Abstract
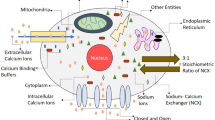
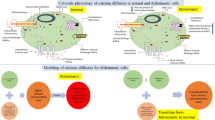
Calcium is a decisive messenger for neuronal vivid functions. The calcium intracellular sequestering major unit is the Endoplasmic Reticulum (ER). Brownian motion of calcium could be bound to different buffers like S100B, calmodulin, etc, and different organelles. Plasma membrane channels like voltage-gated calcium channels (VGCC) and Plasma Membrane Calcium ATPase (PMCA), Orai channel could perturb the calcium concentration. To investigate the calcium interplay for intracellular signaling we have developed the two-dimensional time fractional reaction–diffusion equation. To solve this model analytically, we have used the Laplace and Fourier cosine integral transform method. By using Green’s function we obtained the compact solution in closed form with Mainardi’s function and Wright’s function. Uniqueness and existence proved the more fundamental approach to the fractional reaction–diffusion problem. The fractional Caputo approach gives better insight into this real-life problem by its nonlocal nature. Significant effects of different parameters on free calcium ions were obtained and the results are interpreted with normal and Alzheimeric cells. Non-local property and dynamical aspects are graphically presented which might provide insight into the Stromal interaction molecule (STIM) and S100B parameters.








Similar content being viewed by others
Data availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
LaFerla, F.M.: Calcium dyshomeostasis and intracellular signaling in Alzheimer’s disease. Nat. Rev. Neurosci. 3, 862–872 (2002). https://doi.org/10.1038/nrn960
Kraft, R.: STIM and ORAI proteins in the nervous system. Channels 9, 245–252 (2015). https://doi.org/10.1080/19336950.2015.1071747
Bezprozvanny, I.B.: Calcium Signaling and Neurodegeneration. Acta Nat. 2, 72–80 (2010). https://doi.org/10.32607/20758251-2010-2-1-72-80
Mattson, M.R.: Calcium and neurodegeneration. Aging Cell. 6, 337–350 (2007). https://doi.org/10.1111/j.1474-9726.2007.00275.x
Smith, G.D.: Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophys J. 71, 3064–3072 (1996). https://doi.org/10.1016/S0006-3495(96)79500-0
Smith, G.D., Dai, L., Miura, R.M., Sherman, A.: Asymptotic analysis of buffered calcium diffusion near a point source. SIAM J. Appl. Math. 61, 1816–1838 (2001). https://doi.org/10.1137/S0036139900368996
González-Vélez, V., Piron, A., Dupont, G.: Calcium oscillations in pancreatic \(\alpha \)-cells Rely on noise and ATP-driven changes in membrane electrical activity. Front. Physiol. 11, 602844 (2020). https://doi.org/10.3389/fphys.2020.602844
Dupont, G., Houart, G., De Koninck, P.: Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium 34, 485–497 (2003). https://doi.org/10.1016/S0143-4160(03)00152-0
Friedhoff, V.N., Ramlow, L., Lindner, B., Falcke, M.: Models of stochastic \(\text{ Ca}^{2+}\) spiking. Eur. Phys. J. Spec. Top. 230, 2911–2928 (2021). https://doi.org/10.1140/epjs/s11734-021-00174-1
Schmeitz, C., Hernandez-Vargas, E.A., Fliegert, R., Guse, A.H., Meyer-Hermann, M.: A mathematical model of T lymphocyte calcium dynamics derived from single transmembrane protein properties. Front. Immunol. 4, 277 (2013). https://doi.org/10.3389/fimmu.2013.00277
