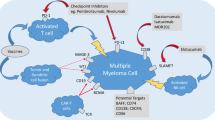
Abstract
Despite the development of various therapeutic agents, multiple myeloma remains incurable. Recently, T-cell redirected immunotherapy has become a promising strategy for the treatment of refractory myeloma. Clinical trials using chimeric antigen receptor (CAR)-T cells and bispecific antibodies have demonstrated successful anti-myeloma responses in triple-class-refractory patients. However, unique and unwanted immune effects associated with on-target/off-target reactivity of activated immune cells need to be considered and properly managed. This review summarizes recent advances in bispecific antibodies for the treatment of refractory myeloma. It outlines the history of their development, along with a discussion of their mechanisms of action and their current and potential future role in myeloma therapy. As more evidence emerges to inform the timing of CAR-T-cell therapy, the results of clinical trials and off-the-shelf nature of bispecifics also suggest the timing of their treatment. These findings will promote further development and application of bispecifics for refractory myeloma in combination with other appropriate agents.




Similar content being viewed by others
References
Anderson KC. Progress and paradigms in multiple myeloma. Clin Cancer Res. 2016;22(22):5419–27.
Bolli N, Avet-Loiseau H, Wedge DC, Van Loo P, Alexandrov LB, Martincorena I, et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun. 2014;5:2997.
Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017;8(1):268.
Ledergor G, Weiner A, Zada M, Wang SY, Cohen YC, Gatt ME, et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat Med. 2018;24(12):1867–76.
Holstein SA, Grant SJ, Wildes TM. Chimeric antigen receptor T-cell and bispecific antibody therapy in multiple myeloma: moving into the future. J Clin Oncol. 2023;41(27):4416–29.
Ludwig H, Terpos E, van de Donk N, Mateos MV, Moreau P, Dimopoulos MA, et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: a consensus report of the European Myeloma Network. Lancet Oncol. 2023;24(6):e255–69.
Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys. 1961;93:460–2.
Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305(5934):537–40.
Mack M, Riethmuller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA. 1995;92(15):7021–5.
Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–7.
Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Blanco B, Dominguez-Alonso C, Alvarez-Vallina L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin Cancer Res. 2021;27(20):5457–64.
Borlak J, Langer F, Spanel R, Schondorfer G, Dittrich C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcgamma receptors. Oncotarget. 2016;7(19):28059–74.
Firestone R, Lesokhin AM, Usmani SZ. An Embarrassment of riches: three FDA-approved bispecific antibodies for relapsed refractory multiple myeloma. Blood Cancer Discov. 2023;4(6):433–6.
O’Neill C, van de Donk N. T-cell redirecting bispecific antibodies in multiple myeloma: current landscape and future directions. Eur J Haematol. 2023;4(3):811–22.
Ravi G, Costa LJ. Bispecific T-cell engagers for treatment of multiple myeloma. Am J Hematol. 2023;98(Suppl 2):S13–21.
Swan D, Murphy P, Glavey S, Quinn J. Bispecific antibodies in multiple myeloma: opportunities to enhance efficacy and improve safety. Cancers (Basel). 2023;15(6):1819.
Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Anti-B-cell maturation antigen BiTE molecule AMG 420 induces responses in multiple myeloma. J Clin Oncol. 2020;38(8):775–83.
Harrison SJ, Minnema MC, Lee HC, Spencer A, Kapoor P, Madduri D, et al. A phase 1 first in human (FIH) study of AMG 701, an anti-B-cell maturation antigen (BCMA) half-life extended (HLE) BiTE® (bispecific T-cell engager) molecule, in relapsed/refractory (RR) multiple myeloma (MM). Blood. 2020;136(Supplement 1):28–9.
Usmani SZ, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab, a B-cell maturation antigen x CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet. 2021;398(10301):665–74.
Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495–505.
Bahlis NJ, Costello CL, Raje NS, Levy MY, Dholaria B, Solh M, et al. Elranatamab in relapsed or refractory multiple myeloma: the MagnetisMM-1 phase 1 trial. Nat Med. 2023;29(10):2570–6.
Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29(9):2259–67.
D’Souza A, Shah N, Rodriguez C, Voorhees PM, Weisel K, Bueno OF, et al. A phase I first-in-human study of ABBV-383, a B-cell maturation antigen x CD3 bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2022;40(31):3576–86.
Vij R, Kumar SK, D’Souza A, Mckay JT, Voorhees PM, Chung A, et al. Updated safety and efficacy results of Abbv-383, a BCMA x CD3 bispecific T-cell redirecting antibody, in a first-in-human phase 1 study in patients with relapsed/refractory multiple myeloma. Blood. 2023;142(Supplement 1):3378–80.
Bar N, Mateos MV, Ribas P, Hansson M, Paris L, Hofmeister CC, et al. Alnuctamab (ALNUC; BMS-986349; CC-93269), a 2+1 B-cell maturation antigen (BCMA) × CD3 T-cell engager (TCE), administered subcutaneously (SC) in patients (Pts) with relapsed/refractory multiple myeloma (RRMM): updated results from a phase 1 first-in-human clinical study. Blood. 2023;142(Supplement 1):2011–4.
