Abstract
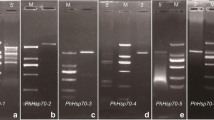
The isolation of cDNAs is described which encode the complete sequence of a precursor protein for a HSP90 homologue consisting of an N-terminal transit peptide of 5850 Da and a mature protein (cpHSP82) of 82 260 Da, located in the plastids of rye leaves (Secale cereale). Hybridization analysis indicated the presence of a single gene in the DNA of rye and a transcript size of 2.8 kb. A phylogenetic tree constructured on the basis of sequence comparisons for HSP90 homologues from different species and compartments indicated that the plastidic HSP82 from rye was more closely related to an eubacterial protein than to HSP90 homologues of the cytosol or ER from both plants and animals. The results suggest that during chloroplast evolution the gene for cpHSP82 was transferred to the nucleus from a prokaryotic endosymbiont. Immunoblots with specific antibodies and Percoll gradient-purified organelles confirmed the location of cpHSP82 in chloroplasts or non-green plastids. In green rye leaves cpHSP82 was constitutively expressed and equally distributed among tissues of different age. The expression of cpHSP82 was enhanced within 2 h by exposure to 42 °C. The cpHSP82 transcript and protein were much more strongly expressed in non-green tissues, such as etiolated, 70S ribosome-deficient 32 °C-grown, or herbicide-bleached, than in normal green leaves. Also chromoplasts from the pericarp of tomato fruits contained high levels of a HSP90 polypeptide while a photosynthetic protein, the large subunit of the ribulose-1,5-bisphosphate carboxylase was largely degraded during ripening.
Similar content being viewed by others
References
Aligue R, Akhavan-Niak H, Russell P: A role for HSP90 in cell cycle control: Weel tyrosine kinase activity requires interaction with Hsp90. EMBO J 13: 6099–6106 (1994).
Bairoch A: PROSITE: a dictionary of sites and patterns in proteins. Nucl Acids Res 20: 2013–2018 (1992).
Bardwell JCA, Craig EA: Eukaryotic Mr 83000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci USA 84: 5177–5181 (1987).
Berberich T, Feierabend J: The onset of 70S chlorplast ribosome formation is determined by an early heat-sensitive stage in the ontogeny of rye leaves. Plant Cell Physiol 35: 907–916 (1994).
Blackman R, Meselson M: Interspecific nucleotide sequence comparisons used to identify regulatory and structural features of the Drosophila hsp82 gene. J Mol Biol 188: 499–515 (1986).
Bogorad L: Evolution of organelle and eukaryotic genomes. Science 188: 891–898 (1975).
Boorstein WR, Ziegelhoffer T, Craig EA: Molecular evolution of the HSP70 multigene family. J Mol Evol 38: 1–17 (1994).
Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S: HSP82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol 9: 3919–3930 (1989).
Brinkmann H, Cerff R, Salomon M, Soll J: Cloning and sequence analysis of cDNAs encoding the cytosolic precursors of subunits GapA and GapB of chloroplast glyceraldehyde-3-phosphate dehydrogenase from pea and spinach. Plant Mol Biol 13: 81–94 (1989).
Conner TW, LaFayette PR, Nagao RT, Key JL: Sequence and expression of a HSP83 from Arabidopsis thaliana. Plant Physiol 94: 1689–1695 (1990).
Denecke J, DeRycke R, Botterman J: Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J 11: 2345–2355 (1992).
Ellis RJ, van der Vies SM: Molecular chaperones. Annu Rev Biochem 60: 321–347 (1991).
Feierabend J: Inhibition of chloroplast ribosome formation by heat in higher plants. In: Edelman M, Hallick RB, Chua N-H (eds) Methods in Chloroplast Molecular Biology, pp. 671–680. Elsevier Biomedical Press, Amsterdam (1982).
Feierabend J, Berberich T: Heat-induced ribosome-deficiency of plastids-mechanism and applications. In: Mache R, Stutz E, Subramanian AR (eds) The Translational Apparatus of Photosynthetic Organelles, pp. 215–227. Springer, Berlin/Heidelberg/New York (1991).
Feierabend J, Winkelhüsener T: Nature of photooxidative envents in leaves treated with chlorosis-inducing herbicides. Plant Physiol 70: 1277–1282 (1982).
