Abstract
Waterflood-assisted oil recovery with sulfate-spiked seawater would cause incompatibility scaling in carbonate reservoirs and reduce economic benefits. This research investigated the benefits of polyphosphate compounds in reducing scaling potential as well as its effect on oil recovery when mixed in high sulfate flood water. Severity of scaling potential of sulfate-spiked water in a carbonate reservoir environment was measured, followed by systematic screening of a polyphosphate compound, which successfully inhibited the sulfate scale precipitation at concentration as low as 100 ppm. The new formulation (seawater with four times sulfate and phosphate, SW4SP) was evaluated and compared with benchmark formulation (modified seawater with four times sulfate, SW4S). Contact angle, ζ-potential and drainage studies show that SW4SP changed the rock wettability from oil wet to water wet to a larger degree compared to SW4S. Improved recovery efficiency of SW4SP was confirmed through a set of core flooding studies in the tertiary and quaternary flood modes. Whereas SW4S recovered 7.7% of original oil in place (OOIP), SW4SP recovered about 8% of OOIP in the tertiary mode under approximately identical flow conditions. Flooding with SW4SP in the quaternary mode following a tertiary flood with SW4S on the same core resulted in 1.7% additional oil recovery, showing improved efficiency of the new flood water formulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Numerous laboratory studies and a few field pilots have shown that both low-salinity waterflooding (LSWF) and ion-modified waterflooding (MWF) could be potential and economic EOR solutions comparable to other chemical EOR techniques (Rotondi et al. 2014; Winoto et al. 2014; AlQuraishi et al. 2015; Jackson et al. 2016; Afekare and Radonjic 2017). The fundamental difference between LSWF and MWF is that in LSWF, the salinity and total dissolved solids (TDS) of the injected water are lowered by diluting with seawater or freshwater (Nasralla and Nasr-El-Din 2011; Shaker and Skauge 2012). On the other hand, modified waterflooding refers to the appropriate optimization of ionic composition of injection water to achieve the favorable attributes of the rock–oil–brine (ROB) system and improve the microscopic displacement efficiency. The base water in modified water could be the seawater or produced water or water from other economically available sources. Most laboratory studies have reported that oil recovery was improved using modified water, especially by spiking SO42− ions. Divalent cations such as Mg2+ and Ca2+ are also found to have a positive impact on oil recovery either individually or in combination with higher SO42− concentration (Fathi et al. 2012; Zahid et al. 2012; Awolayo et al. 2014). Mohsenzadeh et al. (2016), however, reported an additional oil recovery of 33% through core flooding experiments when the injection water salinity was nearly equivalent to distilled water and no additional bivalent ions were added, thus favoring LSWF over MWF. Recent work of Kholood et al. (2018) also favored LSWF over MWF stating that wettability alteration of oil-wet calcite surfaces is more prominent with LSWF compared to high-salinity brines. They also found that the modified brine is effective when Ca2+ ions are substituted by Mg2+ ions and sulfate ions play a catalytic role in wettability alteration in oil-wet calcite plates. The mechanism is attributed to substitution and MgCO3 mineral precipitation. However, the observations were not authenticated through core flooding experiments. Similar observations were earlier reported by Lashkarbolooki et al. (2017). Interestingly, they also observed that among the chloride containing salts, the monovalent cations performed better in terms of the wettability alteration of oil-wet carbonate rock surfaces compared to the divalent cations. The order of performance reported is KCl ≥ DW > NaCl > MgCl2 > CaCl2. Jackson et al. (2016) published a comprehensive review and concluded that low-salinity effect is factual but the required conditions for improving oil recovery are not clear and it may require tuning the composition of the injection water rather than just diluting and injecting low-salinity water.
To understand the underlying mechanisms responsible for improved displacement efficiency of LSWF or MWF, numerous studies have been conducted by adding or removing various cations and anions in brines. In this direction, the work of Zhang et al. (2006) is one of the earlier ones, in which they observed synergetic effects between SO42− and Ca2+ ions and SO42− and Mg2+ ions in assisting oil recovery from chalk. Improved results were found when both Ca2+ and Mg2+ ions are present along with SO42− ions. Mohanty and Chandrasekhar (2013) reported that modified seawater containing Mg2+ and SO42− can potentially change aged oil-wet calcite plates to more water-wet conditions. Awolayo et al. (2014, 2016) suggested that ion-modified brine with four times SO42− in seawater is the optimum which may result in an additional recovery of 9% in carbonate rocks. Awolayo et al. (2018) also suggested that, for improving oil recovery in carbonates by modified water, not only the injected water should be rich in the potential determining ions (PDIs), but also the target reservoir should have minerals that have PDI sources.
As far as the underling chemistry and mechanisms are concerned, most authors cited above attributed the change in wettability toward favorable water wetness as the main factor behind release of trapped oil. A detailed review of low-salinity effect on carbonate rocks conducted by Al-Shalabi and Sepehrnoori (2016) supported this view. Statistical analysis of different proposed mechanisms conducted by Sohal et al. (2016) and Afekare and Radonjic (2017) also suggested favorable wettability alteration as the main underlying factor associated with LSWF/MWF successes. It is worth mentioning here that whereas wettability alteration is just the consequence of oil recovery process, the real mechanism could be a combination of different underlying processes whose net outcome is the visible change in wettability (Purswani et al. 2017). Lashkarbolooki et al. (2016) and Myint and Firoozabadi (2015) conducted extensive reviews of the probable mechanisms of action regarding to the wettability alteration and came to the conclusion that expansion of electric double layer, multicomponent ionic exchange, salt-in effect and dissolution of calcite minerals are possible mechanisms responsible for the wettability alteration of oil-wet carbonate rocks.
