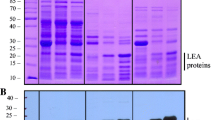
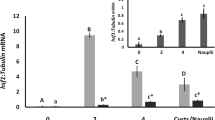
Abstract
Water loss either by desiccation or freezing causes multiple forms of cellular damage. The encysted embryos (cysts) of the crustacean Artemia franciscana have several molecular mechanisms to enable anhydrobiosis—life without water—during diapause. To better understand how cysts survive reduced hydration, group 1 late embryogenesis abundant (LEA) proteins, hydrophilic unstructured proteins that accumulate in the stress-tolerant cysts of A. franciscana, were knocked down using RNA interference (RNAi). Embryos lacking group 1 LEA proteins showed significantly lower survival than control embryos after desiccation and freezing, or freezing alone, demonstrating a role for group 1 LEA proteins in A. franciscana tolerance of low water conditions. In contrast, regardless of group 1 LEA protein presence, cysts responded similarly to hydrogen peroxide (H2O2) exposure, indicating little to no function for these proteins in diapause termination. This is the first in vivo study of group 1 LEA proteins in an animal and it contributes to the fundamental understanding of these proteins. Knowing how LEA proteins protect A. franciscana cysts from desiccation and freezing may have applied significance in aquaculture, where Artemia is an important feed source, and in the cryopreservation of cells for therapeutic applications.





Similar content being viewed by others
References
Amara I, Capellades M, Ludevid MD, Pagès M, Goday A (2013) Enhanced water stress tolerance of transgenic maize plants over-expressing LEA Rab28 gene. J Plant Physiol 170:864–873
National Oceanic and Atmospheric Administration (2011). NOAA’s 1981-2010 Climate Normals. http://www1.ncdc.noaa.gov/pub/data/normals/1981-2010/products/station/USW00024127.normals.txt. Accessed 1 August 2013
Bagshaw JC, Rafiee P, Matthews CO, MacRae TH (1986) Cadmium and zinc reversibly arrest development of Artemia larvae. Bull Environ Contam Toxicol 37:289–296
Bahrndorff S, Tunnacliffe A, Wise MJ, McGee B, Holmstrup M, Loeshcke V (2009) Bioinformatics and protein expression analyses implicates LEA proteins in the drought responses of Collembola. J Insect Physiol 55:210–217
Battista JR, Park M-J, McLemore AE (2001) Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology 43:133–139
Boswell LC, Moore DS, Hand SC (2013) Quantification of cellular protein expression and molecular features of group 3 LEA proteins from embryos of Artemia franciscana. Cell Stress Chaperones. doi:10.1007/s12192-013-0458-3
Browne J, Tunnacliffe A, Burnell A (2002) Anhydrobiosis: plant desiccation gene found in a nematode. Nature 416:38
Buitink J, Leprince O (2004) Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48:215–228
Caprioli M, Katholm AK, Melone G, Ramløv H, Ricci C, Santo N (2004) Trehalose in desiccated rotifers: a comparison between a bdelloid and a monogonont species. Comp Biochem Physiol A 139:527–532
Chakrabortee S, Boschetti C, Walton LJ, Sarkar S, Rubinsztein DC, Tunnacliffe A (2007) Hydrophilic protein associated with desiccation tolerance exhibits board stabilization function. Proc Natl Acad Sci USA 104:19073–18078
Chakrabortee S, Tripathi R, Watson M, Kaminski Schierle GS, Kurniawan DP, Kaminski CF, Wise MJ, Tunnacliffe A (2012) Intrinsically disordered proteins as molecular shields. Mol Biosys 8:210–219
Clegg JS (1986) The physical properties and metabolic status of Artemia cysts at low water contents: the ‘water replacement hypothesis’. In: Leopold AC (ed) Membranes, metabolism and dry organisms. Comstock, Ithaca, pp 169–187
Clegg JS, Trotman CNA (2002) Physiology and biochemistry of Artemia ecology. In: Abatzopolous TJ, Beardmore JA, Clegg JS, Sorgeloos P (eds) Artemia: basic and applied biology. Kluwer, Dordrecht, pp 129–170