Feierabend J, Winkelhüsener T, Kemmerich P, Schulz U: Mechanism of bleaching in leaves treated with chlorosis-inducing herbicides. Z Naturforsch 37c: 898–907 (1982).
Feierabend J, Schlüter W, Tebartz K: Unassembled polypeptides of the plastidic ribosomes in heat-treated 70S ribosome-deficient rye leaves. Planta 174: 542–550 (1988).
Feinberg AP, Vogelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6 (1983).
Felsenstein J: PHYLIP version 3.5c (1993).
Gatenby AA, Ellis RJ: Chaperone function: the assembly of ribulose bisphosphate carboxylase-oxygenase. Annu Rev Cell Biol 6: 125–149 (1990).
Gerhardt B, Beevers H: Influence of sucrose on protein determination by the Lowry procedure. Anal Biochem 24: 337–339 (1968).
Harris EH, Boynton JE, Gillham NW: Chloroplast ribosomes and protein synthesis. Microbiol Rev 58: 700–754 (1994).
Hartl FU, Hlodan R, Langer T: Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biol Sci 19: 20–25 (1994).
von Heijne G, Steppuhn J, Herrmann RG: Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180: 535–545 (1989).
Höinghaus R, Feierabend J: Rapid purification of intact chloroplasts and heat-bleached ribosome-deficient plastids from rye leaves on discontinuous Percoll gradients. Protoplasma 118: 114–120 (1983).
Jakob U, Buchner J: Assisting spontaneity: the role of HSP90 and small HSPs as molecular chaperones. Trends Biol Sci 19: 205–211 (1994).
Keegstra K, Olsen LJ, Theg SM: Chloroplastic precursors and their transport across the envelope membranes. Annu Rev Plant Physiol 40: 471–505 (1989).
Kimura Y, Yahara I, Lindquist S: Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science 268: 1362–1365 (1995).
Koning AJ, Rose R, Comai L: Developmental expression of tomato heat-shock cognate protein 80. Plant Physiol 100: 801–811 (1992).
Krishna P, Sacco M, Cherutti JF, Hill S: Cold-induced accumulation of hsp90 transripts in Brassica napus. Plant Physiol 107: 915–923 (1995).
Kulomaa MS, Weigel NL, Kleinsek DA, Beattie WG, Conneely OM, March C, Zarucki-Schulz T, Schrader WT, O'Malley BW: Amino acid sequence of a chicken heat shock protein derived from the complementary DNA nucleotide sequence. Biochemistry 25: 6244–6251 (1986).
Kurzok HG, Feierabend J: Comparison of a cytosolic and a chloroplast triosephosphate isomerase isoenzyme from rye leaves. II. Molecular properties and phylogenetic relationships. Biochim Biophys Acta 788: 222–233 (1984).
Kyhse-Andersen J: Electroblotting of multiple gels: A simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Meth 10: 203–209 (1984).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Lindquist S, Craig EA: The heat shock proteins. Annu Rev Genet 22: 631–677 (1988).
Logemann J, Schell J, Willmitzer L: Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 (1987).
Mache R, Stutz E, Subramanian AR: The Translational Apparatus of Photosynthetic Organelles. Springer, Berlin/Heidelberg/New York (1991).
Maki RG, Old LJ, Srivastava PK: Human homologue of murine tumor rejection antigen gp96: 5′ regulatory and coding regions and relationship to stress-induced proteins. Proc Natl Acad Sci USA 87: 5658–5662 (1990).
Marrs KA, Casey ES, Capitant SA, Bouchard RA, Dietrich PS, Mettler IJ, Sinibaldi RM: Characterization of two maize HSP90 heat shock protein genes: expression during heat shock, embryogenesis, and pollen development. Devel Genet 14: 27–41 (1993).
Marshall JS, DeRocher AE, Keegstra K, Vierling E: Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci USA 87: 374–378 (1990).
Marshall JS, Keegstra K: Isolation and characterization of a cDNA clone encoding the major HSP70 of the pea chloroplastic stroma. Plant Physiol 100: 1048–1054 (1992).
Martin W, Brinkmann H, Savonna C, Cerff R: Evidence for a chimeric nature of nuclear genomes: eubacterial origin of eukaryotic glyceraldehyde-3-phosphate dehydrogenase genes. Proc Natl Acad Sci USA 90: 8692–8696 (1993).