Some authors also stressed on the importance of lowering interfacial tension (IFT) by LSW/MW as a major factor. Manshad et al. (2016) found that the presence of K2SO4 salt significantly reduced oil–water interfacial tension (IFT), which was attributed to the additional oil recovery. This claim is somewhat contradictory to the detailed study of Ayirala et al. (2018), in which the impact of sulfate and other ions on oil–water interface was analyzed through IFT, interfacial pressure, viscous and elastic moduli, oil droplet crumpling and coalescence behavior and also interfacial film rigidity. The results showed that a reduction in IFT is low and has an insignificant effect on enhanced oil recovery. They also proved that sulfate ions have a measurable impact on the interface only when it is present along with magnesium and calcium ions. Validity of these statements could be found from the extensive studies of the optimization of salt type and concentration conducted by Lashkarbolooki and Ayatollahi (2018a, b), through the calculation of surface free energies (adhesion, cohesion and spreading coefficient) from the measured IFT and contact angle values. Among others, the most relevant information drawn from these studies is: (1) There is a direct correlation between change in contact angle and spreading coefficient; (2) improvement in spreading performance occurred with the reduction in brine salinity and (3) although the dilution of seawater unfavorably changed the IFT, it could favorably change the spreading coefficient and the contact angle. Through this study, they also explained the reasons for higher affinity of SO42− toward the carbonate surface compared to Cl−, thus establishing the higher potential of SO42− as wettability modifier.
The connate water in carbonate reservoirs is usually high in pH, is saturated with Ca2+ and has a significant amount of Sr2+, Ba2+ (the potential scale-forming ions). As evidenced from various laboratory results, to achieve the benefits of MWF, the injection water needs to be spiked with multivalent ions, such as SO42−, Ca2+ or Mg2+, the PDIs. Among these PDIs, it is categorically established that SO42− is one of the essentials for MWF composition. However, it is also well established that the presence of higher concentration of SO42− ions could invite incompatibility scaling problems (Paulo et al. 2001), resulting in reservoir pore blocking and tubing flow restrictions. Formation water in carbonate reservoirs being rich in divalent cations (Ca2+, Ba2+ and Sr2+) and incorporation of higher level of SO42− in injection water may lead to the precipitation and deposition of BaSO4, SrSO4 and/or CaSO4 scales which are extremely difficult to dissolve or remove. Sulfate scaling problems could be even more serious when the downhole temperature is high, as the rate of sulfate scale precipitation increases at elevated temperatures (Yuan 2002; Badr and Azam 2008). In the long run, the resulting phenomena could be pore plugging in the formation near the wellbore, partial or total blocking of production tubing, pressure rise in injection wells, etc. (Andersen et al. 2000; Graham et al. 2001), resulting in overall reduced field productivity, thus losing the economic benefits of MWF.
The work described here is an endeavor toward developing a modified seawater formulation for a high-temperature (120 °C) giant offshore carbonate field in the Middle-East region. The objective is to fulfill the requirement of an efficient MWF, which does not pose the challenge of incompatibility scaling issues at the bottom hole conditions. In this work, we evaluated certain orthophosphates and condensed polyphosphates in combating sulfate scaling issues in MWF, at the same time to evaluate their impacts on the efficiency of MWF in improved oil recovery.
Orthophosphate and polyphosphate are traditionally used in water treatment for mineral removal, scale control and corrosion control. Orthophosphates as scale inhibitors are mostly used for low-temperature application up to 80 °C as there is a possibility of hydrolysis which results in loss of effectiveness. Polyphosphate scale inhibitors, however, have better thermal stability (O’Neil 2013). The rationale behind selecting phosphate-based compounds is briefly described as follows (http://www.phosphatesfacts.org):
- 1.
Strong chelating ability with Ca2+, Ba2+ and Sr2+ ions.
- 2.
Inhibit scale formation by suppressing both nucleation and crystal growth.
- 3.
Works at threshold concentration.
- 4.
Applicable for both carbonate and sulfate scales.
- 5.
Ba and Sr sulfate scale inhibition requires higher pH (> 5.5) which is prevalent in a typical carbonate reservoir.
- 6.
Threshold-level polyphosphates deflocculate suspended scales through reduction in surface adhesion properties, thus helping in the removal of existing scale deposits if any.
Unfortunately, very little research effort is dedicated on the application of phosphate as an ingredient of MWF, though its poly-anionic nature could be expected to have higher impact compared to divalent sulfate ions. The information available on injection water modified with the addition of phosphate ions is meager. The pioneering work of Gupta et al. (2011) reported above 20% additional oil recovery in limestone cores. From several core flooding studies, they demonstrated that whereas seawater with added MgCl2 and borate (without SO42−) resulted in 5.1% and 15.6% additional oil recovery, addition of phosphate recovered 21.3% additional oil. However, no information about the type of the phosphate compound or its concentration is documented. In a subsequent publication, the group reported the issue of Ca phosphate precipitation at concentrations of 250 ppm and above when trisodium phosphate was added to seawater (Loan 2012). More recently, Meng et al. (2015) reported higher oil recovery potential when phosphate ions were present along with sulfate ions. They also claimed that with increasing the phosphate concentration, the IFT was further lowered and the contact angle shifted more toward water-wet conditions. Once again, no information is revealed about the type of phosphate compounds and their compatibility with the water. It must be borne in mind that phosphate precipitation is a high possibility when the brine contains divalent cations. One must be extremely cautious about the selection of phosphate compound type and its threshold concentration to avoid precipitation, which was our prime focus to begin with the project.
From the above discussion, it is evident that substantial opportunity exists either to establish or to negate the usefulness of phosphate compounds as an ingredient of MWF with respect to scale mitigation and EOR. With these objectives, a MW composition is developed and tested at up to 120 °C. A carefully selected polyphosphate compound is introduced into the SO42−-spiked water, which not only arrests the scale precipitation, but also enhances oil recovery potential.
2 Experimental
2.1 Experimental materials
2.1.1 Crude oil
Oil used in this study was from a Middle Eastern carbonate reservoir. The oil sample was degassed, centrifuged at 3000 rpm to remove suspended particles and finally filtered with a 0.45-micron filter before use. The physical and chemical properties of the crude oil are presented in Table 1.