Hans C, Lee NB, Richter JR, Dhodapkar MV, Hoffman JE, Suvannasankha A, et al. LINKER-MM1 study: Linvoseltamab (REGN5458) in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2023;41(Supplement):8006.
Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodriguez-Otero P, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232–44.
Trudel S, Krishnan AY, Fonseca R, Spencer A, Berdeja JG, Lesokhin A, et al. Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): updated results from an ongoing phase I study. Blood. 2021;138(Supplement 1):157–60.
Correnti CE, Laszlo GS, Schueren WJ, Godwin CD, Bandaranayake A, Busch MA, et al. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia. 2018;32(5):1239–43.
Nelson MH, Fritzell S, Miller R, Werchau D, Van Citters D, Nilsson A, et al. The bispecific tumor antigen-conditional 4–1BB x 5T4 agonist, ALGAPV-527, mediates strong T-cell activation and potent antitumor activity in preclinical studies. Mol Cancer Ther. 2023;22(1):89–101.
Muik A, Adams HC 3rd, Gieseke F, Altintas I, Schoedel KB, Blum JM, et al. DuoBody-CD40x4-1BB induces dendritic-cell maturation and enhances T-cell activation through conditional CD40 and 4–1BB agonist activity. J Immunother Cancer. 2022;10(6):e004322.
Geuijen C, Tacken P, Wang LC, Klooster R, van Loo PF, Zhou J, et al. A human CD137xPD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat Commun. 2021;12(1):4445.
Peper-Gabriel JK, Pavlidou M, Pattarini L, Morales-Kastresana A, Jaquin TJ, Gallou C, et al. The PD-L1/4-1BB bispecific antibody-anticalin fusion protein PRS-344/S095012 elicits strong T-cell stimulation in a tumor-localized manner. Clin Cancer Res. 2022;28(15):3387–99.
Liu Z, Xu X, Liu H, Zhao X, Yang C, Fu R. Immune checkpoint inhibitors for multiple myeloma immunotherapy. Exp Hematol Oncol. 2023;12(1):99.
Konishi T, Ochi T, Maruta M, Tanimoto K, Miyazaki Y, Iwamoto C, et al. Reinforced antimyeloma therapy via dual-lymphoid activation mediated by a panel of antibodies armed with bridging-BiTE. Blood. 2023;142(21):1789–805.
Lucca LE. Multiple myeloma treatment: one bridge closer. Blood. 2023;142(21):1763–4.
Pouleau B, Estoppey C, Suere P, Nallet E, Laurendon A, Monney T, et al. Preclinical characterization of ISB 1342, a CD38 x CD3 T-cell engager for relapsed/refractory multiple myeloma. Blood. 2023;142(3):260–73.
Xie B, Li Z, Zhou J, Wang W. Current status and perspectives of dual-targeting chimeric antigen receptor T-cell therapy for the treatment of hematological malignancies. Cancers (Basel). 2022;14(13):3230.
Pihlgren M, Carretero L, Estoppey C, Drake A, Pais D, Loyau J, et al. ISB 2001, a first-in-class trispecific BCMA and CD38 T cell engager designed to overcome mechanisms of escape from treatments for multiple myeloma by targeting two antigens. Blood. 2022;140(Supplement 1):858–9.
Sia H, Garton A, Wolff E, Shah T, Charpentier C, Duchesne D, et al. A phase 1, first-in-human, dose escalation and dose-expansion study of a BCMAxCD38xCD3 targeting trispecific antibody ISB 2001 in subjects with relapsed/refractory multiple myeloma. Blood. 2023;142(Supplement 1):3396–7.
Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Cancer. 2020;1(1):86–98.
Ross T, Wingert S, Haneke T, Klausz K, Otte AK, Schub N, et al. Preclinical characterization of AFM26, a novel B cell maturation antigen (BCMA)-directed tetravalent bispecific antibody for high affinity retargeting of NK cells against myeloma. Blood. 2018;132(Supplement 1):1927.
Plesner T, Harrison SJ, Quach H, Lee C, Bryant A, Vangsted A, et al. Phase I study of safety and pharmacokinetics of RO7297089, an anti-BCMA/CD16a bispecific antibody, in patients with relapsed, refractory multiple myeloma. Clin Hematol Int. 2023;5(1):43–51.
More S, Corvatta L, Manieri VM, Morsia E, Poloni A, Offidani M. Novel immunotherapies and combinations: the future landscape of multiple myeloma treatment. Pharmaceuticals (Basel). 2023;16(11):1628.
Chen H, Yu T, Lin L, Xing L, Cho SF, Wen K, et al. Gamma-secretase inhibitors augment efficacy of BCMA-targeting bispecific antibodies against multiple myeloma cells without impairing T-cell activation and differentiation. Blood Cancer J. 2022;12(8):118.
