Abstract
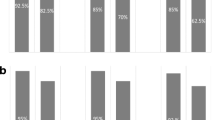
To identify strategies for reducing emesis induced by the CHOP regimen, which includes high-dose steroids, we prospectively evaluated the efficacy of palonosetron in Japanese patients. Palonosetron was administered at a dose of 0.75 mg via intravenous injection over 30 min before chemotherapy on day 1. Patients kept diaries of chemotherapy-induced nausea and vomiting (CINV) incidence from the start of chemotherapy until 168 h afterwards, in which they documented the occurrence and severity of nausea, vomiting, anorexia, and the use of rescue medication. The primary endpoint was the overall occurrence rate of nausea, vomiting, and anorexia; these rates were 56, 12, and 62 %, respectively, including all grades. The rates and severity of symptoms tended to worsen 120–168 h after completing oral prednisolone. We defined complete response (CR) as no vomiting and no use of rescue therapy. The CR rates of post palonosetron 0.75 mg treatment in the acute (0–24 h), delayed (24–168 h), and overall phases (0–168 h) were 86, 66, and 62 %, respectively. Antiemetic strategies of CHOP regimen for day 6 and, thereafter, should be investigated.

Similar content being viewed by others
References
de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, Beurden V, Stoter G, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer. 1997;76:1055–61.
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. Am Soc Clin Oncol J Clin Oncol. 1999;17:2971–94.
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Antiemesis (ver.1, 2012). Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp, Accessed July 16, 2016.
MASCC/ESMO Antiemetic Guideline 2016. Available at http://www.mascc.org/assets/Guidelines-Tools/mascc_antiemetic_guidelines_english_2016_v.1.2.pdf Accessed July 18, 2016.
Wong EH, Clark R, Leung E, Loury D, Bonhaus DW, Jakeman L, et al. The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol. 1995;114:851–9.
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98:2473–82.
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–7.
Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–9.
Di Renzo N, Montanini A, Mannina D, Dondi A, Muci S, Mancuso S, et al. Single-dose palonosetron for prevention of chemotherapy-induced nausea and vomiting in patients with aggressive non-Hodgkin’s lymphoma receiving moderately emetogenic chemotherapy containing steroids: results of a phase II study from the Gruppo Italiano per lo Studio dei Linfomi (GISL). Support Care Cancer. 2011;19:1505–10.
Choi BS, Borsaru GP, Ballinari G, Voisin D, Di Renzo N. Multicenter phase IV study of palonosetron in the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with non-Hodgkin lymphomas undergoing repeated cycles of moderately emetogenic chemotherapy. Leuk Lymphoma. 2014;55:544–50.
Navari RM, Kaplan HG, Gralla RJ, Grunberg SM, Palmer R, Fitts D. Efficacy and safety of granisetron, a selective 5-hydroxytryptamine-3 receptor antagonist, in the prevention of nausea and vomiting induced by high-dose cisplatin. J Clin Oncol. 1994;12:2204–10.
Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in U.S. and Japanese healthy subjects. J Clin Pharmacol. 2004;44:520–31.
Segawa Y, Aogi K, Inoue K, Sano M, Sekine I, Tokuda Y, et al. A phase II dose-ranging study of palonosetron in Japanese patients receiving moderately emetogenic chemotherapy, including anthracycline and cyclophosphamide-based chemotherapy. Ann Oncol. 2009;20:1874–80.
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–24.
Niesler B, Kapeller J, Hammer C, Rappold G. Serotonin type 3 receptor genes: HTR3A, B, C, D, E. Pharmacogenomics. 2008;9:501–4.
Fasching PA, Kollmannsberger B, Strissel PL, Niesler B, Engel J, Kreis H, et al. Polymorphisms in the novel serotonin receptor subunit gene HTR3C show different risks for acute chemotherapy-induced vomiting after anthracycline chemotherapy. J Cancer Res Clin Oncol. 2008;134:1079–86.
Weinstein C, Jordan K, Green SA, Camacho E, Khanani S, Beckford-Brathwaite E, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: results of a randomized, double-blind phase III trial. Ann Oncol. 2016;27:172–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding.
Conflict of interest
The authors have declared no conflicts of interest.
About this article
Cite this article
Miyata, Y., Yakushijin, K., Inui, Y. et al. A prospective study of the antiemetic effect of palonosetron in malignant lymphoma patients treated with the CHOP regimen. Int J Hematol 104, 682–691 (2016). https://doi.org/10.1007/s12185-016-2089-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2089-9