Marhl, M., Haberichter, T., Brumen, M., Heinrich, R.: Complex calcium oscillations and the role of mitochondria and cytosolic proteins. BioSystems 57, 75–86 (2000). https://doi.org/10.1016/S0303-2647(00)00090-3
Brusch, L., Lorenz, W., Or-Guil, M., Bär, M., Kummer, U.: Fold–Hopf bursting in a model for calcium signal transduction. Zeitschrift für Physikalische Chemie. 216, 487 (2002). https://doi.org/10.1524/zpch.2002.216.4.487
Dave, D.D., Jha, B.K.: Modeling the alterations in calcium homeostasis in the presence of protein and VGCC for Alzheimer cell. In: Advances in Intelligent Systems and Computing, (pp. 181-189) (2018). https://doi.org/10.1007/978-981-10-5699-4_18
Jha, A., Adlakha, N.: Two-dimensional finite element model to study unsteady state Ca2+ diffusion in neuron involving ER LEAK and SERCA. Int. J. Biomath. 8, 1550002 (2015). https://doi.org/10.1142/S1793524515500023
Tewari, S.G., Camara, A.K.S., Stowe, D.F., Dash, R.K.: Computational analysis of Ca2+ dynamics in isolated cardiac mitochondria predicts two distinct modes of Ca2+ uptake. J. Physiol. 592, 1917–1930 (2014). https://doi.org/10.1113/jphysiol.2013.268847
Wei, N., Layton, A.T.: Theoretical assessment of the Ca 2+ oscillations in the afferent arteriole smooth muscle cell of the rat kidney. Int. J. Biomath. 11, 1850043 (2018). https://doi.org/10.1142/S1793524518500432
Pawar, A., Pardasani, K.R.: Fractional order interdependent nonlinear chaotic spatiotemporal calcium and \(A\beta \) dynamics in a neuron cell. Phys. Scr. 98, 085206 (2023). https://doi.org/10.1088/1402-4896/ace1b2
Dave, D.D., Jha, B.K.: 2D finite element estimation of calcium diffusion in Alzheimer’s affected neuron. Netw. Model. Anal. Health Inform. Bioinform. 10, 43 (2021). https://doi.org/10.1007/s13721-021-00322-6
Naik, P.A., Pardasani, K.R.: Three-dimensional finite element model to study effect of RyR calcium channel, ER leak and SERCA pump on calcium distribution in oocyte cell. Int. J. Comput. Methods 16, 1850091 (2019). https://doi.org/10.1142/S0219876218500913
Naik, P.A., Eskandari, Z., Yavuz, M., Zu, J.: Complex dynamics of a discrete-time Bazykin–Berezovskaya prey-predator model with a strong Allee effect. J. Comput. Appl. Math. 413, 114401 (2022). https://doi.org/10.1016/j.cam.2022.114401
Pawar, A., Pardasani, K.R.: Effect of disturbances in neuronal calcium and IP3 dynamics on \(\beta \)-amyloid production and degradation. Cogn. Neurodyn. 17, 239–256 (2022). https://doi.org/10.1007/s11571-022-09815-0
Jagtap, Y., Adlakha, N.: Numerical model of hepatic glycogen phosphorylase regulation by nonlinear interdependent dynamics of calcium and \(IP_{3}\). Eur. Phys. J. Plus 138, 399 (2023). https://doi.org/10.1140/epjp/s13360-023-03961-y
Kothiya, A., Adlakha, N.: Simulation of biochemical dynamics of \(C{a}^{2+}\) and \(PLC\) in fibroblast cell. J. Bioenerg. Biomembr. 55, 267–287 (2023). https://doi.org/10.1007/s10863-023-09976-5
Joshi, H., Jha, B.K.: Chaos of calcium diffusion in Parkinson’s infectious disease model and treatment mechanism via Hilfer fractional derivative. Math. Model. Numer. Simul. Appl. 1, 84–94 (2021). https://doi.org/10.53391/mmnsa.2021.01.008
Jethanandanİ, H., Jha, B.K., Ubale, M.: The role of calcium dynamics with amyloid beta on neuron-astrocyte coupling. Math. Model. Numer. Simul. Appl. 3, 376–390 (2023). https://doi.org/10.53391/mmnsa.1398320