Clegg JS, Drinkwater LE, Sorgeloos P (1996) The metabolic status of diapause embryos of Artemia franciscana (SFB). Physiol Zool 69:49–66
Copf T, Schröder R, Averof M (2004) Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci 101:17711–17715
Cornette R, Kanamori Y, Watanabe M, Nakahara Y, Gusev O, Mistumasu K, Kadono-Okuda K, Shimomura M, Mita K, Kikawada T, Okuda T (2010) Identification of anhydrobiosis-related genes from an expressed sequence tag database in the cryptobiotic midge Polypedilum vanderplanki (Diptera; Chironomidae). J Biol Chem 285:35889–35899
Crowe JH, Crowe LM, O’Dell SJ (1981) Ice formation during freezing of Artemia cysts of variable water contents. Mol Physiol 1:145–152
Dai L, Chen D-F, Liu Y-L, Zhao Y, Yang F, Yang J-S, Yang W-J (2011) Extracellular matrix peptides of Artemia cyst shell participate in protecting encysted embryos from extreme environments. PLoS ONE 6(6):e20187
de Chaffoy D, de Maeyer-Criel G, Kondo M (1978) On the permeability and formation of the embryonic cuticle during development in vivo and in vitro of Artemia salina embryos. Differentiation 12:99–109
Drinkwater LE, Crowe JH (1987) Regulation of embryonic diapause in Artemia: environmental and physiological signals. J Exp Zool 241:297–307
Duan J, Cai W (2012) OsLEA3-2, an abiotic stress gene of rice plays a key role in salt and drought tolerance. PLoS ONE 7(9):e45117
Erkut C, Vasilj A, Boland S, Habermann B, Shevchenko A, Kurzchalia TV (2013) Molecular strategies of the Caenorhabditis elegans dauer larva to survive extreme desiccation. PLoS ONE 8(12):e82473
Förster F, Liang C, Shkumatov A, Beisser D, Engelmann JC, Schnölzer M, Frohme M, Müller T, Schill RO, Dandekar T (2009) Tardigrade workbench: comparing stress-related proteins, sequence-similar and functional protein clusters as well as RNA elements in tardigrades. BMC Genomics 10:469
Furuki T, Shimizu T, Chakrabortee S, Yamakawa K, Hatanaka R, Takahashi T, Kikawada T, Okuda T, Mihara H, Tunnacliffe A, Sakurai M (2012) Effects of Group 3 LEA model peptides on desiccation-induced protein aggregation. Biochim Biophys Acta 1824:891–897
Go EC, Pandey AS, MacRae TH (1990) Effect of inorganic mercury on the emergence and hatching of the brine shrimp Artemia franciscana. Mar Biol 107:93–102
Goyal K, Tisi L, Basran A, Browne J, Burnell A, Zurdo J, Tunnacliffe A (2003) Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. J Biol Chem 278:12977–12984
Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388:151–157
Grelet J, Benamar A, Teyssier E, Avelange-Macherel M-H, Grunwald D, Macherel D (2005) Identification in pea seed mitochondria of a late embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol 137:157–167
Hand SC, Jones D, Menze MA, Witt TL (2007) Life without water: expression of plant LEA genes by an anhydrobiotic arthropod. J Exp Zool 307A:62–66
Hand SC, Menze MA, Toner M, Boswell L, Moore D (2011) LEA proteins during water stress: not just for plants anymore. Annu Rev Physiol 73:115–134
Hatanaka R, Hagiwara-Komoda Y, Furuki T, Kanamori Y, Fujita M, Cornette R, Sakurai M, Okuda T, Kikawada T (2013) An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregating protein particles. Insect Biochem Mol Biol 43:1055–1067
Hengherr S, Schill RO, Clegg JS (2011) Mechanisms associated with cellular desiccation tolerance in the animal extremophile Artemia. Physiol Biochem Zool 84:249–257
Hincha DK, Thalhammer A (2012) LEA proteins: IDPs with versatile functions in cellular dehydration tolerance. Biochem Soc Tran 40:1000–1003
Hundertmark M, Popova AV, Rausch S, Seckler R, Hincha DK (2012) Influence of drying on the secondary structure of intrinsically disordered and globular proteins. Biochem Biophys Res Commun 417:122–128