2.1.2 Brines
Synthetic seawater and formation water were prepared based on the compositional data received from the field laboratory. Seawater was used as the base water or diluted as required. Synthetic formation water was used for core saturation, for contact angle measurement and also for compatibility tests. The screening and final selection of the composition of the modified brines were in accordance with the suggestions given by predecessors (Zhang et al. 2007; Fathi et al. 2012; Awolayo et al. 2014). Accordingly, seven different brines were prepared. For convenience, formation water is termed as “FW” and seawater as “SW.” The modified seawaters were termed as, e.g., “0.1SW4S,” meaning ten times diluted seawater with four times the usual sulfate ion found in SW. Table 2 shows the ionic compositions and the total dissolved solids (TDS) of the brines used. All the brines were prepared using laboratory-grade chemicals and deionized water.
2.1.3 Core plugs and trims
Core plugs and trims used in this study were cut from a single core block of about 2 ft long. After extracting, the plugs were subjected to porosity and permeability measurements. The final selection of plugs and trims was based on closeness of these parameters. All the trims used in contact angle measurements were cut from a single piece of core plug to minimize experimental uncertainties.
The plugs and trims were subjected to a two-stage cleaning process using Soxhlet extractors and various solvents. In the first stage of cleaning, toluene was used to remove organic materials present in the pores. The extraction was continued till the effluent was visibly clear. The cores were then removed, dried in an oven and placed in the second unit having methanol as solvent, to extract the inorganic salts. The effluent was titrated with silver nitrate intermittently to ensure complete removal of salts. Plugs and trims were dried in a hot air oven, and a routine core analysis was performed on the whole plug. Wettability restoration of the plugs and trims was performed by saturating the samples with formation brine in vacuum for 48 h and subsequently aging for 30 days in crude oil in a pressure cylinder at 120 °C. Two sets of core plugs and a set of trims of very similar petro-physical properties were chosen for core flooding experiments, drainage test and contact angle measurements. Core plugs of larger length were used for core flooding recovery studies, and the relatively smaller ones were used for drainage studies; details of the core plugs used are given in Table 3.
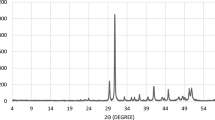
Core plugs were examined by X-ray powder diffraction in both whole-rock and clay-sized powders. Calcite (94.6%), dolomite (4.3%) with minor clay (0.7%) and halite (0.2%) were detected in mixed samples.
2.1.4 Phosphate compounds
A phosphate is a salt of phosphoric acid (H3PO4) which forms polyatomic phosphate ions through the release of one or more hydrogen atoms in basic aqueous solutions. A variety of salts of sodium (Na+) and phosphate (PO43−) are formed as mono- or polyphosphates as condensed anions including di-, tri-, tetra- and even higher phosphates (see Fig. 1).
The diphosphates are also called pyrophosphates and the cyclic polyphosphates are called metaphosphates which include sodium trimetaphosphate (STMP) and higher polyphosphates (Schrodter et al. 2008). STMP and higher metaphosphates are used in water treatment as chelating agents. Because of their poly-anionic character, they can chelate with Ca2+ and other bi- and trivalent ions replacing the native anions (Changa 1983). In a solution, Na triphosphate or STP exists as penta-anionic chains. It binds strongly to metal cations as both a bidentate and tridentate chelating agent as shown in Fig. 2a.
Sodium hexametaphosphate (SHMP) exists in the aqueous solution as hexavalent polyphosphate anion (Fig. 2b). It is widely used as a sequestrant in water treatment and also as a clay dispersing agent (Andreola et al. 2004). Superior antiscaling activity of SHMP is established by Rahman (2013), who screened 20 different antiscalants at 10 ppm dosing in supersaturated CaSO4 solution and found that SHMP’s scale inhibition performance is better than all the generic chemicals studied. In this study, we screened several phosphate compounds as listed in Table 4 and selected the one with highest compatibility with the formation brine.
2.2 Experimental methods
2.2.1 Brine compatibility evaluation
ScaleChem-3 scale prediction simulator was used to study the compatibility between formation water and different injection waters at experimental (90 °C) and reservoir (120 °C) temperatures. The simulations predicted salt precipitation potential at various mixing ratios of formation to injection waters.
To verify the prediction in simulated conditions and to quantify the extent and rapidity of deposition, differential scale loop (DSL) tests were conducted following NACE guidelines (NACE 2005-24225) at 90 °C. Formation water (brine-1, the cationic solution) and injection brines (brine-2, as the anionic solution) were pumped through preheated coils at a rate of 1 mL/min each and then through a capillary test loop (1 m × 1 mm) upon immediate commingling of the two fractions. Scale formation and build-up rates were analyzed from the differential pressure behavior across the test loop. Scale inhibiting chemicals in precise quantities were pumped through a third pump into the loop as per the test deign.
2.2.2 Contact angle measurement
Static contact angles were measured on core trims (approximately 25 mm thick). The trims were polished using a fine grade of sandpaper to minimize the hysteresis effect due to surface roughness. Prior to the tests, the trims were aged in formation water for 24 h, followed by being aged in oil at 120 °C for 4 weeks in a pressure cylinder. The plates were then immersed in preheated test brines (at 90 °C) in a jar, and an oil drop was placed before sealing the jars. The oil drops were subjected to periodic observation, image capturing and analysis by measuring the angle between the baseline and the droplet’s tangent. Figure 3 displays a sample of the images of oil–rock–brine contact angle.
2.2.3 Spontaneous drainage test
Though spontaneous imbibition is traditionally recognized as an empirical method for characterizing reservoir wettability, a spontaneous drainage process (brine replaced by oil) is preferred here because of the fact that these reservoir cores are oil wet in nature. The spontaneous drainage tests were conducted at 90 °C using Amott cells. The brine-saturated core plugs were placed in the cells, filled with oil and placed in a hot air oven. The amount of brine expelled from the cores (spontaneously) was measured periodically. A higher rate and quantity of expelled brine would be indicative of higher oil wettability and vice versa.
2.2.4 ζ-potential measurement
A zeta PALS instrument (Brookhaven Instruments, PALS: phase analysis light scattering) was used to measure ζ-potential of rock–brine and rock–brine–oil systems. The instrument measures the electrophoretic mobility of charged colloidal suspensions. Core pieces were pulverized, sieved (5–10 μm) and aged in oil for 1 week at reservoir temperature. Oil-coated core powders were thoroughly mixed in the test brine, and the ζ-potential was measured while stirring. This set represented a rock–oil–brine (R–O–B) system. A second set of measurements were taken involving bare rock powders suspended in brine, representing a rock–brine (R–B) system. Five measurements were taken for each set of samples, and the average values were considered.