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94.
Meermeier EW, Welsh SJ, Sharik ME, Du MT, Garbitt VM, Riggs DL, et al. Tumor burden limits bispecific antibody efficacy through T cell exhaustion averted by concurrent cytotoxic therapy. Blood Cancer Discov. 2021;2(4):354–69.
Cohen YC, Gatt ME, Sebag M, Kim K, Min CK, Oriol A, et al. First results from the RedirecTT-1 study with teclistamab (tec) + talquetamab (tal) si- multaneously targeting BCMA and GPRC5D in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2023;41(Supplement):abstract8002.
Cohen AD, Mateos MV, Cohen YC, Rodriguez-Otero P, Paiva B, van de Donk N, et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood. 2023;141(3):219–30.
Mouhieddine TH, Van Oekelen O, Melnekoff DT, Li J, Ghodke-Puranik Y, Lancman G, et al. Sequencing T-cell redirection therapies leads to deep and durable responses in relapsed/refractory myeloma patients. Blood Adv. 2023;7(6):1056–64.
Baguet C, Larghero J, Mebarki M. Early predictive factors of failure in autologous CAR T-cell manufacturing and/or efficacy in hematologic malignancies. Blood Adv. 2024;8(2):337–42.
Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898–911.
Ghassemi S, Durgin JS, Nunez-Cruz S, Patel J, Leferovich J, Pinzone M, et al. Rapid manufacturing of non-activated potent CAR T cells. Nat Biomed Eng. 2022;6(2):118–28.
Arcangeli S, Bove C, Mezzanotte C, Camisa B, Falcone L, Manfredi F, et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J Clin Invest. 2022;132(12):e150807.
Dickinson MJ, Barba P, Jager U, Shah NN, Blaise D, Briones J, et al. A novel autologous CAR-T therapy, YTB323, with preserved T-cell stemness shows enhanced CAR T-cell efficacy in preclinical and early clinical development. Cancer Discov. 2023;13(9):1982–97.
Philipp N, Kazerani M, Nicholls A, Vick B, Wulf J, Straub T, et al. T-cell exhaustion induced by continuous bispecific molecule exposure is ameliorated by treatment-free intervals. Blood. 2022;140(10):1104–18.
Friedrich MJ, Neri P, Kehl N, Michel J, Steiger S, Kilian M, et al. The pre-existing T cell landscape determines the response to bispecific T cell engagers in multiple myeloma patients. Cancer Cell. 2023;41(4):711–25.
Cohen AD, Susanibar-Adaniya SP, Vogl DT, Garfall AL, Waxman A, Zubka D, et al. Sequential T-cell engagement for myeloma (“STEM”) trial: a phase 2 study of cevostamab consolidation following BCMA CAR T cell therapy. Blood. 2023;142(Supplement 1):3389–91.
Blache U, Popp G, Dunkel A, Koehl U, Fricke S. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat Commun. 2022;13(1):5225.
Qasim W. Genome-edited allogeneic donor “universal” chimeric antigen receptor T cells. Blood. 2023;141(8):835–45.
Cichocki F, van der Stegen SJC, Miller JS. Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood. 2023;141(8):846–55.
Berrien-Elliott MM, Jacobs MT, Fehniger TA. Allogeneic natural killer cell therapy. Blood. 2023;141(8):856–68.
Karahan ZS, Aras M, Sutlu T. TCR-NK cells: a novel source for adoptive immunotherapy of cancer. Turk J Haematol. 2023;40(1):1–10.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38.
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–8.
Leclercq G, Haegel H, Schneider A, Giusti AM, Marrer-Berger E, Boetsch C, et al. Src/lck inhibitor dasatinib reversibly switches off cytokine release and T cell cytotoxicity following stimulation with T cell bispecific antibodies. J Immunother Cancer. 2021;9(7):e002582.
Lancman G, Sastow DL, Cho HJ, Jagannath S, Madduri D, Parekh SS, et al. Bispecific antibodies in multiple myeloma: present and future. Blood Cancer Discov. 2021;2(5):423–33.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C) from the JSPS, Grant Number 18K08331 (to T.O.), the Takeda Science Foundation (to T.O.), the SENSHIN Medical Research Foundation (to T.O.), and the Center for Clinical and Translational Research of Kyushu University Hospital (to T.O.).
Funding
This study was funded by Japan Society for the Promotion of Science, 18K08331, Toshiki Ochi, Takeda Science Foundation, SENSHIN Medical Research Foundation, Center for Clinical and Translational Research of Kyushu University Hospital.
Author information
Authors and Affiliations
Contributions
T.O., T.K., and K.T. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ochi, T., Konishi, T. & Takenaka, K. Bispecific antibodies for multiple myeloma: past, present and future. Int J Hematol (2024). https://doi.org/10.1007/s12185-024-03766-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12185-024-03766-4