Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) has been used to treat patients with inherited metabolic disorders (IMDs) for more than 40 years. In the first two decades, various IMDs were treated by HSCT with a wide variety of donor sources and conditioning regimens selected at the institutional level. However, HSCT was not always successful due to post-transplant complications such as graft failure. In the third decade, myeloablative conditioning with targeted busulfan-based pharmacokinetic monitoring was established as an optimal conditioning regimen, and unrelated cord blood was recognized as an excellent donor source. During the fourth decade, further improvements were made to transplant procedures, including modification of the conditioning regimen, and the survival rate after HSCT markedly improved. Simultaneously, several long-term observational studies for patients after HSCT clarified its therapeutic effects on growth and development of cognitive function, fine motor skills, and activities of daily living when compared with enzyme replacement therapy. Although immune-mediated cytopenia was newly highlighted as a problematic morbidity after HSCT for IMDs, especially in younger patients who received unrelated cord blood, a recent study with rituximab added to the conditioning raised expectations that this issue can be overcome.


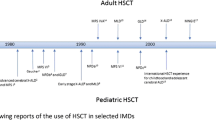
Quoted from Bone Marrow Transplantation 2021; 56: 1238–1247. Copyright 2021 by Springer Nature. Reprinted with permission (License number 5276540342735)
Similar content being viewed by others
References
Peters C, Balthazor M, Shapiro EG, King RJ, Kollman C, Hegland JD, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–902.
Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, et al. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. Blood. 1998;91:2601–8.
Peters C, On behalf of the NMDP, IBMTR, and the Working Party on Inborn Errors of the EBMT. Steward CG Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31:229–39.
Peters C, Charnas LR, Tan Y, Ziegler RS, Shapiro EG, DeFor T, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–8.
Kato S, Yabe H, Takakura H, Mugishima H, Ishige M, Tanaka A, et al. Hematopoietic stem cell transplantation for inborn errors of metabolism: a report from the research committee on transplantation for inborn errors of metabolism of the Japanese ministry of health, labour and welfare and the working group of the Japan society for hematopoietic cell transplantation. Pediatr Transplant. 2016;20:203–14.
Bartelink IH, Reij EML, Gerhardt CE, Maarseveen EM, Wildt A, Versluys B, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014;20:345–53.
Rodgers NJ, Kaizer AM, Miller WP, Rudser KD, Orchard PJ, Braunlin EA. Mortality after hematopoietic stem cell transplantation for severe mucopolysaccharidosis type I: the 30-year University of Minnesota experience. J Inherit Metab Dis. 2017;40:271–80.
Aldenhoven M, Jones SA, Bonney D, Borrill RE, Coussons M, Mercer J, et al. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines. Biol Blood Marrow Transplant. 2015;21:1106–9.
Fratantoni JC, Hall CW, Neufeld EF. The defect in Hurler and Hunter syndromes. II. Deficiency of specific factors involved in mucopolysaccharide degradation. Proc Natl Acad Sci USA. 1969;64:360–6.
Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, et al. Reversal of clinical features of Hurler’s disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet. 1981;2:709–12.
Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status, and future directions. Br J Haematol. 2009;148:356–72.
Boelens JJ, Orchard PJ, Robert Wynn RF. Transplantation in inborn errors of metabolism: current considerations and future perspectives. Br J Haematol. 2014;167:293–303.
Tan EY, Boelens JJ, Jones SA, Wynn RF, On behalf of the Inborn Errors Working Party of the EBMT. Hematopoietic stem cell transplantation in inborn errors of metabolism. Front Pediatr. 2019;7:433. https://doi.org/10.3389/fped2019.00433.
Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–9.
Aldenhoven M, Wynn RF, Orchard PJ, O’Meara A, Veys P, Alain Fischer A, et al. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood. 2015;125:2164–72.
Tanaka A, Okuyama T, Suzuki Y, Sakai N, Takakura H, Sawada T, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab. 2012;107:513–20.
Barth AL, Magalhãesa TSPC, Reis ABR, Oliveira ML, Scalco FB, Cavalcanti NC, et al. Early hematopoietic stem cell transplantation in a patient with severe mucopolysaccharidosis II: a 7 years follow-up. Mol Genet Metab Rep. 2017;12:62–8.
Kubaski F, Yabe H, Suzuki Y, Seto T, Hamazaki T, Mason RW, et al. Hematopoietic stem cell transplantation for patients with mucopolysaccharidosis II. Biol Blood Marrow Transplant. 2017;23:1795–803.
Yabe H, Tanaka A, Chinen Y, Kato S, Sawamoto K, Yasuda E, et al. Hematopoietic stem cell transplantation for morquio a syndrome. Mol Genet Metab. 2016;117:84–94.
Allewelt H, Taskindoust M, Troy J, Page K, Wood S, Parikh S, et al. Long-term functional outcomes after hematopoietic stem cell transplant for early infantile Krabbe disease. Biol Blood Marrow Transplant. 2018;24:2233–8.
