Abstract
The clinical features of patients with secondary hemophagocytic lymphohistiocytosis (sHLH) complicated with pleural effusion have rarely been evaluated. We retrospectively analyzed 203 patients newly diagnosed with sHLH from July 2015 to July 2019 according to the HLH-2004 protocol. Baseline characteristics, laboratory results, and imaging were reviewed. Pleural effusion was found in 58.6% of the studied sHLH population, and characteristic imaging findings were minimal volume and bilaterality. Patients with pleural effusion had lower PLT counts, HB levels and ALB levels as well as higher sCD25 levels than those without pleural effusion (all p values < 0.05). Multivariate analyses showed that lg(sCD25) and PLT ≤ 65 × 109/L were significant risk factors for developing pleural effusion in sHLH. Regarding prognostic value, survival analysis showed a lower survival probability for patients with pleural effusion than for those without pleural effusion (median OS, 90 vs. 164 days, p = 0.028). In multivariate analysis, pleural effusion was an independent prognostic factor for overall survival (OS) (HR 2.68; 95% CI 1.18–6.11, p = 0.019). Pleural effusion is frequently found in patients with sHLH and is associated with greater inflammation and worse outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophagocytic lymphohistiocytosis (HLH), caused by cytokine-dependent accumulation and aberrant activation of macrophages and cytotoxic T cells, is a life-threatening and severe hyperinflammatory syndrome [1]. It is recognized as primary HLH (pHLH) or secondary HLH (sHLH) [2]. pHLH is accompanied by inherited mutations affecting lymphocyte cytotoxicity and immune regulation. sHLH is triggered by various pathologies, mainly infections, malignancies, autoimmune disorders or unknown aetiologies, without a family history or known genetic predisposition [3].
Clinically, sHLH symptoms is characterised by sustained fever, cytopenia, coagulopathy, and hepatosplenomegaly that may rapidly progress to terminal multiple organ failure [4]. In the most serious cases, cytokine storms can result in progressive multiple organ failure involving the neurologic, cardiovascular, hepatic, and/or respiratory systems [5]. Experimental and epidemiological data suggest that the spleen and liver are the most frequently involved organs, and nearly 60% of HLH patients have altered liver tests [6]. Recent clinical studies have shown that pulmonary involvement is also frequent, and symptoms can include cough, dyspnoea, pleural effusion, and respiratory failure [7].
To the best of our knowledge, few studies to date have investigated the association between sHLH and pleural effusion. Here, we aimed to show the incidence, distribution, possible mechanisms and prognostic value of pleural effusion in sHLH.
Materials and methods
Study design and data collection
Two hundred and three consecutive patients with newly diagnosed sHLH between July 2015 and July 2019 were admitted to the First Affiliated Hospital of Nanjing Medical University were included in our retrospective study. Detailed chest imaging was obtained for all patients recruited. Forty-one patients were excluded for meeting the exclusion criteria: (a) Comorbidities conditions which could directly cause pleural effusion: congestive heart failure, pneumonia, cancer, severe hepatic disease (liver cirrhosis or a Model for End-Stage Liver Disease score > 20), renal failure(estimated creatinine clearance < 15 mL/min per 1.73 m2), pulmonary embolism, acute pancreatitis; (b) History of drugs and operation known to cause pleural effusion at admission: amiodarone, dasatinib, methotrexate, post-cardiac surgery, lung operation and/or radiation; (c) Under 18 years or Refuse to any treatment or HScore < 90. Diagnosis of sHLH was based on the HLH-2004 diagnostic criteria [8], Patients were divided into the positive pleural effusion (PE+) group and the negative pleural effusion (PE−) group according to the presence or absence of pleural effusion at the initial diagnosis of sHLH.
Baseline clinical characteristics and laboratory data were collected by reviewing their medical records. Blood samples were obtained on admission, and the tests were performed by laboratory technologists at Nanjing Medical University Hospital. The results of chest X-ray, computed tomography (CT), positron emission tomography/computed tomography (PET-CT), ultrasonography, and thoracentesis were also evaluated.
Diagnosis and definitions
The diagnosis of HLH was established based on the 2004 HLH Diagnostic Criteria. PET-CT, combined with biopsy of suspicious lesions, revealed lymphoma-associated hemophagocytic lymphohistiocytosis (LHLH) and hemophagocytic lymphohistiocytosis of unknown origin (NHLH) [9].
