Abstract
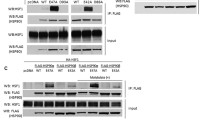
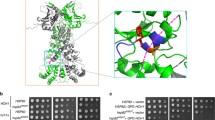
Two isoforms of the 90-kDa heat-shock protein (Hsp90), i.e., Hsp90α and Hsp90β, are expressed in the cytosol of mammalian cells. Although Hsp90 predominantly exists as a dimer, the dimer-forming potential of the β isoform of human and mouse Hsp90 is less than that of the α isoform. The 16 amino acid substitutions located in the 561–685 amino acid region of the C-terminal dimerization domain should be responsible for this impeded dimerization of Hsp90β (Nemoto T, Ohara-Nemoto Y, Ota M, Takagi T, Yokoyama K. Eur J Biochem 233: 1–8, 1995). The present study was performed to define the amino acid substitutions that cause the impeded dimerization of Hsp90β. Bacterial two-hybrid analysis revealed that among the 16 amino acids, the conversion from Ala558 of Hsp90β to Thr566 of Hsp90α and that from Met621 of Hsp90β to Ala629 of Hsp90α most efficiently reversed the dimeric interaction, and that the inverse changes from those of Hsp90α to Hsp90β primarily explained the impeded dimerization of Hsp90β We conclude that taken together, the conversion of Thr566 and Ala629 of Hsp90α to Ala558 and Met621 is primarily responsible for impeded dimerization of Hsp90β.


Similar content being viewed by others
Abbreviations
- Hsp:
-
heat-shock protein
- Hsp90:
-
90-kDa heat shock protein
- hHsp90:
-
human Hsp90
- hHsp90α and hHsp90β:
-
α and β isoforms, respectively, of hHsp90
- Grp94:
-
94-kDa glucose-regulated protein/endoplasmic reticulum paralog of Hsp90
- Trap1:
-
Trap1/mitochondrial paralog of Hsp90
- HtpG:
-
bacterial ortholog of eukaryotic Hsp90
- PAGE:
-
polyacrylamide gel electrophoresis
- SDS:
-
sodium dodecyl sulfate
References
Bardwell JC, Craig EA (1987) Eukaryotic M r 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sc. U S A 84:5177–5181
Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S (1989) Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol 9:3919–3930
Farrelly FW, Finkelstein DB (1984) Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem 259:5745–5751
Felts SJ, Owen BAL, Nguyen P-M, Trepe J, Donner DT, Toft DO (2000) The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem 275:3305–3333
Harris SF, Shiau AK, Agard DA (2004) The crystal structure of the carboxy-terminal dimerization domain of htpG, the Escherichia coli Hsp90, reveals a potential substrate binding site. Structure 12:1087–1097
Hickey E, Brandon SE, Smale G, Lloyd D, Weber LA (1989) Sequence and regulation of a gene encoding a human 89-kDa heat-shock protein. Mol Cell Biol 9:2615–2626
Imai Y, Matsushima Y, Sugimura T, Terada M (1991) A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res 19:2785
Karimova G, Ullmann A, Ladant D (2001) Protein–protein interactions between Bacillus stearothernophilus tyrosyl-tRNA-synthase subdomains revealed by a bacterial two-hybrid system. J Mol Microbiol Biotechnol 3:73–82
Kawano T, Kobayakawa T, Fukuma Y et al (2004) A comprehensive study on the immunological reactivity of Hsp90 molecular chaperone. J Biochem 136:711–722
MacLean MJ, Martinez M, Bot N, Picard D (2006) A yeast-based assay revealed a functional defect of the Q488H polymorphism in human Hsp90α. Biochem Biophys Res Commun 337:133–137
Mendel DB, Orti E (1988) Isoform composition and stoichiometry of the approximately 90-kDa heat shock protein associated with glucocorticoid receptors. J Biol Chem 263:6695–6702
Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, Piper PW, Pearl LH (2003) Structural and functional analysis of the middle segment of Hsp90: implications for ATP hydrolysis and client–protein and co-chaperone interactions. Mol Cell 11:647–658