Laule C, Vavasour IM, Shahinfard E, Madler B, Zhang J, Li DKB, et al. Hematopoietic stem cell transplantation in late-onset Krabbe disease: no evidence of worsening demyelination and axonal loss 4 years post-allograft. J Neuroimaging. 2018;28:252–5.
Malm G, Ringdén O, Winiarski J, Gröndahl E, Uyebrant P, Eriksson U, et al. Clinical outcome in four children with metachromatic leukodystrophy treated by bone marrow transplantation. Bone Marrow Transplant. 1996;17:1003–8.
Boelens JJ, Aldenhoven M, Purtill D, Ruggeri A, Defor T, Wynn R, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–7.
Even-Or E, NaserEddin A, Dinur Schejter Y, Shadur B, Zaidman I, Stepensky P. Haploidentical stem cell transplantation with post-transplant cyclophosphamide for osteopetrosis and other nonmalignant diseases. Bone Marrow Transplant. 2021;56:434–41.
Yabe H, Inoue H, Matsumoto M, Hamanoue S, Hiroi A, Koike T, Sako M, Fujiwara M, Ueda Y, Maruya E, Saji H, Kato S, Yabe M. Unmanipulated HLA-haploidentical bone marrow transplantation for the treatment of fatal, nonmalignant diseases in children and adolescents. Int J Hematol. 2004;79:78–82.
Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8.
Boelens JJ, Rocha V, Aldenhoven M, Wynn R, O’Meara A, Michel G. Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with hurler syndrome. Biol Blood Marrow Transplant. 2009;15:618–25.
Nakasone H, Tabuchi K, Uchida N, Ohno Y, Matsuhashi Y, Takahashi S, Onishi Y, Onizuka M, Kobayashi H, Fukuda T, Ichinohe T, Takanashi M, Kato K, Atsuta Y, Yabe H, Kanda Y. Which is more important for the selection of cord blood units for haematopoietic cell transplantation: the number of CD34-positive cells or total nucleated cells? Br J Haematol. 2019;185(1):166–9.
Rocha V, Gluckman E, Eurocord-Netcord registry and European blood and marrow transplant group. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147:262–74.
Parikh SH, Mendizabal A, Benjamin CL, Komanduri KV, Antony J, Petrovic A, et al. A novel reduced-intensity conditioning regimen for unrelated umbilical cord blood transplantation in children with nonmalignant diseases. Biol Blood Marrow Transplant. 2014;20:326–36.
Mallhi KK, Smith AR, DeFor TE, Lund TC, Orchard PJ, Miller WP. Allele-level HLA matching impacts key outcomes following umbilical cord blood transplantation for inherited metabolic disorders. Biol Blood Marrow Transplant. 2017;23:119–25.
Tokimasa S, Ohta H, Takizawa S, Kusuki S, Hashii Y, Sakai N, et al. Umbilical cord-blood transplantations from unrelated donors in patients with inherited metabolic diseases: single-institute experience. Pediatr Transplant. 2008;12:672–6.
Kato K, Yabe H, Shimozawa N, Adachi S, Kurokawa M, Hashii Y, et al. Stem cell transplantation for pediatric patients with adrenoleukodystrophy: a nationwide retrospective analysis in Japan. Pediatr Transplant. 2022;26: e14125. https://doi.org/10.1111/petr.14125.
Yamamoto H, Kato D, Uchida N, Ishikawa K, Araoka H, Takagi S, et al. Successful sustained engraftment after reduced-intensity umbilical cord blood transplantation for adult patients with severe aplastic anemia. Blood. 2011;117:3240–2.
Barker JN, Kurtzberg J, Ballen K, Boo M, Brunstein C, Cutler C, et al. Optimal practices in unrelated donor cord blood transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2017;23:882–96.
Lum SH, Orchard PJ, Lund TC, Miller WP, Boelens JJ, Wynn R, et al. Outcome after cord blood transplantation using busulfan pharmacokinetics-targeted myeloablative conditioning for hurler syndrome. Transplant Cell Ther. 2021;27(1):91.e1-91.e4. https://doi.org/10.1016/j.bbmt.2020.08.033.
Boelens JJ, Wynn RF, O’Meara A, Veys P, Bertrand Y, Souillet G, et al. Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transplant. 2007;40:225–33.
Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35.
Chua SJ, Bielecki R, Wong CJ, Yamanaka N, Rogers IM, Casper RF. Neural progenitors, neurons and oligodendrocytes from human umbilical cord blood cells in a serum-free, feeder-free cell culture. Biochem Biophys Res Commun. 2009;379:217–21.
Yabe H, Inoue H, Matsumoto M, Hamanoue S, Koike T, Ishiguro H, et al. Allogeneic haematopoietic cell transplantation from alternative donors with a conditioning regimen of low-dose irradiation, fludarabine and cyclophosphamide in Fanconi anaemia. Br J Haematol. 2006;134:208–12.
