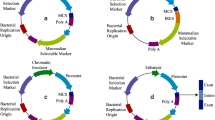
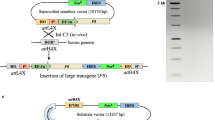
Abstract
Human blood coagulation factor VIII (hFVIII) is used in hemostatic and prophylactic treatment of patients with hemophilia A. Biotechnological innovations have enabled purification of the culture medium of rodent or human cells harboring the hFVIII expression cassette. However, cell lines express hFVIII protein derived from an exogenous expression vector at a lower level than most other proteins. Here, we describe hFVIII production using piggyBac transposon and the human-derived expi293F cell line. Use of a drug selection protocol, rather than transient expression protocol, allowed cells harboring hFVIII expression cassettes to efficiently produce hFVIII. In heterogeneous drug-selected cells, the production level was maintained even after multiple passages. The specific activity of the produced hFVIII was comparable to that of the commercial product and hFVIII derived from baby hamster kidney cells. We also applied codon optimization to the hFVIII open reading frame sequences in the transgene, which increased production of full-length hFVIII, but decreased production of B-domain-deleted human FVIII (BDD-hFVIII). Low transcriptional abundance of the hF8 transgene was observed in cells harboring codon-optimized BDD-hFVIII expression cassettes, which might partially contribute to decreased hFVIII production. The mechanism underlying these distinct outcomes may offer clues to highly efficient hFVIII protein production.





Similar content being viewed by others
References
Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–44.
Kingdon HS, Lundblad RL. An adventure in biotechnology: the development of haemophilia A therapeutics—from whole-blood transfusion to recombinant DNA to gene therapy. Biotechnol Appl Biochem. 2002;35(2):141–8.
Cao W, Dong B, Horling F, Firrman JA, Lengler J, Klugmann M, et al. Minimal essential human factor VIII alterations enhance secretion and gene therapy efficiency. Mol Ther Methods Clin Dev. 2020;19:486–95.
Kumar SR. Industrial production of clotting factors: challenges of expression, and choice of host cells. Biotechnol J. 2015;10(7):995–1004.
Soukharev S, Hammond D, Ananyeva NM, Anderson JA, Hauser CA, Pipe S, et al. Expression of factor VIII in recombinant and transgenic systems. Blood Cells Mol Dis. 2002;28(2):234–48.
Swiech K, Picanco-Castro V, Covas DT. Production of recombinant coagulation factors: are humans the best host cells? Bioengineered. 2017;8(5):462–70.
Sandberg H, Kannicht C, Stenlund P, Dadaian M, Oswaldsson U, Cordula C, et al. Functional characteristics of the novel, human-derived recombinant FVIII protein product, human-cl rhFVIII. Thromb Res. 2012;130(5):808–17.
Butler M, Spearman M. The choice of mammalian cell host and possibilities for glycosylation engineering. Curr Opin Biotechnol. 2014;30:107–12.
Ghaderi D, Taylor RE, Padler-Karavani V, Diaz S, Varki A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. 2010;28(8):863–7.
Durocher Y, Butler M. Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol. 2009;20(6):700–7.
Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. 2012;28:147–75.
Sandberg H, Almstedt A, Brandt J, Gray E, Holmquist L, Oswaldsson U, et al. Structural and functional characteristics of the B-domain-deleted recombinant factor VIII protein, r-VIII SQ. Thromb Haemost. 2001;85(1):93–100.
Ding S, Wu X, Li G, Han M, Zhuang Y, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122(3):473–83.
Yusa K, Zhou L, Li MA, Bradley A, Craig NL. A hyperactive piggyBac transposase for mammalian applications. Proc Natl Acad Sci U S A. 2011;108(4):1531–6.
Jain NK, Barkowski-Clark S, Altman R, Johnson K, Sun F, Zmuda J, et al. A high density CHO-S transient transfection system: comparison of ExpiCHO and Expi293. Protein Expr Purif. 2017;134:38–46.
Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6(5):363–9.
Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–9.
Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407(3):313–9.
Jenkins PV, Freas J, Schmidt KM, Zhou Q, Fay PJ. Mutations associated with hemophilia A in the 558–565 loop of the factor VIIIa A2 subunit alter the catalytic activity of the factor Xase complex. Blood. 2002;100(2):501–8.
Muto A, Yoshihashi K, Takeda M, Kitazawa T, Soeda T, Igawa T, et al. Anti-factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost. 2014;12(2):206–13.