Joshi, H., Jha, B.K.: On a reaction–diffusion model for calcium dynamics in neurons with Mittag–Leffler memory. Eur. Phys. J. Plus 136, 623 (2021). https://doi.org/10.1140/epjp/s13360-021-01610-w
Luchko, Y., Suzuki, A., Yamamoto, M.: On the maximum principle for the multi-term fractional transport equation. J. Math. Anal. Appl. 505, 125579 (2022). https://doi.org/10.1016/j.jmaa.2021.125579
Vatsal, V.H., Jha, B.K., Singh, T.P.: To study the effect of ER flux with buffer on the neuronal calcium. Eur. Phys. J. Plus 138(494), 1–14 (2023). https://doi.org/10.1140/epjp/s13360-023-04077-z
Naik, P.A., Pardasani, K.R.: Finite element model to study calcium distribution in oocytes involving voltage gated Ca2+ channel, ryanodine receptor and buffers. Alex. J. Med. 52, 43–49 (2016). https://doi.org/10.1016/j.ajme.2015.02.002
Naik, P.A., Pardasani, K.R.: Three-dimensional finite element model to study calcium distribution in oocytes. Netw. Model. Anal. Health Inform. Bioinform. 6(16), 1–11 (2017). https://doi.org/10.1007/s13721-017-0158-5
Naik, P.A., Farman, M., Zehra, A., Nisar, K.S., Hincal, E.: Analysis and modeling with fractal-fractional operator for an epidemic model with reference to COVID-19 modeling. Partial Differ. Equ. Appl. Math. 10, 100663 (2024). https://doi.org/10.1016/j.padiff.2024.100663
Singh, T., Adlakha, N.: Numerical investigations and simulation of calcium distribution in the alpha-cell. Bull. Biomath. 1, 40–57 (2023). https://doi.org/10.59292/bulletinbiomath.2023003
Kumar, H., Naik, P.A., Pardasani, K.R.: Finite element model to study calcium distribution in T lymphocyte involving buffers and ryanodine receptors. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 88, 585–590 (2018). https://doi.org/10.1007/s40010-017-0380-7
Naik, P.A.: Modeling the mechanics of calcium regulation in T lymphocyte: a finite element method approach. Int. J. Biomath. 13, 2050038 (2020). https://doi.org/10.1142/S1793524520500382
Jha, B.K., Joshi, H., Dave, D.D.: Portraying the effect of calcium-binding proteins on cytosolic calcium concentration distribution fractionally in nerve cells. Interdiscip. Sci. 10, 674–685 (2018). https://doi.org/10.1007/s12539-016-0202-7
Joshi, H., Yavuz, M.: Numerical analysis of compound biochemical calcium oscillations process in hepatocyte cells. Adv. Biol. (2024). https://doi.org/10.1002/adbi.202300647
Joshi, H., Yavuz, M., Stamova, I.: Analysis of the disturbance effect in intracellular calcium dynamic on fibroblast cells with an exponential kernel law. Bull. Biomath. 1, 24–39 (2023). https://doi.org/10.59292/bulletinbiomath.2023002
Jethanandani, H., Jha, B.K., Ubale, M.: Bifurcation analysis of calcium dynamics in nerve cell. Eur. Phys. J. Plus 138, 1159 (2023). https://doi.org/10.1140/epjp/s13360-023-04699-3
Bhattacharyya, R., Jha, B.K.: Analyzing fuzzy boundary value problems: a study on the influence of mitochondria and ER fluxes on calcium ions in neuron cells. J. Bioenerg. Biomembr. (2024). https://doi.org/10.1007/s10863-023-09994-3
Podlubny, I.: Fractional Differential Equations: An Introduction to Fractional Derivatives, Fractional Differential Equations, to Methods of Their Solution and Some of Their Applications. Academic Press (1998)
Mainardi, F., Pagnini, G.: The wright functions as solutions of the time-fractional diffusion equation. Appl. Math. Comput. 141, 51–62 (2003)
Keener, J., Sneyd, J. (eds.): Mathematical Physiology. Springer, New York, New York, NY (2009)