The diagnosis of pleural effusion was based on chest X-ray, CT, PET-CT, and thoracic ultrasound. As there are no accepted definitions to describe the extent of pleural effusion, we decided to use the following validated criteria: minimal: a small amount of liquid, below the fourth rib level; moderate: liquid involves the fourth to second anterior ribs without clinical symptoms, or a crescent-shaped low density area is observed on chest CT, with mild compression of local lung tissue; massive: liquid involves the upper part of the second anterior rib, accompanied by clinical symptoms, or pleural effusion causes significant compression of lung tissue, a reduction if volume, close to the lung door, and a mediastinal shift to the opposite side. In addition, early-onset effusion was defined as effusion occurring at the time of sHLH diagnosis or during the first chemotherapy cycle. Late-onset effusion was defined as any effusion occurring after the first cycle of chemotherapy.
Outcome and follow-up
Overall survival (OS) was defined as the time between the first day of diagnosis and the date of death from any cause or the last follow-up until June 2020. Follow-up was conducted by reviewing inpatient medical records and making phone calls.
Statistical analysis
Data analysis was performed using SPSS version 22.0 (Chicago, IL, USA), GraphPad Prism 6 (GraphPad Software, La Jolla, CA), and STATA/MP statistical software (version 16.0; StataCorp, TX, USA). Quantitative variables are reported as medians with interquartile ranges (IQRs), and categorical data are presented as frequencies and percentages. Survival functions were estimated by the Kaplan–Meier method and Univariable Cox. A two-sided p < 0.05 was used to define statistical significance for all comparisons.
Results
Incidence and risk factors for pleural effusion
From July 2015 to July 2019, a total of 162 subjects with sHLH were enrolled in this study, of whom 95 (58.6%) patients developed pleural effusion at the time of diagnosis. The median time between sHLH onset and pleural effusion onset was 2 days (0–3.5 days). To determine the risk of pleural effusion, we retrospectively analyzed the demographic, clinical and laboratory characteristics of subjects with and without pleural effusion (Table 1). For demographic and clinical parameters, there was no significant difference in age, gender, aetiology, maximum temperature, splenomegaly or treatment regimen between the PE+ group and PE− group. Regarding laboratory examinations, patients with pleural effusion had lower PLT counts, HB levels and ALB levels as well as higher sCD25 levels than those without pleural effusion (all p values < 0.05). Of significance, lg(sCD25) (p = 0.011; OR 13.27; 95% CI 1.81–97.11) and PLT ≤ 65 × 109/L (p = 0.027; OR 4.03; 95% CI 1.17–13.88) were found to be risk factors for developing pleural effusion. These results were confirmed using a reduced model multivariate analysis.
Distribution of pleural effusion
We reviewed the radiological reports at the diagnosis of 95 sHLH patients with pleural effusion and found that the most common radiologic findings were small amounts of bilateral pleural effusion with no specific pattern. In the PE+ group, there were 68 (71.6%) cases of minimal pleural effusion, 19 (20.0%) cases of moderate, 8 (8.4%) cases of massive, 73 (76.8%) cases of bilateral pleural effusion, 11 (11.6%) cases of left and 11 (11.6%) cases of right. In addition, we further explored the correlations between pleural effusion levels and other laboratory data parameters, as shown in Table 2. There were significant correlations between pleural effusion levels and HB, PLT, ALB, and sCD25. Another analysis showed that there was a statistically significant difference in the incidence of pelvic effusion, pericardial effusion and ascites between the PE+ group and the PE− group, indicating that patients with pleural effusion have a higher probability of pelvic effusion, pericardial effusion, and ascites (Table 3).
Prognostic value of pleural effusion in sHLH
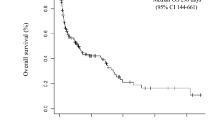
After a median follow-up of 107 (interquartile range 36–425) days, 66 (69.5%) deaths occurred in the PE+ group, whereas 40 (59.7%) deaths occurred in the PE− group. In the Kaplan–Meier analysis (Fig. 1A), OS was significantly worse in the PE+ group than in the PE− group (median OS, 90 vs. 164 days, p = 0.028). X-tile was conducted to assess biomarkers and calculate the optimal survival cut-off level [10]. Table 4 summarizes the univariate and multivariate Cox regression analyses of OS for potential risk predictors in sHLH. By univariate analysis, age > 72 years, EBV infection, PLT < 30 × 109/L, FIB ≤ 1.3 g/L, TG ≥ 3.0 mmol/L, ALB < 31.7 g/L, ADA > 134.3 U/L, β 2-MG > 6.7 mg/L, ferritin > 1500 ng/mL, and LHLH were also associated with a worse outcome. Upon multivariable adjustment, pleural effusion (HR 2.68; 95% CI 1.18–6.11), PLT < 30 × 109/L (HR 2.78; 95% CI 1.41–5.49), and EBV infection (HR 2.36; 95% CI 1.18–4.74) were significantly associated with poor survival.