2.2.5 NMR porosity studies
An NMR Rock Core Analyzer (Magritek) was used to measure the pore size distribution (PSD) and cumulative porosity of the core plugs. The measurements were taken before and after core flooding experiments in order to investigate the change in pore structures. In both cases, the core plugs were cleaned of any oil and soluble salts and fully saturated with formation water prior to NMR studies.
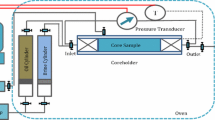
2.2.6 Core flooding experiments
Temco CFS-830-10000-HC core flooding apparatus was used to evaluate the oil recovery efficiency of brines. Seawater was used as standard secondary recovery fluid, and two ion-modified brines (SW4S and SW4SP, the most potent brine established through previous studies) were used as the tertiary injection fluids.
Oil-saturated core plugs with known initial saturation were installed in the core holder and stabilized at desired temperature and confinement pressure. Seawater was injected (in the secondary recovery mode) at a flow rate of 0.1 mL/min till there was no further oil production. The flow rate was later increased to 0.5 mL/min as a practice of bump flooding to overcome capillary end effects. Differential pressure across the core was continuously measured in this process. However, the pressure readings were found to be fluctuating and erratic, which was later found to be due to faulty pressure transducers and the associated electronic circuits. (A sample core flood pressure–permeability plot is shown in Fig. 4.) The oil displacement efficiency and residual oil saturation, Sor, were calculated with utmost precessions by measuring the weight and volume of the effluents using precession balance and micro-burettes, respectively. After reaching Sor (secondary), the tertiary flood was conducted at the same flow rate (0.1 mL/min) for 2 or more pore volumes of injection fluids till no further oil was produced. The flow rate was later increased to 0.5 mL/min (five times) as a practice of bump flooding; however, upon increasing the flow rate no perceivable additional oil was recovered. The third core flooding was conducted in the following sequence. Seawater was injected in the secondary mode, SW4S brine was injected in the tertiary recovery mode, and finally, SW4SP was injected in the quaternary recovery mode. This sequence was designed in order to ascertain the real impact of SHMP over and above SW4S flood recovery. During the changeover between two flood brines, extra precautions were taken to measure the produced oil volume by using a funnel fitted micro-burette having 0.01 mL graduation, ensuring error limit not exceeding 5%.
3 Results and discussion
3.1 Brine compatibility
In waterflood designing, one of the necessary components for consideration is the compatibility between injection water and formation water. This can be predicted reasonably well through laboratory testing and through computer simulation. In the present study, we studied water incompatibility through computer simulation at 120 °C (reservoir temperature) and 90 °C (experimental temperature). The scaling tendency (ST) was calculated and is plotted in Fig. 5.
Figure 5 indicates that the scaling tendency (ST) increased with an increase in SO42− concentration, increasing from 2000 to 12,000 mg/L when the SO42− concentration in injection water increased from 0.034 to 0.202 mol/L. For the mixing of formation and seawater (Fig. 5a), the ST at 90 °C was about 3% less than that at 120 °C. No significant difference in ST was observed when the TDS of the base water was reduced to one-tenth through dilution with deionized water (Fig. 5b). Thus, it can be concluded that the ST is mainly dependent on the concentration of SO42− in injection water and less on the TDS. It can also be noticed that the undiluted seawater has a minor self-scaling tendency too. (ST is above zero at 100% seawater.) Maximum solid precipitation potential is shifted toward the right when the SO42− concentration increases, suggesting that the scaling problem will not only be aggravated with higher SO42− concentration in the displacing water but also will manifest for a longer duration compared to injection water of lower SO42− concentration.
Based on the above observations, further compatibility tests were performed in DSL to investigate the kinetics of scale formation in dynamic conditions. In these experiments, formation water and injection water with different ratios were injected into the test loop separately and the differential pressure across the loop was plotted against time. An early rise of differential pressure indicated faster scale deposition and higher level of incompatibility between the two brines. Figure 6a–c shows the differential pressure across the loop when injection waters were SW2S, SW4S and SW6S, respectively.
Figure 6d represents the time at which the scales start to build up in the loop. It is evident from Fig. 6a–c that scaling may start at a very early stage of water injection at the flood water front. Whereas for SW2S brine scales were formed after about 90 min, for SW4S and SW6S brines scales began to appear after 13 and 8 min, respectively. Figure 6d clearly shows that the scale build-up time can be correlated with the SO42− concentration in the brine. These findings were the basis and motivation for the rest of the study.
3.2 Inhibitor screening and optimization
As discussed earlier, polyphosphates are known to have sequestrant properties and phosphate ions are reported to have enhanced oil recovery potential too (Gupta et al. 2011). However, the type of phosphate compounds which can serve both the purposes is not being studied in detail. Phosphates are known to exist as a variety of complex molecules starting from monophosphate (e.g., trisodium phosphate) to polyphosphates (having hundreds of PO4 groups in a single molecule). This research was restricted from mono- to hexametaphosphate, the molecular structures of which are shown in Fig. 1.
Initial screening and dose optimization were conducted through static jar tests using a 50:50 mixture of FW and SW4S brine mixed with one of the phosphates (dosed from 100 to 500 ppm at an increment of 100 ppm). Samples were prepared in duplicates and incubated at 25 and 95 °C for 24 h. The results are presented in Table 4. The tests ruled out usefulness of lower phosphates and indicated that only tri- and hexametaphosphates have the ability to inhibit sulfate scaling. However, it is evident from Table 4 that the dose requirement for trimetaphosphate would be much higher than for hexametaphosphate. Thus, sodium hexametaphosphate (SHMP) was selected for the subsequent studies.
