Abstract
Augmented renal clearance (ARC) is a phenomenon characterized by increased renal functionality, which can impact the pharmacokinetics and pharmacodynamics of antimicrobial drugs eliminated by the kidneys. It is a potential concern for infection treatment. Cord blood transplantation (CBT) is primarily impeded by delayed neutrophil recovery and immune reconstitution, thereby increasing susceptibility to infection. However, the clinical implications of ARC following CBT remain unexplored. We retrospectively assessed the influence of ARC on post-transplant outcomes at various time points in 194 adult recipients of single-unit unrelated CBT between 2007 and 2022 at our institution. ARC was observed in 52.9% of patients at 1 day, 39.8% at 15 days, and 26.5% at 29 days post-CBT. ARC was not significantly associated with bloodstream infection, acute graft-versus-host disease, or veno-occlusive disease/sinusoidal obstruction syndrome at any time point. ARC at 1 day, 15 days, and 29 days post-CBT was not significantly associated with overall survival, non-relapse mortality, or relapse rates. These findings suggest that ARC is common in adults during the early stages of CBT, but does not discernibly influence clinical outcomes or post-CBT complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Augmented renal clearance (ARC) is a phenomenon characterized by enhanced renal functionality frequently observed among critically ill individuals [1,2,3]. ARC possesses the potential to induce therapeutic inefficacy and reduce systemic drug exposure for renally cleared medications, thereby potentially compromising the efficacy of infection treatment [4,5,6]. Noteworthy risk factors for ARC include younger age, male gender, trauma, traumatic brain injury, hematological malignancies, and neutropenia [1, 2].
Cord blood transplantation (CBT) from unrelated donors represents an alternative approach for adult patients lacking human leukocyte antigen (HLA)-matched related or unrelated donors [7,8,9]. CBT is primarily hindered by delayed neutrophil recovery and immune reconstitution, heightening the risk of infectious complications [10,11,12,13,14]. Furthermore, since the glomerular filtration rate will ultimately determine the fate of the drug clearance, even for drugs that are first metabolized by the liver or intestine, such as chemotherapeutic drugs, immunosuppressants, and antifungal drugs, it is necessary to take into account the potential impact of ARC on the majority of drugs used in CBT [1]. Thus, the substantial effect of ARC on medication clearance might have consequences for post-transplant complications, including bloodstream infection (BSI), acute graft-versus-host disease (GVHD), and veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after CBT. Nonetheless, the clinical implications of ARC after CBT remain unexplored. We postulated that ARC could influence outcomes following CBT and impact post-transplant complications. To investigate this, we conducted a retrospective analysis to assess whether ARC influences clinical outcomes and post-transplant complications in adults who underwent CBT.
Methods
Patients and CBT procedures
This retrospective study incorporated data from 194 adult patients who underwent single-unit unrelated CBT as their initial allogeneic hematopoietic cell transplantation (HCT) at our institution between March 2007 and December 2022. Unrelated cord blood units were procured from cord blood banks in Japan. Treating physicians determined the conditioning regimens and GVHD prophylaxis. Supportive care, including antibacterial, antifungal, and antiviral prophylaxis, as well as transfusion practices, were largely standardized across all patients [7, 14]. The Institutional Review Board of the Institute of Medical Science, University of Tokyo, granted approval for this retrospective study (2021–110-0331).
Objectives and definitions
The primary objective of this retrospective study was to explore the influence of ARC at various time points on overall survival (OS), non-relapse mortality (NRM), and relapse rates following CBT. The secondary objectives were to examine the association between ARC and post-transplant complications, including BSI, acute GVHD, and VOD/SOS following CBT. BSI was defined as the isolation of bacteria from blood cultures between the day of CBT and 30 days after CBT. Confirmation of BSI caused by coagulase-negative Staphylococcus required the presence of two separate positive blood cultures [14]. The diagnosis of acute GVHD and VOD/SOS was based on previously established standard criteria.
Creatinine clearance (CrCl) was assessed through 24-h urine collection at least once a week during hospitalization for allogeneic HCT as a standardized procedure in our hospital. CrCl was calculated using the conventional formula: CrCl (mL/min) = urine volume (ml/min) × urinary creatinine (mg/dl)/serum creatinine (mg/dl), with adjustment for body surface area (BSA) determined by the Du Bois formula: corrected CrCl = CrCl × (1.73 m2/BSA). ARC was defined as a CrCl value of ≥ 130 mL/min/1.73m2 [2].