Subgroup analysis on the predictive power of pleural effusion in sHLH
In subgroup analysis, etiology-stratified analysis suggested that pleural effusion in LHLH patients was significantly associated with poor survival (median OS, 90 vs. 229 days, p = 0.037), rather than non-LHLH patients (Fig. 1C,D). Furthermore, we performed subgroup analyses to eliminate the effect of confounding factors, including age, gender, pathogenesis, EBV infection, neutrophils, haemoglobin, platelets, fibrinogen, and albumin. As shown in Fig. 2, the positive associations between pleural effusion and poor survival were stronger among males and patients with ANC < 1.0 × 109/L. Nevertheless, in the overall subgroup analysis, the predictive efficiency of pleural effusion combined with baseline characteristics showed no significant change.
Therapeutic methods
The sHLH treatment methods varied depending on etiology. No statistically significant difference was observed in treatment regimen between the PE+ group and the PE− group. All 95 patients in the PE+ group received active treatment for the primary diseases and appropriate supplementation with diuretics, oxygen therapy, colloidal fluid and crystalloids.
In our 94 malignancy-associated hemophagocytic lymphohistiocytosis (MHLH) patients, 56 patients had received systemic combination chemotherapy, such as EPOCH, CHOP, and DEP; 24 patients were treated with HLH-94 or HLH-04 as the initial therapy; and 12 patients were treated with only GC + IVIg. In our 68 non-MHLH patients, 13 patients were given HLH-94 first-line treatment, GC + IVIg was administered in 12 patients, GC was administered in 10 patients, GS + IVIg + cyclophosphamide was administered in 3 patients, GC + etoposide was administered in 2 patients, and GS + IVIg + cyclosporine was administered in 2 patients.
In the PE+ group, after 2 weeks of treatment, 30 patients with pleural effusion decreased or disappeared, 9 patients with pleural effusion increased. In the PE− group, 4 patients state worsened with the onset of pleural effusion. Survival analysis showed a lower survival probability for patients with pleural effusion increased group than pleural effusion decreased group (median OS, 46 vs. 152 days, p = 0.015) (Fig. 1B).
Discussion
To our knowledge, this retrospective study is the first systematic study on pleural effusion in sHLH. In the present study, we observed the distribution and possible mechanism of pleural effusion in sHLH, and demonstrated that pleural effusion was associated with worse survival in sHLH.
Pleural effusion was found in 58.6% of the studied sHLH population at diagnosis, a higher percentage than the range of previously reported data for critical illness and haematologic malignancies [11, 12]. The incidence of pleural effusion in sHLH patients was high, which was consistent with previous studies [13, 14]. Our findings suggest that pleural effusion developed more often in patients with lower PLT counts, HB and ALB levels and higher sCD25 levels. Moreover, through multivariate analyses, we determined that PLT ≤ 65 × 109/L and high levels of sCD25 were associated with an increased risk of pleural effusion. It is well known that platelets and sCD25 play an important role when evaluating sHLH and are the diagnostic criteriaset in the HLH-2004 criterion [15]. Cytopenia, especially persistent severe thrombocytopenia, is a key laboratory marker of HLH and is mainly related to severe cytokine-mediated inflammation [6, 16]. Reportedly, the rapid onset of cytopenia suggests a consumptive process critically driven by TNF-α and INF-γ. Uncontrolled activation of macrophages will result in phagocytosis of platelets and other haematopoietic components by macrophages [16,17,18]. Indeed, a high level of sCD25 linked the diagnosis of adult HLH with the defining features of hypercytokinemia [19, 20]. It is reasonable to presume that pleural effusion may be associated with a higher inflammation state. Therefore, maintaining awareness of the possibility of pleural effusion is important, especially in sHLH patients with significantly decreased haemoglobin, platelet, and albumin and significantly elevated sCD25.