Minami Y, Kawasaki H, Miyata Y, Suzuki K, Yahara I (1991) Analysis of native forms and their isoform compositions of the mouse 90-kDa heat-shock protein, HSP90. J Biol Chem 266:10099–10103
Minami Y, Kimura Y, Kawasaki H, Suzuki K, Yahara I (1994) The carboxy-terminal region of mammalian HSP90 is required for its dimerization and function in vivo. Mol Cell Biol 14:1459–1464
Moore SK, Kozak C, Robinson EA, Ullrich SJ, Appella E (1989) Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem 264:5343–5351
Nemoto T, Ohara-Nemoto Y, Ota M, Takagi T, Yokoyama K (1995) Mechanism of dimer formation of the 90-kDa heat-shock protein. Eur J Biochem 233:1–8
Nemoto T, Matsusaka T, Ota M, Takagi T, Collinge DB, Walther-Larsen H (1996) Dimerization characteristics of the 94-kDa glucose-regulated protein. J Biochem 120:249–256
Nemoto T, Sato N, Iwanari H, Yamashita H, Takagi T (1997) Domain structures and immunogenic regions of the 90-kDa heat-shock protein (HSP90): probing with a library of anti-HSP90 monoclonal antibodies and limited proteolysis. J Biol Chem 272:26179–26187
Nemoto TK, Ono T, Kobayakawa T, Tanaka E, Baba TT, Tanaka K, Takagi T, Gotoh T (2001) Domain–domain interactions of HtpG, an Escherichia coli homologue of eukaryotic HSP90 molecular chaperone. Eur J Biochem 268:5258–5269
Passarino G, Cavalleri GL, Stecconi R, Franceschi C, Altomare K, Dato S, Greco V, Sforza LLC, Underhill PA, Benedictis G (2003) Molecular variation of human HSP90α and HSP90β genes in Caucasians. Hum Mutat 21:554–555
Pelham HRB (1986) Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell 46:959–961
Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90:65–75
Rebbe NF, Ware J, Bertina RM, Modrich P, Sttafford DW (1987) Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene 53:235–245
Roi R (1998) Structures and expression of HSP90 family proteins. Jpn J Oral Biol 40:528–541 (in Japanese)
Schmitz G, Schmidt M, Feierabend J (1996) Characterization of a plastid-specific HSP90 homologue: identification of a cDNA sequence, phylogenetic descendence and analysis of its mRNA and protein expression. Plant Mol Biol 30:479–492
Shiau AK, Harris SF, Southworth DR, Agard DA (2006) Structural analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell 127:329–340
Song HY, Dunbar JD, Zhang YX, Guo D, Toft DO (1996) Identification of a protein with homology to hsp90 that binds to type 1 tumor necrosis factor receptor. J Biol Chem 270:3574–3581
Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP (1997) Crystal structure of an hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89:239–250
Tanaka E, Nemoto TK, Ono T (2001) Liberation of the intra-molecular interaction as the mechanism of heat-induced activation of HSP90 molecular chaperone. Eur J Biochem 268:5270–5277
Welch WJ, Feramisco JR (1982) Purification of the major mammalian heat shock proteins. J Biol Chem 257:14949–14959
Yamada S, Ono T, Mizuno A, Nemoto TK (2003) A hydrophobic segment within the C-terminal domain is essential for both client-binding and dimer formation of the HSP90-family molecular chaperone. Eur J Biochem 270:146–154
Acknowledgments
We greatly appreciate Dr. T. Ono for the technical assistance. This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (to T. K.N.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayakawa, T., Yamada, Si., Mizuno, A. et al. Substitution of only two residues of human Hsp90α causes impeded dimerization of Hsp90β. Cell Stress and Chaperones 13, 97–104 (2008). https://doi.org/10.1007/s12192-008-0017-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-008-0017-5