Abstract
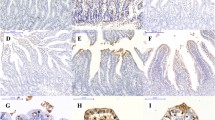
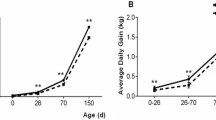
The objective of this study is to investigate the expression and distribution of heat shock protein 70 (Hsp70) in the intestine of intrauterine growth retardation (IUGR) piglets. Samples from the duodenum, prejejunum, distal jejunum, ileum, and colon of IUGR and normal-body-weight (NBW) piglets were collected at birth. The results indicated that the body and intestine weight of IUGR piglets were significantly lower than NBW piglets. The villus height and villus/crypt ratio in jejunum and ileum of IUGR piglets were significantly reduced compared to NBW piglets. These results indicated that IUGR causes abnormal gastrointestinal morphologies and gastrointestinal dysfunction. The mRNA of hsp70 was increased in prejejunum (P < 0.05), distal jejunum (P < 0.05), and colon in IUGR piglets. However, the hsp70 mRNA in ileum of piglets with IUGR was decreased. Similar to hsp70 mRNA, the protein levels of Hsp70 in prejejunum (P < 0.05), distal jejunum, and colon (P < 0.05) in IUGR piglets were higher than those in NBW piglets. These results indicated that the expression of Hsp70 in the intestinal piglets was upregulated by IUGR, and different intestinal sites had different responses to stress. Meanwhile, the localization of Hsp70 in the epithelial cells of the whole villi and intestinal gland rather than in the lamina propria and myenteron suggested that Hsp70 has a cytoprotective role in epithelial cell function and structure.




Similar content being viewed by others
Abbreviations
- IUGR:
-
Intrauterine growth retardation
- Hsp:
-
Heat shock protein
- NBW:
-
Normal body weight
- NRC:
-
National Research Council
- SD:
-
Standard deviation
- OD:
-
Optical density
- HSF:
-
Heat shock factor
References
Ahmad RH, Craig CC, Arlan R (1995) Expression of heat shock genes in hepatocytes is affected by age and food restriction in rats. J Nutri 125:410–418
Anckar J, Sistonen L (2007) Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol 594:78–88
Arispe N, Doh M, Maio AD (2002) Lipid interaction differentiates the constitutive and stress induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones 7(4):330–338
Arvans DL, Vavricka SR, Ren HY et al (2005) Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol 288:G696–G704
Bao ED, Sultan KR, Nowak B, Hartung J (2008) Localization of heat shock proteins and histopathological changes in the kidneys of transported pigs. Livest Sci 118(3):231–237
Baserga M, Bertolottob C, Maclennanc NK et al (2004) Uteroplacental insufficiency decreases small intestine growth and alters apoptotic homeostasis in term intrauterine growth retarded rats. Early Hum Dev 79:93–105
Bruce JL, Price BD, Coleman CN, Calderwood SK (1993) Oxidative injury rapidly activates the heat shock transcription factor but fails to increase levels of heat shock proteins. Cancer Res 53:12–15
Da SP, Aitken RP, Rhind SM, Racey PA, Wallace JM (2001) Influence of placental mediated foetal growth restriction on the onset of puberty in male and female lambs. Reproduction 122:375–383
Daugaard M, Rohde M, Jäättelä M (2007) The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett 581:3702–3710
David JC, Grognet JF, Lalles JP (2002) Weaning affects the expression of heat shock proteins in different regions of the gastrointestinal tract of piglets. J Nutrition 132:2551–2561
Fowden AL, Giussani DA, Forhead AJ (2005) Endocrine and metabolic programming during intrauterine development. Early Hum Dev 81:723–734
Gasbarrini A, Esposti SD, Di CC, Di NS, Loffredo S, Abraham A, Simoncini M, Pola R, Colantoni A, Trevisani F, Bernardi M, Gasbarrini G (1998) Effect of ischemia–reperfusion on heat shock protein 70 and 90 gene expression in rat liver: relation to nutritional status. Dig Dis Sci 43:2601–2605
Giussani DA, Forhead AJ, Gardner DS, Fletcher AJ, Allen WR, Fowden AL (2003) Postnatal cardiovascular function after manipulation of fetal growth by embryo transfer in the horse. J Physiol 547:67–76
Goloubinoff P, De Los Rios P (2007) The mechanism of Hsp70 chaperones (entropic) pulling the models together. Trends Biochem Sci 32(8):372–380
Gower VC, Thompson AM (1997) Location of inducible heat shock protein mRNA in the guinea pig cochlea with a nonradioactive in situ hybridization technique. Laryngoscope 107(2):228–232
Jones B, Gores GJ (1997) Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol 273:G1174–G1188
Kabakov AE, Gabai VL (1997) Heat shock proteins and cytoprotection: ATP-deprived mammalian cells. RG Landes, Austin
Khassaf M, Child RB, McArdle A, Brodie D, Esanu C, Jackson MJ (2001) Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol 90:1031–1035
Kojima M, Hoshimaru M, Aoki T, Takahashi JB, Ohtsuka T, Asahi M, Matsuura N, Kikuchi H (1996) Expression of heat shock proteins in the developing rat retina. Neurosci Lett 205:215–217
Liu YX, Li NN, You L (2008) Hsp70 is associated with endothelial activation in placental vascular diseases. Mol Med 14(9–10):561–566
Mao L, Shelden EA (2006) Developmentally regulated gene expression of the small heat shock protein Hsp27 in zebrafish embryos. Gene Expr Patterns 6:127–133
Marshman E, Ottewell PD, Potten CS, Watson AJ (2001) Caspase activation during spontaneous and radiation induced apoptosis in the murine intestine. J Pathol 195:285–292
Martin J, Masri J, Bernath A, Nishimura RN, Gera J (2008) Hsp70 associates with Rictor and is required for mTORC2 formation and activity. Biochem and Bioph Res Co 372:578–583