The pathogenesis of sHLH-induced pleural effusion is still not clear. One theory holds that the onset of pleural effusion is caused by excessive inflammatory cytokines resulting from HLH. The release of a large number of inflammatory factors leads to widespread increases in vascular permeability that can result in progressive subcutaneous and body cavity oedema, including pleural effusion [21]. Previous studies have suggested that cytokines and other inflammatory mediators could induce gaps between endothelial cells by disassembling intercellular junctions, altering the cellular cytoskeletal structure, or directly damaging the cell monolayer. This creation of gaps can result in microvascular leak and pleural effusion [21,22,23]. Moreover, HLH was also described in severely ill patients during the COVID-19 pandemic, presenting with vascular injury resulting from hyperinflammation in the alveoli [24]. Weaver et al. [25] reported that hyperinflammation, rather than hemophagocytosis, appears to be the driving cause of HLH pathology. In our study, we obtained evidence that the incidence of pleural effusion in patients with different aetiologies of sHLH was not statistically significant. An immune-mediated mechanism is more likely responsible for the sHLH-related pleural effusion, as our multivariate analysis has reported.
We reviewed the radiological reports at diagnosis of 162 patients with sHLH, noting presence of pleural effusion and its characteristic. A notable observation in our study was that the incidence of early-onset effusion (58.6%) was higher than that of late-onset effusion (11.7%). Pleural effusion may be the first presentation of sHLH. The early onset of pleural effusion and its association with HLH severity support a direct link to HLH or suggest that pleural effusion is in part due to HLH itself. Moreover, pleural effusion is characterized by minimal amounts and bilateral in the diagnosis of sHLH, consistent with two paediatric HLH studies [13, 26].
More recent studies have shown that pleural effusion is an important prognostic factor for overall survival in critically ill patients and those with haematologic malignancies [27, 28]. It is generally believed that the prognosis of sHLH is known to be heavily dependent on the aetiology and treatment. Based on some case reports, it appears that pleural effusion was an ominous sign [29, 30]. Our study showed that the cumulative death rate was significantly higher in sHLH patients with pleural effusion than in those without (p = 0.028), and the presence of pleural effusion was independently associated with worse survival among patients with sHLH. In the etiology analysis, 79 LHLH patients also found the same results. However, the mechanism leading to this higher mortality rate remains unclear. One hypothesis could be that the pleural effusion could sometimes only be the reflection of a more severe form of HLH (with a more intense cytokine storm and a more intense hemophagocytic activity). Therefore, deeper knowledge of pleural effusion in sHLH is important for guiding physicians to evaluate the condition and formulate treatment in these patients. Additionally, our present results strongly imply that patients with moderate to massive amounts of pleural effusion have worse survival than patients with minimal amounts of pleural effusion, and the increase of pleural effusion is accompanied the severity of the disease. There was no difference in prognosis between patients with bilateral pleural effusion and patients with unilateral pleural effusion. Currently, there is no specific treatment for pleural effusion in sHLH, and active treatment of primary disease based on fluid therapy is a key measure. After the initial effective treatment of HLH, the pleural effusion can be completely alleviated.
Our study also suffers from some limitations. First, the results are a retrospective cohort study from a single center, which may not be representative of the general sHLH population. Nevertheless, this also implies an advantage in terms of consistency in diagnosis, treatment and follow-up. Second, pleural effusion levels are a dynamic process, and their analyses did not account for development over time. In addition, only three of the 95 patients with pleural effusion in this study underwent pleural puncture and drainage. Due to the small sample size, this paper cannot summarize the biochemical indexes and cytological characteristics of sHLH combined with pleural effusion. Thus, our results must be interpreted with some caution.
Conclusions
Our data indicate that the incidence of pleural effusion is relatively high in sHLH patients, and suggest that the pathogenesis of pleural effusion may be directly related to excessive inflammatory cytokines in sHLH. Additionally, sHLH patients with pleural effusion had a higher inflammatory state and poor prognosis.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
La Rosée P, Horne A, Hines M, von Bahr GT, Machowicz R, Berliner N, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465–77.
Al-Samkari H, Berliner N. Hemophagocytic lymphohistiocytosis. Annu Rev Pathol. 2018;13:27–49.
Risma KA, Marsh RA. Hemophagocytic lymphohistiocytosis: clinical presentations and diagnosis. J Allergy Clin Immunol Pract. 2019;7:824–32.
Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515.
Yildiz H, Van Den Neste E, Defour JP, Danse E, Yombi JC. Adult haemophagocytic lymphohistiocytosis: a review. QJM. 2020;14:hcaa011.
Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–16.
Seguin A, Galicier L, Boutboul D, Lemiale V, Azoulay E. Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis. Chest. 2016;149:1294–301.
Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31.
Zhang LJ, Xu J, Liu P, Ding CY, Li JY, Qiu HX, et al. The significance of 18F-FDG PET/CT in secondary hemophagocytic lymphohistiocytosis. J Hematol Oncol. 2012;5:40.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9.
Oudart JB, Maquart FX, Semouma O, Lauer M, Arthuis-Demoulin P, Ramont L. Pleural effusion in a patient with multiple myeloma. Clin Chem. 2012;58:672–4.
Alexandrakis MG, Passam FH, Kyriakou DS, Bouros D. Pleural effusions in hematologic malignancies. Chest. 2004;125:1546–55.
Fitzgerald NE, MacClain KL. Imaging characteristics of hemophagocytic lymphohistiocytosis. Pediatr Radiol. 2003;33:392–401.
Shieh AC, Guler E, Smith DA, Tirumani SH, Beck RC, Ramaiya NH. Hemophagocytic lymphohistiocytosis: a primer for radiologists. AJR Am J Roentgenol. 2020;214(1):W11–9.
Ramachandran S, Zaidi F, Aggarwal A, Gera R. Recent advances in diagnostic and therapeutic guidelines for primary and secondary hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2017;64:53–7.
Wang Y, Wang Z, Wu L, Zhang J, Wang J, Yan L. Recombinant human thrombopoietin is an effective treatment for thrombocytopenia in hemophagocytic lymphohistiocytosis. Ann Hematol. 2013;92(12):1695–9.
Zoller EE, Lykens JE, Terrell CE, Aliberti J, Filipovich AH, Henson PM, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208:1203–14.
Trottestam H, Berglöf E, Horne A, et al. Risk factors for early death in children with haemophagocytic lymphohistiocytosis. Acta Paediatr. 2012;101:313–8.
Humblet-Baron S, Franckaert D, Dooley J, Ailal F, Bousfiha A, Deswarte C, et al. IFN-γ and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis. J Allergy Clin Immunol. 2019;143(6):2215–26.
Hayden A, Lin M, Park S, Pudek M, Schneider M, Jordan MB, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1:2529–34.
Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689–91.
London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:19r–23r.
Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020;202:361–70.
Ding J, Hostallero DE, El Khili MR, Fonseca GJ, Milette S, Noorah N, et al. A network-informed analysis of SARS-CoV-2 and hemophagocytic lymphohistiocytosis genes’ interactions points to Neutrophil extracellular traps as mediators of thrombosis in COVID-19. PLoS Comput Biol. 2021;17: e1008810.
Weaver LK, Behrens EM. Hyperinflammation, rather than hemophagocytosis, is the common link between macrophage activation syndrome and hemophagocytic lymphohistiocytosis. Curr Opin Rheumatol. 2014;26(5):562–9.
Jin YK, Xie ZD, Yang S, Lu G, Shen KL. Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis: a retrospective study of 78 pediatric cases in mainland of China. Chin Med J (Engl). 2010;123:1426–30.
Binder C, Duca F, Binder T, Rettl R, Dachs TM, Seirer B. Prognostic implications of pericardial and pleural effusion in patients with cardiac amyloidosis. Clin Res Cardiol. 2021;110:532–43.
Ryu JS, Lim JH, Lee JM, Kim WC, Lee KH, Memon A, et al. Minimal pleural effusion in small cell lung cancer: proportion, mechanisms, and prognostic effect. Radiology. 2016;278(2):593–600.
Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125:2908–14.
Karapinar B, Yilmaz D, Balkan C, Akin M, Ay Y, Kvakli K. An unusual cause of multiple organ dysfunction syndrome in the pediatric intensive care unit: hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2009;10:285–90.
Acknowledgements
The authors express their gratitude for all persons who were involved in reporting on patients and gathering data.
Funding
This work was supported by the National Natural Science Foundation of the People’s Republic of China [No. 81570175].
Author information
Authors and Affiliations
Contributions
WYC and HXQ designed the experiment. WYC and XG performed the experiments. WYC, XG, GLY, CFM and HXQ organized the clinical materials. WYC and XG performed the data analysis. WYC wrote the paper. All authors contributed to the final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical standards
All procedures in studies were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. It was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Number: 2019-SR-446).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Cheng, W., Gao, X., Yin, G. et al. Characteristics and prognostic value of pleural effusion in secondary hemophagocytic lymphohistiocytosis. Int J Hematol 116, 102–109 (2022). https://doi.org/10.1007/s12185-022-03333-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03333-9