Zhang, H., Sun, S., Wu, L., Pchitskaya, E., Zakharova, O., Tacer, K.F., Bezprozvanny, I.: Store-operated calcium channel complex in postsynaptic spines: a new therapeutic target for Alzheimer’s disease treatment. J. Neurosci. 36, 11837–11850 (2016). https://doi.org/10.1523/JNEUROSCI.1188-16.2016
Gil, D., Guse, A.H., Dupont, G.: Three-dimensional model of sub-plasmalemmal Ca2+ microdomains evoked by the interplay between ORAI1 and InsP3 receptors. Front. Immunol. 12, 659790 (2021). https://doi.org/10.3389/fimmu.2021.659790
Manhas, N., Sneyd, J., Pardasani, K.R.: Modelling the transition from simple to complex Ca2+ oscillations in pancreatic acinar cells. J. Biosci. 39, 463–484 (2014). https://doi.org/10.1007/s12038-014-9430-3
Sneyd, J., Tsaneva-Atanasova, K., Bruce, J.I.E., Straub, S.V., Giovannucci, D.R., Yule, D.I.: A model of calcium waves in pancreatic and parotid acinar cells. Biophys. J. 85, 1392–1405 (2003). https://doi.org/10.1016/S0006-3495(03)74572-X
Dave, D.D., Jha, B.K.: Analytically depicting the calcium diffusion for Alzheimer’s affected cell. Int. J. Biomath. 11, 1850088 (2018). https://doi.org/10.1142/S1793524518500882
Berrocal, M., Mata, A.M.: The plasma membrane Ca2+-ATPase, a molecular target for Tau-induced cytosolic calcium dysregulation. Neuroscience 518, 112–118 (2022). https://doi.org/10.1016/j.neuroscience.2022.04.016
Marambaud, P., Dreses-Werringloer, U., Vingtdeux, V.: Calcium signaling in neurodegeneration. Mol. Neurodegener. 4, 1–15 (2009). https://doi.org/10.1186/1750-1326-4-20
Yagami, T., Kohma, H., Yamamoto, Y.: L-type voltage-dependent calcium channels as therapeutic targets for neurodegenerative diseases. Curr. Med. Chem. 19, 4816–4827 (2012). https://doi.org/10.2174/092986712803341430
Jha, B.K., Adlakha, N., Mehta, M.N.: Finite volume model to study the effect of ER flux on cytosolic calcium distribution in astrocytes. J. Comput. 3, 74–80 (2011)
Miller, K.S., Ross, B.: An Introduction to The Fractional Calculus and Fractional Differential Equations, (1993)
Diethelm, K.: The analysis of fractional differential equations: an application-oriented exposition using differential operators of caputo type. Lecture Notes in Mathematics. 2004 (2010)
Du, A.T., Schuff, N., Amend, D., Laakso, M.P., Hsu, Y.Y., Jagust, W.J., Yaffe, K., Kramer, J.H., Reed, B., Norman, D., Chui, H.C., Weiner, M.W.: Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 71, 441–447 (2001). https://doi.org/10.1136/jnnp.71.4.441
Dave, D.D., Jha, B.K.: 3D mathematical modeling of calcium signaling in Alzheimer’s disease. Netw. Model. Anal. Health Inform. Bioinform. 9, 1–10 (2020). https://doi.org/10.1007/s13721-019-0207-3
Cristóvaõ, J.S., Gomes, C.M.: S100 proteins in Alzheimer’s disease. Front. Neurosci. 13, 446874 (2019). https://doi.org/10.3389/fnins.2019.00463
Acknowledgements
The authors are highly thankful to the SHODH scheme, the education department, Government of Gujarat, India for financial support for carrying out this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no Conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vatsal, V.H., Jha, B.K. & Singh, T.P. Deciphering two-dimensional calcium fractional diffusion of membrane flux in neuron. J. Appl. Math. Comput. (2024). https://doi.org/10.1007/s12190-024-02115-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12190-024-02115-2