Based on the positive results shown in static tests, dynamic compatibility tests were conducted with 250, 125, 100, 75, 50 and 25 ppm of SHMP dosing in SW4S brine. Figure 7 shows that the differential pressure across the flow loop started to increase when the SHMP dosing fell below the 100 ppm level. Thus, establishing the minimum inhibitor concentration (MIC) required to suppress the scaling tendency is about 100 ppm. Hence, for all further studies, 100 ppm SHMP is considered for mixing with the smart brines, and its impact on the rock–fluid parameters and oil recovery are studied. This new brine is denoted as SW4SP.
3.3 Static contact angle
The changes in contact angle for all the brine–oil–rock systems against contact time are presented in Fig. 8, and the changes in contact angle (Δθ) achieved by different brines are given in Table 5.
Figure 8 depicts the change in contact angle with time when the oil-aged core trims were exposed to different brines. For most brines, the contact angle ceased to change at or before 45 h. No further change in contact angle was observed after further exposure. Table 5 shows that a maximum change in contact angle (77°) was achieved with SW4S which is the maximum change seen among all the sulfate brines tested. Thus, the optimum sulfate ion concentration considered for this study was 0.134 mol/L or nearly four times that of the sulfate concentration in seawater. These results are in agreement with the wettability and core flood recovery results reported by Strand et al. (2006), Zhang et al. (2007) and Awolayo et al. (2016). It is also evident from the above figure and table that the Δθ value of 100 ppm SHMP-mixed brine (SW4SP) is 97°, which is higher than that of SW4S brine, indicating a positive impact of SHMP on the SW4S brine in altering carbonate rock wettability toward water wetness.
3.4 ζ-potential
ζ-potential results of various rock–brine (RB) and rock–oil–brine (ROB) systems are shown graphically in Fig. 9.
It could be seen that the all the rock–brine systems showed positive ζ-potential values whereas for the rock–oil–brine systems the ζ-potential values changed from a high negative value of −32 mV (for deionized water, out of scale in Fig. 9) to positive values (2.42, 3.15 mV) for SW4S and SW4SP brines compared to other brines with or without phosphate ions.
The stability of water film between the oil and rock interfaces determines the rock wettability, which in turn is controlled by the charges at oil–brine and rock–brine interfaces. If the water film is thick, the stable water wetness on the rock surface prevails. It is easy to change the rock wettability toward oil-wet state if the water film is thin and unstable (Sheng 2013). Thus, the rock wettability depends on the sign and magnitude of the electrical charges at the two interfaces which govern the attractive or repulsive forces within the interface.
Carbonate rock surfaces are known to have excess positive charges; thus, when the carbonate particles are aged in oil, the negative sites of the polar oil molecules will tend to attach on the rock surfaces and yield a net negative ζ-potential. As seen from the study, the oil–deionized water system yielded a high negative ζ-potential value (−32 mV), whereas in the absence of oil the ζ-potential is positive or close to neutral (0.36 mV). Thus, it can be safely assumed that the rock has an affinity toward oil wetness (which is also evidenced through contact angle measurements). As the oil is expelled, brine would start to occupy the space and the ζ-potential would tend to become more positive as seen for the rock–brine systems. Figure 9 indicates that the diluted low-salinity brines have lesser ability to convert the oil-aged particles to water wet compared to the SW4S and SW4SP brines. It is interesting to see that the SW4S and SW4SP have raised ζ-potential of the rock–oil–brine systems to a larger extent compared to other brines. This is somewhat in agreement with the findings of Song et al. (2017) who concluded a similar effect due to the addition of Na2SO4 to brine. In addition, it can also be seen that the addition of phosphate to SW4S brine has a little more impact on increasing the ζ-potential of the rock–oil–brine system. This observation is in agreement with the contact angle measurement where we observed that these two brines (SW4S and SW4SP) have the maximum impact on wettability reversal. Similar results were reported by Jackson and Vinogradov (2012) and Jackson et al. (2016). When the mineral surfaces were returned to water wet, the ζ-potential of the mineral–brine interface was found to be positive. As the sample was converted to increasingly oil wet, the ζ-potential became increasingly more negative. In our cases, the objective of ζ-potential measurement is to see how close the ζ-potential of oil-wet rock particles approaches toward the brine-wet rock, which could be an indication of release of stuck oil and help us determine the relative potential of the modified brine. It must be mentioned, however, that we used these values as indication only and used to ascertain the relative efficiency of the modified brines used for the study.
3.5 Spontaneous drainage
Spontaneous drainage experiments were conducted on core plugs as given in Table 3, using five different modified seawaters. The objective was to evaluate the impact of brine chemistry on imbibing oil into brine-saturated core plugs and the resulting drainage of brine. Higher spontaneous imbibition of oil would take place if the rock surface has a higher affinity toward oil and vice versa.
The selected core plugs were saturated with different brines (DIW, SW, FW, SW4S and SW4SP) for 24 h. Spontaneous drainage volume of brine was measured at 90 °C at regular interval till no further brine was expelled out of the core (observed for 8 days). The drainage volume (in percentage) against time (in days) is plotted (Fig. 10), which shows that except for FW, the spontaneous drainage of brine stopped after 6 days for all other brines.
The experimental data suggest that among all the five brines investigated, the SW4SP is seen to be the most potent brine in retaining water wettability followed by SW4S, thereby releasing minimum brine from the pore spaces. Results from this drainage test are in compliance with those from contact angle and ζ-potential studies in which SW4SP is proven to be the most efficient compared to other brines tested.
3.6 Core flood recovery
Three core flood experiments were conducted at 120 °C to investigate the displacement efficiency of brines at residual formation brine saturation (as described in previous section). Carefully selected core plugs of very similar petro-physical properties were used in these displacement experiments. The properties of the core plugs and the recovery data are listed in Table 6, and the displacement efficiency against pore volumes (PVs) of brine injected is presented in Fig. 11.
In the first core flooding experiment CF-1, seawater was used as the secondary flooding medium and SW4S was used in the tertiary mode. The experimental results of CF-1 were considered as benchmarks for comparison with the subsequent core flooding experiments. A cumulative recovery of 63.1% and 70.8% of original oil in place (OOIP) was recovered during the secondary and tertiary recovery modes, respectively (which includes the bump-flood recovery), showing an additional recovery of 7.7% during the tertiary flood. This result is in agreement with previous works of Fathi et al. (2012) who reported an incremental recovery of 5% to 18% of OOIP using SW4S brine, compared to SW. However, they used chalk cores, but not limestone cores. Awolayo et al. (2014) also used SW4S brine and achieved 10% incremental recovery in the tertiary recovery mode, using cores and crude oil from the same reservoir as ours.