Mottram JC, Murphy WJ, Agabian N: A transcriptional analysis of the Trypanosoma brucei hsp83 gene cluster. Mol Biochem Parasitol 37: 115–128 (1989).
Neumann D, Nover L, Parthier B, Rieger R, Scharf KD, Wollgiehn R, Nieden UZ: Heat shock and other stress response systems of plants. Biol Zentralbl 108: 1–156 (1989).
Nover L: Heat Shock Response. CRC Press, Boca Raton, FL (1991).
Otto S, Feierabend J: Enzymes of starch and sugar phosphate metabolism in achlorophyllous ribosome-deficient plastids from high-temperature-grown rye leaves. Physiol Plant 76: 65–73 (1989).
Palmer JD: Plastid chromosomes: structure and evolution. In: Bogorad L, Vasil IK (eds) The Molecular Biology of Plastids, pp. 5–53. Academic Press, San Diego (1991).
Rebbe NF, Ware J, Bertring RM, Modrich P, Stafford DW: Nucleotide sequence of a cDNA for a member of the human 90 kDa heat shock protein. Gene 53: 235–242 (1987).
Reiß T, Bergfeld R, Link G, Thien W, Mohr H: Photo-oxidative destruction of chloroplasts and its consequences for cytosolic enzyme levels and plant development. Planta 159: 518–528 (1983).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463 (1977).
Schmidt M, Svendsen I, Feierabend J: Analysis of the primary structure of the chloroplast isozyme of triosephosphate isomerase from rye leaves by protein and cDNA sequencing indicates a eukaryotic origin of its gene. Biochim Biophys Acta 1261: 256–264 (1995).
Schröder G, Beck M, Eichel J, Vetter HP, Schröder J: HSP90 homologue from Madagascar periwinkle (Catharanthus roseus): cDNA sequence, regulation of protein expression and location in the endoplasmic reticulum. Plant Mol Biol 23: 583–594 (1993).
Shapira M, Pedraza G: Sequence analysis and transcriptional activation of heat shock protein 83 of Leishmania mexicana amazonensis. Mol Biochem Parasitol 42: 247–256 (1990).
Shih MC, Lazar G, Goodman HM: Evidence in favor of the symbiotic origin of chloroplasts: primary structure and evolution of tobacco glyceraldehyde-3-phosphate dehydrogenase. Cell 47: 73–80 (1986).
Thomas PS: Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA 77: 5201–5205 (1980).
Thomas PS: Hybridization of denatured RNA transferred or dotted to nitrocellulose paper. Meth Enzymol 100: 255–266 (1983).
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354 (1979).
Vierling E: The roles of heat shock proteins in plants. Annu Rev Plant Mol Biol 42: 579–620 (1991).
Waegemann K, Soll J: Characterization of the protein import apparatus in isolated outer envelopes of chloroplasts. Plant J 1: 149–158 (1991).
Walther-Larsen H, Brandt J, Collinge DB, Thordal-Christensen H: A pathogen-induced gene of barley encodes a HSP90 homologue showing striking similarity to vertebrate forms resident in the endoplasmic reticulum. Plant Mol Biol 21: 1097–1108 (1993).
Weeden NF: Evolutionary affinities of plant phosphoglucose isomerase and fructose-1,6-bisphosphatase isozymes. In: Ratazzi MC, Scandalios JG, Whitt GS (eds) Isozymes. Current Topics in Biological and Medical Research, Vol 8. Cellular Localization, Metabolism, and Physiology, pp. 53–66. Alan R. Liss, New York, NY (1983).
Weil JH: Organization and expression of the chloroplast genome. Plant Sci 49: 149–157 (1987).
Winter U, Feierabend J: Multiple coordinate controls cintribute to a balanced expression of ribulose-1,5-bisphosphate carboxylase/oxygenase subunits in rye leaves. Eur J Biochem 187: 445–453 (1990).
Yamazaki M, Akaogi K, Miwa T, Imai T, Soeda E, Yokoyama K: Nucleotide sequence of a full-length cDNA for 90 kDa heat-shock protein from human peripheral blood lymphocytes. Nucl Acids Res 17: 7108 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmitz, G., Schmidt, M. & Feierabend, J. Characterization of a plastid-specific HSP90 homologue: identification of a cDNA sequence, phylogenetic descendence and analysis of its mRNA and protein expression. Plant Mol Biol 30, 479–492 (1996). https://doi.org/10.1007/BF00049326
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00049326