Sugita C, Yamashita A, Moriguchi-Goto S, Furukoji E, Takahashi M, Harada A, et al. Factor VIII contributes to platelet-fibrin thrombus formation via thrombin generation under low shear conditions. Thromb Res. 2009;124(5):601–7.
Swiech K, Kamen A, Ansorge S, Durocher Y, Picanco-Castro V, Russo-Carbolante EM, et al. Transient transfection of serum-free suspension HEK 293 cell culture for efficient production of human rFVIII. BMC Biotechnol. 2011;11:114.
Ward NJ, Buckley SM, Waddington SN, Vandendriessche T, Chuah MK, Nathwani AC, et al. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117(3):798–807.
Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72(6):5085–92.
Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73(4):2886–92.
Shestopal SA, Hao JJ, Karnaukhova E, Liang Y, Ovanesov MV, Lin M, et al. Expression and characterization of a codon-optimized blood coagulation factor VIII. J Thromb Haemost. 2017;15(4):709–20.
Orlova NA, Kovnir SV, Gabibov AG, Vorobiev II. Stable high-level expression of factor VIII in Chinese hamster ovary cells in improved elongation factor-1 alpha-based system. BMC Biotechnol. 2017;17(1):33.
Spencer HT, Denning G, Gautney RE, Dropulic B, Roy AJ, Baranyi L, et al. Lentiviral vector platform for production of bioengineered recombinant coagulation factor VIII. Mol Ther. 2011;19(2):302–9.
Grancha S, Navajas R, Maranon C, Paradela A, Albar JP, Jorquera JI. Incomplete tyrosine 1680 sulphation in recombinant FVIII concentrates. Haemophilia. 2011;17(4):709–10.
Chun H, Pettersson JR, Shestopal SA, Wu WW, Marakasova ES, Olivares P, et al. Characterization of protein unable to bind von Willebrand factor in recombinant factor VIII products. J Thromb Haemost. 2021;19(4):954–66.
Gangadharan B, Ing M, Delignat S, Peyron I, Teyssandier M, Kaveri SV, et al. The C1 and C2 domains of blood coagulation factor VIII mediate its endocytosis by dendritic cells. Haematologica. 2017;102(2):271–81.
Hartholt RB, van Velzen AS, Peyron I, Ten Brinke A, Fijnvandraat K, Voorberg J. To serve and protect: The modulatory role of von Willebrand factor on factor VIII immunogenicity. Blood Rev. 2017;31(5):339–47.
Muczynski V, Casari C, Moreau F, Ayme G, Kawecki C, Legendre P, et al. A factor VIII-nanobody fusion protein forming an ultrastable complex with VWF: effect on clearance and antibody formation. Blood. 2018;132(11):1193–7.
Kuchipudi SV, Tellabati M, Nelli RK, White GA, Perez BB, Sebastian S, et al. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol J. 2012;9:230.
Kimchi-Sarfaty C, Schiller T, Hamasaki-Katagiri N, Khan MA, Yanover C, Sauna ZE. Building better drugs: developing and regulating engineered therapeutic proteins. Trends Pharmacol Sci. 2013;34(10):534–48.
Acknowledgements
This work was partly supported by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to Takuji Yoshimura (grant number 17K08632), Keiji Nogami (grant numbers 18K07885 and 21K07804), and Investigator-Research Support grant (Sanofi AS).
Funding
This study were funded by Japan Society for the Promotion of Science (Grant Nos. 17K08632, 18K07885, 21K07804), Sanofi AS (Investigator-Research Support grant).
Author information
Authors and Affiliations
Contributions
TY conceived and designed the research, performed experiments, analyzed and interpreted the data, prepared figures, and wrote and edited the manuscript; KaH performed experiments; NS helped in vector construction; KO supplied resource; KyH supplied resource; MS supervised the research; KN supervised the research and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
TY has received research funding from Bioverativ Inc./Sanofi S.A.; KaH, KO, and KyH have no conflict of interest; NS teaches a course endowed by CSL Behring; MS and KN have received research funding from Bioverativ Inc./Sanofi S.A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yoshimura, T., Horiuchi, K., Shimonishi, N. et al. Modified expi293 cell culture system using piggyBac transposon enables efficient production of human FVIII. Int J Hematol 117, 56–67 (2023). https://doi.org/10.1007/s12185-022-03468-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03468-9