Measurement of drug trough levels
The serum vancomycin (VCM) trough levels were measured at our hospital using two techniques, depending on the treatment period: the fluorescence polarization immunoassay (FPIA) (March 2007 to January 2012) and the chemiluminescent enzyme immunoassay (from February 2012), as previously described [15]. In a commercial laboratory (SRL, Tokyo, Japan), serum teicoplanin (TEIC) trough levels were tested using two techniques depending on the treatment period: the FPIA (from March 2007 to March 2013) and the latex agglutination turbidimetric immunoassay (from April 2013). Our hospital monitored serum cyclosporine (CSP) trough levels using two techniques, depending on the treatment period: the FPIA (from March 2007 to February 2012) and the chemiluminescent immunoassay (from March 2012).
Statistical analysis
Group comparisons were conducted using Fisher's exact test for categorical variables. Continuous variables were compared using the Mann–Whitney U test. The probability of OS was estimated utilizing the Kaplan–Meier method, with differences assessed via the log-rank test. NRM and relapse probabilities were estimated using cumulative incidence curves to account for competing risks, and differences were evaluated using Gray's test. Regarding ARC at 15 and 29 days, the landmark days were set at 14 and 28 days after CBT, respectively, to evaluate the corresponding ARC values. Multivariate analysis was conducted employing a Cox proportional hazards model for overall mortality and a Fine and Gray model for NRM and relapse. The multivariate analysis included the following factors as covariates: ARC (yes vs. no), age (< 45 vs. ≥ 45 years), gender (male vs. female), HCT-Specific Comorbidity Index (< 3 vs. ≥ 3), refined disease risk index (low/intermediate vs. high/very high), cryopreserved cord blood total nucleated cell dose (< 2.5 × 107/kg vs. ≥ 2.5 × 107/kg), HLA disparities defined as high-resolution for HLA-A, -B, and -DR (< 3 vs. ≥ 3), and conditioning regimen (total body irradiation [TBI] ≥ 10 Gy-based vs. TBI < 10 Gy-based). GVHD prophylaxis (CSP plus methotrexate [MTX] vs. CSP plus mycophenolate mofetil) was not included in the variables of the multivariate analysis because selection for GVHD prophylaxis was associated with the type of conditioning regimen (P < 0.001 by Fisher's exact test). The significance level was set at P < 0.05, and all statistical analyses were performed using EZR software version 1.61 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [16] and GraphPad Prism 9 for Mac OS X (GraphPad Software Inc, San Diego, CA).
Results
Patient characteristics
Table 1 illustrates the characteristics of the patients enrolled in this study. The median age of patients at the time of CBT was 46.5 years. Acute myeloid leukemia accounted for the majority of cases, comprising 51% of the total. The predominant conditioning regimens employed were myeloablative regimens based on TBI with a dose of ≥ 10 Gy (78%), while CSP plus MTX were the most commonly utilized GVHD prophylaxis (78%).
CrCl values and ARC at each time point following CBT
The median CrCl values at various time points after CBT were as follows: 133.6 ml/min (Interquartile range [IQR], 104.4–165.8 ml/min) at 1 day, 118.0 ml/min (IQR, 88.6–147.6 ml/min) at 15 days, and 105.1 ml/min (IQR, 74.1–132.8 ml/min) at 29 days. ARC was observed in 100 (52.9%) of the 189 assessable patients at 1 day, 77 (39.8%) of the 193 assessable patients at 15 days, and 50 (26.5%) of the 188 assessable patients at 29 days after CBT.
Among adult patients who received allogeneic HCT from a matched sibling donor (n = 22), a matched unrelated donor (n = 18), or a haploidentical donor (n = 1) during the study period in our hospital, ARC was observed in 19 (48.7%) of the 39 assessable patients at 1 day, 13 (32.5%) of the 40 assessable patients at 15 days, and 9 (22.5%) of the 40 assessable patients at 29 days after HCT. The incidences of ARC at each time point were comparable between CBT and HCT from adult donors (Table 2).