The experiment CF-2 was conducted under similar experimental conditions. Seawater as the secondary recovery fluid was followed by phosphate-mixed SW4S brine (i.e., SW4SP) as the tertiary recovery fluid. The cumulative recoveries were found to be 66.4% and 74.4%, respectively, from the secondary and tertiary flood modes, showing an incremental recovery by 8%, which is very close to the oil recovery displaced by SW4S brine and may fall within experimental error margin. These results could not be validated with literature because no published work could be found in which SHMP brine was used as one of the MWF ingredients.
From the above two core flooding experiments, it remains inconclusive whether phosphate has any real impact on additional oil recovery. Thus, a third core flooding experiment CF-3 was conducted under exactly the same experimental conditions. Seawater was injected in the secondary mode, the SW4S brine was injected in the tertiary mode and the SW4SP brine was injected in the quaternary mode. In the secondary mode, the cumulative oil recovery was 64.7% of OOIP which is close to the previous two experiments. In the tertiary mode with SW4S brine, the cumulative recovery increased to 73.4% of OOIP, i.e., an additional 8.7% was achieved which was very similar to the previous core flooding results. In the quaternary flood mode with SW4SP brine, the cumulative recovery increased to 75.1%, i.e., 1.7% additional recovery. This incremental recovery could be attributed to the presence of phosphate in the flood water.
3.7 Pore size distribution (PSD) in core samples obtained by NMR
In the core displacement process, we captured the differential pressure (DP) data and calculated the real-time permeability of cores. The objective is to observe pore blocking, if any, due to scales or fines deposits in the displacement process. However, as stated earlier, due to erratic readings of the pressure transducer no definitive conclusion could be made. To compensate this, we investigated the pore structures before and after core flooding through NMR pore size distribution (PSD) and cumulative porosity analysis. NMR-PSD and cumulative porosity of the core plugs are shown in Figs. 12, 13 and 14.
A vertical bar is placed on 90-ms readings. The left side of this bar represents micro- and mesoporosity while the right side represents macroporosity. From the T2 distribution curve, it can be seen that the selected core plugs, cores 1C, 3A and 2B used in experiments CF-1, CF-2 and CF-3, respectively, were mostly comprised of macro-pores and there were a little micro- and meso-pores. Thus, it can be expected that the majority of the injection water had flowed through the macro-pores.
The PSD of core 1C after core flooding experiment (Fig. 12a) shows that there was a minor pore blockage in the 90–120-ms region (corresponding to average pore throat size of 1–2 microns (Tawfik et al. 2019) as correlated from mercury injection pressure), which could be attributed to pore damage due to sulfate scale deposition. It could be seen that in the rest of the macro-pore domain there is a minor porosity enlargement. Overall porosity of this core increased by nearly 0.6% during the entire flood process. Though this value is low, it could still be considered as an indication of carbonate dissolution, a process observed by many of the predecessors and attributed to one of the mechanisms responsible for additional recovery by SW4S brine.
A comparison of porosity distribution of core 3A before and after core flooding experiment CF-3, in which the SW4SP brine was used as flood water in the tertiary mode, is shown in Fig. 13. From these figures, it is evident that there was no pore blockage either in meso- or macro-pore regions. Rather there was a noticeable pore size enhancement from about 500 to 2000 ms (relaxation time T2). The cumulative porosity increased by nearly 1.5%. These observations lead to the conclusion that there is no pore blockage due to scale deposition during core flooding. The incremental pore space could be attributed to enhanced carbonate dissolution possibly due to the presence of metaphosphate ions, known to have calcium sequestering properties.
The PSD of core 2B after core flooding experiment CF-3 shows the overall increase in pore size distribution throughout the macro-pore region. The cumulative porosity increased by nearly 2.8%. This large incremental porosity cannot be justified by carbonate dissolution alone. Some amount of fines migration may also contribute to the incremental porosity; however, there is no confirming evidence for this claim. It may be noted that in experiment CF-3 the core has been flooded with additional 2.5 PV of brine compared to the experiments CF-1 and CF-2. The longer brine flood may also be responsible for higher pore enlargement.
4 Conclusions
The possibilities of inhibiting sulfate scale deposition during sulfate-spiked smart waterflooding using polyphosphate compound are studied. Strong scaling tendency of sulfate-spiked seawater is established through DSL studies and computer simulation. SHMP is introduced as scale inhibitor and a possible PDI for enhanced oil recovery. Based on the experimental findings, the following conclusions are drawn:
- (1)
All the sulfate-spiked brines would cause sulfate scale precipitation and block pore channels upon mixing with formation water in carbonate reservoirs.
- (2)
Increasing sulfate concentration by four times to that of seawater showed better performance compared to two or six times higher sulfate concentration.
- (3)
From a suite of phosphate and polyphosphate compounds, sodium hexametaphosphate (SHMP) is found to be the most potent compound which can inhibit scaling tendency of the smart waters even at elevated temperatures.
- (4)
Contact angle measurement, ζ-potential and drainage studies show that the addition of SHMP to SW4S brine not only reduced the scaling possibility but also changed the carbonate wettability from oil wet to water wet and helped in releasing the trapped oil more than SW4S brine. Core flooding studies show an additional recovery of 1.7% is achieved in the quaternary flood mode with SHMP-mixed smart brine (SW4SP). This can be attributed to the poly-anionic character of metaphosphate ions.
- (5)
Porosity distribution analysis shows minor pore blockage in the small pore regions when SW4S brine is used. No pore blockage is observed when SHMP is present in the flood water.
References
Afekare DA, Radonjic M. From mineral surfaces and coreflood experiments to reservoir implementations: comprehensive review of low-salinity water flooding. Energy Fuels. 2017;31(12):13043–62. https://doi.org/10.1021/acs.energyfuels.7b02730.