King AM, MacRae TH (2012) The small heat shock protein p26 aids development of encysting Artemia embryos, prevents spontaneous diapause termination and aids in stress. PLoS ONE 7(8):e43723
King AM, Toxopeus J, MacRae TH (2013) Functional differentiation of small heat shock proteins in diapause-destined Artemia embryos. FEBS J 280:4761–4772
King AM, Toxopeus J, MacRae TH (2014) Artemin, a diapause-specific chaperone, contributes to stress tolerance of Artemia cysts and influences their release from females. J Exp Biol. doi:10.1242/eb100081
Lapinski J, Tunncliffe A (2003) Anhydrobiosis without trehalose in bdelloid rotifers. FEBS Lett 553:387–390
Li S, Chakraborty N, Borcar A, Menze MA, Toner M, Hand S (2012) Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. Proc Natl Acad Sci USA 100:20859–20864
Liang P, MacRae TH (1999) The synthesis of small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol 207:445–456
Loi P, Iuso D, Czernik M, Zacchini F, Ptak G (2013) Towards storage of cells and gametes in dry form. Trends Biotech 31:688–695
Ma W-M, Li H-W, Dai Z-M, Yang J-S, Yang F, Yang W-J (2013) Chitin-binding proteins of Artemia diapause cysts participate in formation of the embryonic cuticle layer of cyst shells. Biochem J 449:285–294
Manfre AJ, Lanni LM, Marcotte WR (2006) The Arabidopsis group 1 LATE EMBRYOGENESIS ABUNDANT protein ATEM6 is required for normal seed development. Plant Physiol 140:140–149
Marunde MR, Samarajeewa DA, Anderson J, Li S, Hand SC, Menze MA (2013) Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J Insect Physiol 59:377–386
Menze MA, Boswell L, Toner M, Hand SC (2009) Occurrence of mitochondria-targeted late embryogenesis abundant (LEA) gene in animals increases organelle resistance to water stress. J Biol Chem 284:10714–10719
Mowla SB, Cuypers A, Driscoll SP, Kiddle G, Thomson J, Foyer CH, Theodoulou FL (2006) Yeast complementation reveals a role for an Arabidopsis thaliana late embryogenesis abundant (LEA)-like protein in oxidative stress tolerance. Plant J 48:743–756
Pandey AS, MacRae TH (1991) Toxicity of organic mercury compounds to the developing brine shrimp, Artemia. Ecotoxic Environ Saf 21:68–79
Qiu Z, MacRae TH (2008) ArHsp22, a developmentally regulated small heat shock protein produced in diapause-destined Artemia embryos, is stress inducible in adults. FEBS J 275:3556–3566
Qiu Z, MacRae TH (2010) A molecular overview of diapause in embryos of the crustacean, Artemia franciscana. In: Clark M, Cerda J, Lubzens E (eds) Dormancy and resistance in harsh environments. Springer, Berlin, pp 165–187
Rafiee P, Matthews CO, Bagshaw JC, MacRae TH (1986) Reversible arrest of Artemia development by cadmium. Can J Zool 64:1633–1641
Ramløv H, Hvidt A (1992) Artemia cysts at subzero temperatures studied by differential scanning calorimetry. Cryobiology 29:131–137
Reyes JL, Rodrigo M-J, Colmenero-Flores JM, Gil J-V, Garay-Arroyo A, Campos F, Salamini F, Bartels D, Covarrubias AA (2005) Hydrophilins from distant organisms can protect enzymatic activities from water limitation effects in vitro. Plant Cell Environ 28:709–718
Robbins HM, Van Stappen G, Sorgeloos P, Sung YY, MacRae TH, Bossier P (2010) Diapause termination and development of encysted Artemia embryos: roles for nitric oxide and hydrogen peroxide. J Exp Biol 213:1464–1470
Sakurai M, Furuki T, Akao K, Tanaka D, Nakahara Y, Kikawada T, Watanabe M, Okuda T (2008) Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki. Proc Natl Acad Sci USA 105:5093–5098
Sharon MA, Kozarova A, Clegg JS, Vacratsis PO, Warner AH (2009) Characterization of a group 1 late embryogenesis abundant protein in encysted embryos of the brine shrimp Artemia franciscana. Biochem Cell Biol 87:415–430