VCM, TEIC, and CSP trough levels according to ARC
VCM were administered, and VCM trough levels were evaluated within 4 days at 1 day (n = 92), 15 days (n = 136), and 29 days (n = 57) after CBT. TEIC was administered, and TEIC trough levels were evaluated within 4 days at 1 day (n = 12), 15 days (n = 32), and 29 days (n = 9) after CBT. CSP was administered, and CSP trough levels were evaluated within 4 days at 1 day (n = 183), 15 days (n = 170), and 29 days (n = 159) after CBT.
The patient group exhibiting ARC at 29 days displayed slightly lower VCM (P = 0.112) and TEIC (P = 0.190) trough levels compared to those without ARC, although these differences did not reach statistical significance (Fig. 1a, b). The patient group exhibiting ARC did not affect VCM and TEIC trough levels at 1 day or 15 days (Fig. 1a, b). There were no significant associations between ARC and CSP trough levels at each time point (Fig. 1c).
Impact of ARC on BSI, acute GVHD, and VOD/SOS
The patient group exhibiting ARC at 1 day displayed a slightly higher proportion of BSI compared to those without ARC (19.0% vs. 10.1%, P = 0.102), although this difference did not reach statistical significance. Additionally, there were no significant associations between ARC at each time point and the occurrence of BSI, acute GVHD, or VOD/SOS (Table 3).
Impact of ARC on OS, NRM, and relapse
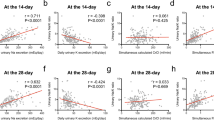
Univariate analysis demonstrated that ARC at 1 day, 15 days, and 29 days post-CBT was not associated with the probability of OS or the cumulative incidences of NRM and relapse (Fig. 2). In the multivariate analysis, ARC following CBT at each time point was also not significantly associated with the probabilities of OS, NRM, or relapse rates (Table 4).
The impact of augmented renal clearance (ARC) on the overall survival (OS), non-relapse mortality (NRM), and relapse rate in adult patients who underwent single-unit cord blood transplantation (CBT). Kaplan–Meier survival curves were employed to depict OS, while cumulative incidence curves were used to represent NRM and relapse. These curves were plotted both without landmark (a–c) and with a conditional landmark analysis conducted at both 14 (d–f) and 28 days (g–i) after CBT. Group comparisons were conducted using the log-rank test for OS and Gray's test for NRM and relapse
Discussion
Our study demonstrated that ARC is frequently observed in adults during the early stages following CBT, with a prevalence ranging from 26.5 to 52.9%, comparable to that seen in intensive care unit (ICU) patients [2]. ARC has been shown to have an impact on the pharmacokinetics and pharmacodynamics of antimicrobial drugs eliminated by the kidneys, such as meropenem, piperacillin/tazobactam, and VCM, potentially leading to therapeutic failure in infection treatment [3,4,5,6]. In fact, consistent with a previous report indicating the influence of ARC on VCM clearance in children with febrile neutropenia after HCT [17], our previous study revealed a correlation between ARC and lower initial trough levels of VCM in recipients of CBT [15]. However, our current study revealed no influence of ARC on post-transplant complications or clinical outcomes in CBT recipients. While most previous studies have shown no impact of ARC on mortality in patients receiving certain antibiotics [18, 19], a Spanish group demonstrated lower ICU mortality in patients with ARC [20]. It is plausible that the presence of ARC indicates preserved renal function, potentially leading to improved clinical outcomes. Overall, these findings suggest that the prognostic implications of ARC in various clinical settings remain unclear.
Apart from antibiotics, it is conceivable that ARC might also contribute to therapeutic failure of chemotherapeutic drugs eliminated by the kidneys. However, the exact impact of ARC on the efficacy of such drugs remains uncertain. Thus, we hypothesized that ARC might be associated with an increased risk of relapse after CBT. Nonetheless, our findings revealed that ARC did not elevate the risk of relapse following CBT.
Our study could not demonstrate higher drug clearance among patients with ARC. This might be partly due to the adjustment of some drug dosages according to drug trough levels by treating physicians. These findings could contribute to the lack of clinical implications of ARC after CBT in adults.