AlQuraishi AA, AlHussinan SN, AlYami HQ. Efficiency and recovery mechanisms of low salinity water flooding in sandstone and carbonate reservoirs. In: SPE Offshore Mediterranean Conference and Exhibition, 25–27 March, Ravenna, Italy; 2015. Document ID OMC-2015-223.
Al-Shalabi EW, Sepehrnoori K. A comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J Pet Sci Eng. 2016;139:137–61. https://doi.org/10.1016/j.petrol.2015.11.027.
Andersen KI, Halvorsen E, Sælensminde T, Østbye NO. Water management in a closed loop—problems and solutions at brage field. In: SPE European Petroleum Conference, 24–25 October, Paris, France; 2000. https://doi.org/10.2118/65162-MS.
Andreola F, Castellini E, Manfredini T, Romagnoli M. The role of sodium hexametaphosphate in the dissolution process of kaolinite and kaolin. J Eur Ceram Soc. 2004;24(7):2113–24. https://doi.org/10.1016/S0955-2219(03)00366-2.
Awolayo A, Sarma H, AlSumaiti AM. A laboratory study of ionic effect of smart water for enhancing oil recovery in carbonate reservoirs. In: SPE EOR Conference at Oil and Gas West Asia, 31 March–2 April, Muscat, Oman; 2014. https://doi.org/10.2118/169662-MS.
Awolayo A, Sarma H, AlSumaiti A. An experimental investigation into the impact of sulfate ions in smart water to improve oil recovery in carbonate reservoirs. Transp Porous Media. 2016;111(3):649–68. https://doi.org/10.1007/s11242-015-0616-4.
Awolayo A, Sarma H, Nghiem L. Brine-dependent recovery processes in carbonate and sandstone petroleum reservoirs: review of laboratory-field studies, interfacial mechanisms and modeling attempts. Energies. 2018;11(11):3020. https://doi.org/10.3390/en11113020.
Ayirala SC, Al-Yousef AA, Li Z, Xu Z. Water ion interactions at crude-oil/water interface and their implications for smart waterflooding in carbonates. SPE J. 2018;23(05):1817–32. https://doi.org/10.2118/183894-PA.
Badr A, Azam A. Laboratory study and prediction of calcium sulphate at high-salinity formation water. Open Pet Eng J. 2008;1:62–73. https://doi.org/10.2174/1874834100801010062.
Changa DM. The binding of free calcium ions in aqueous solution using chelating agents, phosphates and poly(acrylic acid). J Am Oil Chem Soc. 1983;60(3):618–22. https://doi.org/10.1007/BF02679800.
Fathi SJ, Austad T, Strand S. Water-based enhanced oil recovery (EOR) by “smart water” in carbonate reservoirs. In: SPE EOR Conference at Oil and Gas West Asia, 16–18 April, Muscat, Oman; 2012. https://doi.org/10.2118/154570-MS.
Graham GM, Boak LS, Hobden CM. Examination of the effect of generically different scale inhibitor species (PPCA and DETPMP) on the adherence and growth of barium sulphate scale on metal surfaces. In: SPE International Symposium on Oilfield Scale, 30–31 January, Aberdeen, United Kingdom; 2001. https://doi.org/10.2118/68298-MS.
Gupta R, Smith GG, Hu L, Willingham T, Lo Cascio M, Shyeh JJ, Harris CR. Enhanced waterflood for carbonate reservoirs—impact of injection water composition. In: SPE Middle East Oil and Gas Show and Conference, 25–28 September, Manama, Bahrain; 2011. https://doi.org/10.2118/142668-MS.
Jackson MD, Vinogradov J. Impact of wettability on laboratory measurements of streaming potential in carbonates. Colloids Surf A Physicochem Eng Asp. 2012;393:86–95. https://doi.org/10.1016/j.colsurfa.2011.11.005.
Jackson MD, Vinogradov J, Hamon G, Chamerois M. Evidence, mechanisms and improved understanding of controlled salinity waterflooding part 1: sandstones. Fuel. 2016;185:772–93. https://doi.org/10.1016/j.fuel.2016.07.075.
Kholood N, Peyman P, Nader M, Ali S. Effect of initial wettability on performance of smart water flooding in carbonate reservoirs—an experimental investigation with IOR implications. Energies. 2018;11(6):1394. https://doi.org/10.3390/en11061394.
Lashkarbolooki M, Riazi M, Hajibagheri F, Ayatollahi S. Low salinity injection into asphaltenic-carbonate oil reservoir, mechanistical study. J Mol Liq. 2016;216:377–86. https://doi.org/10.1016/j.molliq.2016.01.051.
Lashkarbolooki M, Ayatollahi S, Riazi M. Mechanistical study of effect of ions in smart water injection into carbonate oil reservoir. Process Saf Environ Prot. 2017;105:361–72. https://doi.org/10.1016/j.psep.2016.11.022.
Lashkarbolooki M, Ayatollahi S. Investigating injection of low salinity brine in carbonate rock with the assist of works of cohesion and adhesion and spreading coefficient calculations. J Pet Sci Eng. 2018a;161:381–9. https://doi.org/10.1016/j.petrol.2017.12.010.
Lashkarbolooki M, Ayatollahi S. Performance of sea water dilution on the surface free energies of the crude oils in water-flooded carbonate rock. J Adhes Sci Technol. 2018b;32(12):1359–68. https://doi.org/10.1080/01694243.2017.1415050.
Loan TV. Ion chromatography analysis of advanced ion management carbonate coreflood experiments. In: Abu Dhabi International Petroleum Conference and Exhibition, 11–14 November, Abu Dhabi, UAE; 2012. https://doi.org/10.2118/161821-MS.
Manshad AK, Olad M, Taghipour SA, Nowrouzi I. Effects of water soluble ions on interfacial tension (IFT) between oil and brine in smart and carbonated smart water injection process in oil reservoirs. J Mol Liq. 2016;223:987–93. https://doi.org/10.1016/j.molliq.2016.08.089.