Hansen MD, Filipovich AH, Davies SM, Mehta P, Bleesing J, Jodele S, et al. Allogeneic hematopoietic cell transplantation (HCT) in Hurler’s syndrome using a reduced intensity preparative regimen. Bone Marrow Transplant. 2008;41:349–53.
Marsh RA, Rao MB, Gefen A, Bellman D, Mehta PA, Khandelwal P, et al. Experience with alemtuzumab, fludarabine, and melphalan reduced-intensity conditioning hematopoietic cell transplantation in patients with nonmalignant diseases reveals good outcomes and that the risk of mixed chimerism depends on underlying disease, stem cell source, and alemtuzumab regimen. Biol Blood Marrow Transplant. 2015;21:1460–70.
Baxter MA, Wynn RF, Schyma L, Holmes DK, Wraith JE, Fairbairn LJ, et al. Marrow stromal cells from patients affected by MPS I differentially support haematopoietic progenitor cell development. J Inherit Metab Dis. 2005;28:1045–53.
Watson HA, Holley RJ, Langford-Smith KJ, Wilkinson FL, van Kuppevelt TH, Wynn RF, et al. Heparan sulfate inhibits hematopoietic stem and progenitor cell migration and engraftment in mucopolysaccharidosis I. J Biol Chem. 2014;289:36194–203.
Xie Y, Parekh J, Tang Z, Wu D, Wu X. Donor-specific antibodies and primary graft failure in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Transplant Cell Ther. 2021;27(8):687.e1-687.e7. https://doi.org/10.1016/j.jtct.2021.04.030.
Yabe H, Morimoto T, Takakura H, Okuya M, Ikegaya R, Kato S, et al. Post-transplantation-emerging anti-HLA DQA1/DQB1 antibody possibly responsible for graft rejection after myeloablative-unrelated marrow grafting. Bone Marrow Transplant. 2016;51(4): 601–3.
Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. Rituximab for desensitization during HLA-mismatched stem cell transplantation in patients with a positive donor-specific anti-HLA antibody. Bone Marrow Transplant. 2020;55(7):1326–36. https://doi.org/10.1038/s41409-020-0928-z.
Haines HL, Bleesing JJ, Davies SM, Hornung L, Jordan MB, Marsh RA, et al. Outcomes of donor lymphocyte infusion for treatment of mixed donor chimerism after a reduced-intensity preparative regimen for pediatric patients with nonmalignant diseases. Biol Blood Marrow Transplant. 2015;21:288–92.
Gupta AO, Boelens JJ, Ebens CL, Kurtzberg J, Lund TC, Smith AR, et al. Consensus opinion on immune-mediated cytopenias after hematopoietic cell transplant for inherited metabolic disorders. Bone Marrow Transplant. 2021;56:1238–47.
Page KM, Mendizabal AM, Prasad VK, Martin PL, Parikh S, Susan Wood S, et al. Posttransplant autoimmune hemolytic anemia and other autoimmune cytopenias are increased in very young infants undergoing unrelated donor umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2008;14:1108–17.
Faraci M, Zecca M, Pillon M, Rovelli A, Menconi MC, Ripaldi M, et al. Autoimmune hematological diseases after allogeneic hematopoietic stem cell transplantation in children: an Italian multicenter experience. Biol Blood Marrow Transplant. 2014;20:272–8.
Kruizinga MD, van Tol MJD, Bekker V, Netelenbos T, Smiers FJ, Bresters D, et al. Risk factors, treatment, and immune dysregulation in autoimmune cytopenia after allogeneic hematopoietic stem cell transplantation in pediatric patients. Biol Blood Marrow Transplant. 2018;24:772–8.
Scordo M, Hsu M, Jakubowski AA, Shah GL, Cho C, Maloy MA, et al. Immune Cytopenias after Ex Vivo CD34+-Selected Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2019;25:1136–41.
Galvin RT, Cao Q, Miller WP, Knight-Perry J, Smith AR, Ebens CL. Characterizing immune-mediated cytopenias after allogeneic hematopoietic cell transplantation for pediatric nonmalignant disorders. Transplant Cell Ther. 2021;27(316):e1-316.e8.
Daikeler T, Labopin M, Ruggeri A, Crotta A, Abinun M, Hussein AA, et al. New autoimmune diseases after cord blood transplantation: a retrospective study of EUROCORD and the autoimmune disease working party of the European group for blood and marrow transplantation. Blood. 2013;121:1059–64.
Nataraj R, Hiwarkar P, Bonney D, Campbell H, Jones S, Deambrosis D, et al. B-cell depletion abrogates immune mediated cytopenia and rejection of cord blood transplantation in Hurler syndrome. Bone Marrow Transplant. 2022;57:38–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yabe, H. Allogeneic hematopoietic stem cell transplantation for inherited metabolic disorders. Int J Hematol 116, 28–40 (2022). https://doi.org/10.1007/s12185-022-03383-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03383-z