In conclusion, our data underscore the common occurrence of ARC in adults during the early stages after CBT. However, we could not demonstrate higher drug clearance, such as VCM, TEIC, and CSP, among patients with ARC. ARC was not significantly associated with the development of BSI, acute GVHD, or VOD/SOS at any time point. Furthermore, ARC at each time point did not discernibly influence clinical outcomes, including OS, NRM, and relapse following CBT. Nevertheless, it is important to note that our study was a retrospective, single-institute analysis conducted in Japan, with a limited number of patients. Further research is warranted to elucidate the effects of ARC on clinical outcomes in the field of hematology and HCT.
Data availability statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
Udy AA, Roberts JA, Lipman J. Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol. 2011;7:539–43.
Mahmoud SH, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics. 2017;9:36.
Hefny F, Sambhi S, Morris C, Kung JY, Stuart A, Mahmoud SH. Drug dosing in critically Ill adult patients with augmented renal clearance. Eur J Drug Metab Pharmacokinet. 2022;47:607–20.
Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28:695–700.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83.
Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45:385–92.
Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–30.
Konuma T, Kanda J, Inamoto Y, Hayashi H, Kobayashi S, Uchida N, et al. Improvement of early mortality in single-unit cord blood transplantation for Japanese adults from 1998 to 2017. Am J Hematol. 2020;95:343–53.
Konuma T, Mizuno S, Kondo T, Arai Y, Uchida N, Takahashi S, et al. Improved trends in survival and engraftment after single cord blood transplantation for adult acute myeloid leukemia. Blood Cancer J. 2022;12:81.
Tomonari A, Takahashi S, Ooi J, Tsukada N, Konuma T, Kobayashi T, et al. Bacterial bloodstream infection in neutropenic adult patients after myeloablative cord blood transplantation: experience of a single institution in Japan. Int J Hematol. 2007;85:238–41.
Yazaki M, Atsuta Y, Kato K, Kato S, Taniguchi S, Takahashi S, et al. Incidence and risk factors of early bacterial infections after unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2009;15:439–46.
Ballen K, Woo Ahn K, Chen M, Abdel-Azim H, Ahmed I, Aljurf M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1636–45.
Takagi S, Ogura S, Araoka H, Uchida N, Mitsuki T, Yuasa M, et al. The impact of graft cell source on bloodstream infection in the first 100 days after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56:1625–34.
Mizusawa M, Konuma T, Kato S, Isobe M, Shibata H, Suzuki M, et al. Clinical outcomes of persistent colonization with multidrug-resistant Gram-negative rods in adult patients undergoing single cord blood transplantation. Int J Hematol. 2020;111:858–68.
Yasu T, Konuma T, Oiwa-Monna M, Kato S, Isobe M, Takahashi S, et al. Lower vancomycin trough levels in adults undergoing unrelated cord blood transplantation. Leuk Lymphoma. 2021;62:348–57.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Shimamoto Y, Verstegen RHJ, Mizuno T, Schechter T, Allen U, Ito S. Population pharmacokinetics of vancomycin in paediatric patients with febrile neutropenia and augmented renal clearance: development of new dosing recommendations. J Antimicrob Chemother. 2021;76:2932–40.
Burnham JP, Micek ST, Kollef MH. Augmented renal clearance is not a risk factor for mortality in Enterobacteriaceae bloodstream infections treated with appropriate empiric antimicrobials. PLoS ONE. 2017;12: e0180247.
Kawano Y, Maruyama J, Hokama R, Koie M, Nagashima R, Hoshino K, et al. Outcomes in patients with infections and augmented renal clearance: a multicenter retrospective study. PLoS ONE. 2018;13: e0208742.
Tomasa-Irriguible TM, Sabater-Riera J, Pérez-Carrasco M, et al. Augmented renal clearance. An unnoticed relevant event. Sci Prog. 2021;104:368504211018580.
Acknowledgements
The authors thank all of the physicians and staff at our hospital and the cord blood banks in Japan.
Funding
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
TK conceived the project, designed the research, collected data, analyzed data, and wrote the paper. KT collected data. All the other authors participated in the treatment of the patients, acquired the clinical data, and contributed to writing the paper. All authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Konuma, T., Takano, K., Monna-Oiwa, M. et al. Clinical implications of augmented renal clearance after unrelated single cord blood transplantation in adults. Int J Hematol 118, 718–725 (2023). https://doi.org/10.1007/s12185-023-03669-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03669-w