2):2866–2877. doi:https://doi.org/10.1080/21655979.2021.2024970
Acknowledgements
We extend special thanks to Dr. Wei Huili for inspiring this study and for her patience and timely help during the study.
Funding
This study was funded by National Natural Science Foundation of China (grant numbers 81774273 and 82004275).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, H., Shi, Y., Zhou, Y. et al. TMBIM6 promotes diabetic tubular epithelial cell survival and albumin endocytosis by inhibiting the endoplasmic reticulum stress sensor, IRE1α. Mol Biol Rep 49, 9181–9194 (2022). https://doi.org/10.1007/s11033-022-07744-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07744-z