Shimizu T, Kanamori Y, Furuki T, Kikawada T, Okuda T, Takahashi T, Mihara H, Sakurai M (2010) Desiccation-induced structuralization and glass formation of group 3 late embryogenesis abundant protein model peptides. Biochemistry 49:1093–1104
Soulages JL, Kim K, Walters C, Cushman JC (2002) Temperature-induced extended helix/random coil transitions in a group 1 late embryogenesis-abundant protein from soybean. Plant Physiol 128:822–832
Storey KB, Storey JM (2013) Molecular biology of freezing tolerance. Comp Physiol 3:1283–1308
Tolleter D, Hincha DK, Macherel D (2010) A mitochondrial late embryogenesis abundant protein stabilizes model membranes in the dry state. Biochim Biophys Acta 1798:1926–1933
Tompa K, Bokor M, Tompa P (2010) Hydration of intrinsically disordered proteins from wide-line NMR. In: Uversky V, Longhi S (eds) Instrumental analysis of intrinsically disordered proteins: assessing structure and conformation. Wiley, Hoboken, pp 345–368
Trotman CNA, Mansfield BC, Tate WP (1980) Inhibition of emergence, hatching, and protein biosynthesis in embryonic Artemia salina. Dev Biol 80:167–174
Tunnacliffe A, Wise MJ (2007) The continuing conundrum of the LEA proteins. Naturwissenschaften 94:791–812
Tunnacliffe A, Lapinski J, McGee B (2005) A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia 546:315–321
Tyson T, O’Mahony Zamora G, Wong S, Skelton M, Daly B, Jones JT, Mulvihill ED, Elsworth B, Phillips M, Blaxter M, Burnel AM (2012) A molecular analysis in the anhydrobiotic nematode Panagrolaimus superbus using expressed sequence tags. BMC Res Notes 5:68–91
Van Stappen G, Lavens P, Sorgeloos P (1998) Effects of hydrogen peroxide treatment in Artemia cysts of different geographical origin. Arch Hydrobiol Spec Issues Advanc Limnol 52:281–296
Veeramani S, Baskaralingam V (2011) Shell-bound iron dependant nitric oxide synthesis in encysted Artemia parthenogenetica embryos during hydrogen peroxide exposure. Biometals 24:1035–1044
Warner AH, Miroshnychenko O, Kozarova A, Vacratsis PO, MacRae TH, Kim J, Clegg JS (2010) Evidence for multiple group 1 late embryogenesis abundant proteins in encysted embryos of Artemia and their organelles. J Biochem 148:581–592
Warner AH, Chakrabortee S, Tunnacliffe A, Clegg JS (2012) Complexity of the heat-soluble LEA proteome in Artemia species. Comp Biochem Physiol D 7:260–267
Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA (2001) Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim Biophys Acta 1544:196–206
Wu G, Zhang H, Sun J, Liu F, Ge X, Chen W-H, Yu J, Wang W (2011) Diverse LEA (late embryogenesis abundant) and LEA-like genes and their responses to hypersaline stress in post-diapause embryonic development of Artemia franciscana. Comp Biochem Physiol B 160:32--39
Yoshida T, Arii Y, Hino K, Sawatani I, Tanaka M, Takahashi R, Bando T, Mukai K, Fukuo K (2011) High hatching rates after cryopreservation of hydrated cysts of the brine shrimp A. franciscana. Cryoletters 32:206–215
Zhao Y, Ding X, Xiang Y, Dai Z-M, Yang J-S, Yang W-J (2012) Involvement of cyclin K posttranscriptional regulation in the formation of Artemia diapause cysts. PLoS ONE 7(2):e32129
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN/7661-2011) to THM, and an NSERC Julie Payette Scholarship and Killam Pre-doctoral Scholarship awarded to JT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toxopeus, J., Warner, A.H. & MacRae, T.H. Group 1 LEA proteins contribute to the desiccation and freeze tolerance of Artemia franciscana embryos during diapause. Cell Stress and Chaperones 19, 939–948 (2014). https://doi.org/10.1007/s12192-014-0518-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-014-0518-3