McMillen IC, Robinson JS (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85:571–633
Michael D, Hnat JW, Meadows DE, Brochman et al (2005) Heat shock protein-70 and 4-hydroxy-2-nonenal adducts in human placental villous tissue of normotensive, preeclamptic and intrauterine growth restricted pregnancies. Am J Obster and Gynecol 193:836–840
Miller L, Qureshi MA (1992) Molecular changes associated with heat shock treatment in avian mononuclear and lymphoid lineage cells. Poult Sci 71:473–481
Morimoto RI (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22:427–1438
Musch MW, Sugi K, Straus D, Chang EB (1999) Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology 117:115–122
National Research Council (NRC) (1998) Nutrient requirements of swine, 10th edn. National Academy Press, Washington, DC revised
Njemini R, Bautmans I, Lambert M, Demanet C, Mets T (2007) Heat shock proteins and chemokine/cytokine secretion profile in ageing and inflammation. Mech Ageing Dev 128:450–454
Oyake J, Otaka M, Jin M et al (2006) Overexpression of 70-kDa heat shock protein confers protection against monochloramine-induced gastric mucosal cell injury. Life Sci 79:300–305
Pierzchalski P, Krawiec A, Ptak-Belowska A, Baranska A, Konturek SJ, Pawlik WW (2006) The mechanism of heat-shock protein 70 gene expression abolition in gastric epithelium caused by Helicobacter pylori infection. Helicobacter 11:96–104
Ren H, Musch MW, Kojima K, Boone D, Ma A, Chang EB (2001) Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology 121:631–639
Salokhe S, Sarkar A, Kulkarni A, Mukherjee S, Pal JK (2006) Flufenoxuron, an acylurea insect growth regulator, alters development of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) by modulating levels of chitin, soluble protein content, and HSP70 and p34cdc2 in the larval tissues. Pestic Biochem Physiol 85:84–90
San YH, Ming FT, Yu TH et al (2005) Developmental changes of heat shock proteins in porcine testis by a proteomic analysis. Theriogenology 64:1940–1955
Sarge KD, Murphy SP, Morimoto RI (1993) Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol 13:1392–1407
Schwerin M, Maak S, Kalbe C, Fuerbass R (2001) Functional promoter variants of highly conserved inducible hsp70 genes significantly affect stress response. Biochimica et Biophysica Acta 1522:108–111
Selak MA, Storey BT, Peterside I, Simmons R (2003) Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab 285:E130–E137
Sepponen K, Pösö AR (2006) The inducible form of heat shock protein 70 in the serum, colon and small intestine of the pig: comparison to conventional stress markers. Vet J 171:519–524
Shah M, Stanek J, Handwerger S (1998) Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. J Histochem 30:509–518
Simmons RA, Irena SK, Selak MA (2005) Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to cell failure β-cell failure. J Biol Chem 28(31):28785–28791
Sistonen L, Sarge KD, Phillips B, Abravaya K, Morimoto RI (1992) Activation of heat shock factor 2 during hemin induced differentiation of human erythroleukemia cells. Mol Cell Biol 12:4104–4111
Takenaka IM, Hightower LE (1993) Regulation of chicken Hsp70 and Hsp90 family gene expression by transforming growth factor-b1. J Cell Physiol 155:54–62
Trahair JF, DeBarro TM, Robinson JS, Owens JA (1997) Restriction of nutrition in utero selectively inhibits gastrointestinal growth in fetal sheep. J Nutr 127:637–641
Wada I, Otaka M, Jin M (2006) Expression of HSP72 in the gastric mucosa is regulated by gastric acid in rats—correlation of HSP72 expression with mucosal protection. Biochem Bioph Res Co 349:611–618
Wang T, Huo YJ, Shi FX, Xu RJ, Hutz RJ (2005) Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate 88:66–72
Wang JJ, Chen LX, Li DF et al (2008) Intrauterine growth restriction affects the proteomes of the small intestine, liver and skeletal muscle in newborn pigs. J Nutr 138:60–66
Wataba K, Saito T, Takeuchi M et al (2004) Changed expression heat shock proteins in various pathological findings in placentas with intrauterine fetal growth restriction. Med Electron Microsc 37:170–176
Watanabe D, Otaka M, Mikami K et al (2004) Expression of a 72-kDa heat shock protein, and its protective function, in gastric mucosa in cirrhotic rats. J Gastroenterol 39:724–733
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu GY, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84:2316–2337
Xu RJ, Mellor DJ, Tungthanathanich P, Birtles MJ, Reynolds GW, Simpson HV (1992) Growth and morphological changes in the small and the large intestine in piglets during the first three days after birth. J Dev Physiol 18:161–172
Yu JM, Bao ED, Yan JY, Lei L (2008) Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones 13:327–335
Zhu L, Bao ED, Zhao RQ et al (2009) Expression of heat shock protein 60 in the tissues of transported piglets. Cell Stress Chaperones 14:61–69
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (30771569). We would like to thank Yuanxiao Wang and Jianjun Wang for their assistance regarding sample collection. We also thank Caiyong Chen for critical discussions and reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, X., Wang, T., Zhang, X. et al. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress and Chaperones 15, 335–342 (2010). https://doi.org/10.1007/s12192-009-0148-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-009-0148-3