Meng W, Haroun MR, Sarma HK, Adeoye JT, Aras P, Punjabi S, Al Kobaisi M. A novel approach of using phosphate-spiked smart brines to alter wettability in mixed oil-wet carbonate reservoirs. In: SPE Abu Dhabi International Petroleum Exhibition and Conference, 9–12 November, Abu Dhabi, UAE; 2015. https://doi.org/10.2118/177551-MS.
Mohanty KK, Chandrasekhar S. Wettability alteration with brine composition in high temperature carbonate reservoirs. In: SPE Annual Technical Conference and Exhibition, 30 September–2 October, New Orleans, Louisiana, USA; 2013. https://doi.org/10.2118/166280-MS.
Mohsenzadeh A, Pourafshary P, Al-Wahaibi Y. Oil recovery enhancement in carbonate reservoirs via low saline water flooding in presence of low concentration active ions: a case study. In: SPE EOR Conference at Oil and Gas West Asia, 21–23 March, Muscat, Oman; 2016. https://doi.org/10.2118/179767-MS.
Myint PC, Firoozabadi A. Thin liquid films in improved oil recovery from low-salinity brine. Curr Opin Colloid Int Sci. 2015;20(2):105–14. https://doi.org/10.1016/j.cocis.2015.03.002.
Nasralla RA, Nasr-El-Din HA. Coreflood study of low salinity water injection in sandstone reservoirs. In: SPE/DGS Saudi Arabia Section Technical Symposium and Exhibition, 15–18 May, Al-Khobar, Saudi Arabia; 2011. https://doi.org/10.2118/149077-MS.
O’Neil MJ, editor. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. RSC Publishing; 2013. https://www.rsc.org/merck-index.
Paulo J, Mackay EJ, Menzies N, Poynton N. Implications of brine mixing in the reservoir for scale management in the Alba Field. In: SPE International Symposium on Oilfield Scale, 30–31 January, Aberdeen, United Kingdom; 2001. https://doi.org/10.2118/68310-MS.
Purswani P, Tawfik MS, Karpyn ZT. Factors and mechanisms governing wettability alteration by chemically tuned waterflooding: a review. Energy Fuels. 2017;31(8):7734–45. https://doi.org/10.1021/acs.energyfuels.7b01067.
Rahman F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination. 2013;319:79–84. https://doi.org/10.1016/j.desal.2013.03.027.
Rotondi M, Callegaro C, Masserano F, Bartosek M. Low salinity water injection: eni’s experience. In: SPE Abu Dhabi International Petroleum Exhibition and Conference, 10–13 November, Abu Dhabi, UAE; 2014. https://doi.org/10.2118/171794-MS.
Schrodter K, Bettermann G, Staffel T, Wahl F, Klein T, Hofmann T. Phosphoric acid and phosphates. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH; 2008. https://doi.org/10.1002/14356007.a19_465.pub3.
Shaker SB, Skauge A. Wettability and oil recovery by low salinity injection. In: SPE EOR Conference at Oil and Gas West Asia, 16–18 April, Muscat, Oman; 2012. https://doi.org/10.2118/155651-MS.
Sheng JJ. Review of surfactant enhanced oil recovery in carbonate reservoirs. Adv Pet Explor Dev. 2013;6(1):1–10. https://doi.org/10.3968/j.aped.1925543820130601.1582.
Sohal MA, Thyne G, Sogaard EG. Review of recovery mechanisms of ionically modified waterflood in carbonate reservoirs. Energy Fuels. 2016;30(3):1904–14. https://doi.org/10.1021/acs.energyfuels.5b02749.
Song J, Zeng Y, Wang L, Duan X, Puerto M, Chapman WG, Biswal SL, Hirasaki GJ. Surface complexation modeling of calcite zeta potential measurements in brines with mixed potential determining ions (Ca2+, CO32−, Mg2+, SO42−) for characterizing carbonate wettability. J Colloid Interface Sci. 2017;506:169–79. https://doi.org/10.1016/j.jcis.2017.06.096.
Strand S, Hognesen EJ, Austad T. Wettability alteration of carbonates-effects of potential determining ions (Ca2+ and SO42−) and temperature. Colloids Surf A Physicochem Eng Asp. 2006;275:1–10. https://doi.org/10.1016/j.colsurfa.2005.10.061.
Tawfik MS, Karpyn ZT, Johns R. Multiscale study of chemically-tuned waterflooding in carbonate rocks using micro-computed tomography. In: IOR 2019-20th European Symposium on Improved Oil Recovery, April 8–11, Pau, France; 2019. https://doi.org/10.3997/2214-4609.201900074.
Winoto W, Loahardjo N, Morrow NR. Assessment of oil recovery by low salinity waterflooding from laboratory tests. In: SPE Improved Oil Recovery Symposium, 12–16 April, Tulsa, Oklahoma, USA; 2014. https://doi.org/10.2118/169886-MS.
Yuan M. Effect of temperature on barium sulfate scale inhibition of diethylene triamine penta (methylene phosphonic acid). In: Amjad Z, editor. Advances in crystal growth inhibition technologies; 2002. p. 151–163.
Zahid A, Stenby EH, Shapiro AA. Smart waterflooding (high sal/low sal) in carbonate reservoirs. In: SPE Europec/EAGE Annual Conference, 4–7 June, Copenhagen, Denmark; 2012. https://doi.org/10.2118/154508-MS.
Zhang P, Tweheyo M, Austad T. Wettability alteration and improved oil recovery in chalk: the effect of calcium in the presence of sulfate. Energy Fuels. 2006;20(5):2056–62. https://doi.org/10.1021/ef0600816.
Zhang P, Tweheyo MT, Austad T. Wettability alteration and improved oil recover by spontaneous imbibition of sea water into chalk: impact of the potential determining ions Ca2+, Mg2+, and SO42−. Colloids Surf A Physicochem Eng. 2007;301(1–3):199–208. https://doi.org/10.1016/j.colsurfa.2006.12.058.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Yan-Hua Sun
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ghosh, B., Sun, L. & Thomas, N.C. Compatibility evaluation of modified seawater for EOR in carbonate reservoirs through the introduction of polyphosphate compound. Pet. Sci. 17, 393–408 (2020). https://doi.org/10.1007/s12182-019-00380-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-019-00380-6