Abstract
This study paves the way on reducing smoke emission and NOx emissions of research diesel engine by detailing the effect of water addition in biodiesel. Fuel samples were prepared with different concentrations of water in orange peel oil biodiesel (94% waste orange peel oil biodiesel + 4% water + 2% Span 80 (WOPOBDE1) and 90% waste orange peel oil biodiesel + 8% water + 2% Span 80 (WOPOBDE2). Span 80 was employed as a nonionic surfactant, which emulsifies water in biodiesel. Experimental results revealed that the nitrogen oxides and smoke emission of orange peel oil biodiesel emulsion were reduced by 11%–19% and 3%–21%, respectively, compared to that of neat orange peel oil biodiesel (WOPOBD). In addition, the introduction of orange peel oil–water emulsions in the diesel engine considerably reduced the emissions of unburned hydrocarbons and carbon monoxide. The overall hydrocarbon emission of WOPOBDE2 was 12.2% lower than that of WOPOBD and 16.3% lower than that of diesel. The overall CO emission of WOPOBDE2 was 17% lower than that of base fuel (WOPOBD) and 21.8% lower than that of diesel. Experimental results revealed that modified fuel had higher brake thermal efficiency and lower brake specific fuel consumption than that of base fuel at all engine brake power levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is a growing number of on-road vehicles because of an exponential surge in population and enhanced lifestyle. This puts huge pressure on fossil petroleum fuel, energy security, and environmental stability. The exhaust emitted from a diesel engine is a major concern for the researchers to focus on a viable alternative. Pollutants namely carbon monoxide (CO), nitrogen oxides (NOx), carbon dioxide (CO2), unburned hydrocarbons (UBHC), and particulate matter (PM) is to be minimized to the maximum possible extent from existing diesel engine. In this regard, biodiesel as a supplement in diesel engine fuel seems appropriate. Nontoxic, biodegradable, higher flash point, the absence of aromatic compounds, negligible sulfur, and carbon neutral characteristics are some of the major advantages of biodiesel as a diesel engine fuel (Mahalingam et al. 2018).
Biodiesel derived from palm kernel oil, orange peel oil or mustard oil is employed as a neat fuel or in blends with diesel in a compression ignition (CI) engine (Joy et al. 2017; Venu and Madhavan 2017). The major drawback of using neat biodiesel in CI engines is its superior NOx emissions and lower thermal efficiency (Pandian et al. 2018). NOx emissions should be controlled by suitable methods to meet the emission standards (Anbarasu et al. 2015). Among many techniques, an emulsion process is preferred for the instantaneous decrease in PM and NOx emissions for a biodiesel-fueled diesel engine (Ravikumar and Saravanan 2016). In this process, water is mixed with the biodiesel in a certain percentage in the presence of a nonionic surfactant, which emulsifies water and biodiesel to improve the stability of the mixture (Melo-Espinosa et al. 2015; Anbarasu and Karthikeyan 2016; Appavu and Venkata Ramanan 2018). Many authors have agreed there are reductions in nitrogen oxides emissions when diesel engines run on diesel–biodiesel–water blends (Yuvarajan and Venkata Ramanan 2016; Annamalai et al. 2016; Devarajan et al. 2016; Vellaiyan and Amirthagadeswaran 2017b).
The aim of this experimental investigation is to elucidate the performance and emission patterns of an engine fueled by neat waste orange peel oil biodiesel (WOPOBD) and its various water blends. In this study, Span 80 as a nonionic surfactant was used for emulsifying water and biodiesel. Earlier reported literature is the motivation for choosing Span 80 as a surfactant (Devarajan and Madhavan 2017; Vellaiyan and Amirthagadeswaran 2017a; Arul Gnana Dhas et al. 2018). This study employed four fuels, namely diesel, waste orange peel oil biodiesel (WOPOBD), fuel comprising 4% (in volume) of water, 94% (in volume) of neat waste orange peel oil biodiesel and 2% (in volume) span 80 (WOPOBDE1), and fuel comprising 8% (in volume) of water, 90% (in volume) of neat waste orange peel oil biodiesel and 2% (in volume) Span 80 (WOPOBDE2) in a constant speed compression ignition engine. The emission and performance characteristics of all the test fuels were investigated and compared with the baseline diesel at ambient conditions.
2 Materials and methods
2.1 Preparation of orange peel oil biodiesel and its emulsion
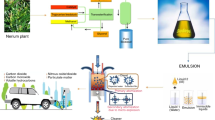
A steam distillation plant was used for the extraction of orange peel oil. The experimental setup consists of two chambers namely heating and steam preparation chamber. Figure 1 shows the photographic view of the distillation setup. A 1.2 kg of orange peel was placed in the steam chamber and heated to 110 °C. The fumes consisting of orange essence and steam vapor are routed to a condensing chamber for cooling purpose. The mixture of liquid water and orange peel oil was collected in a collection tank. Orange peel oil was separated from the mixture due to its density difference. 1.2 kg of orange peel yielded 700 mL of orange peel oil. A 500 g sample of orange peel oil in the reactor was heated to the temperature of 65 °C. A molar ratio of 5:1 (methanol to orange peel oil) and catalysts of 0.3% (wt/wt) to orange peel oil was used in the transesterification process. A solution containing sodium hydroxide dissolved in methanol was then added and mixed at a constant stirring speed of 340 rpm for 45 min. This ensured uniform reactivity of solution and accelerated the reaction rate. The mixture was allowed to cool in the vessel yielding two distinct layers of ester and glycerol. The modified fuel which consists of base fuel, water and surfactants are prepared by altering its proportions. For blending purpose, ultrasonicator, and magnetic stirrer were employed (Pandian et al. 2017). Various properties of prepared and modified fuels are established by ASTM techniques. Table 1 illustrates the properties of test fuels. Table 2 shows the fatty acid compositions of tested fuels.
2.2 Experimental setup
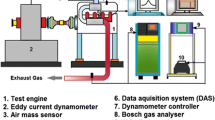
A four-stroke, single-cylinder, vertical, air-cooled diesel engine is used for the experimental analysis. The specification of the engine is listed in Table 3. The layout of the engine setup is shown in Fig. 2. The research engine consisted of a data acquisition unit, smoke opacimeter, fuel supply system, emission analyzer, and a dynamometer. An AVL emission analyzer was employed to measure HC, NOx, and CO emissions. An AVL 437C model opacimeter was employed to measure smoke opacity. All these investigations were performed at steady state conditions in order to ensure the reliability of recordings. Table 4 shows the gas analyzer range, accuracy and its uncertainties details.
3 Results and discussion
The chapter details the performance and emission pattern of a research diesel engine fueled with base and modified fuels at different brake powers.
3.1 Carbon monoxide (CO)
Figure 3 shows the variation of carbon monoxide emission with brake power (BP) for all test fuels. CO emissions from biofuels are comparatively less than that of diesel at all brake power levels. This is because of the abundant availability of inbuilt oxygen in orange peel oil biodiesel and water blends (Joy et al. 2017; Devarajan et al. 2017a). The inherent oxygen content of orange peel biodiesel and water blends also accelerates the oxidation reaction and reduces the CO emissions (Venkata Ramanan and Yuvarajan 2015). CO emission decreases with an increase in water content for orange peel biodiesel. CO emission for WOPOBDE1 and WOPOBDE2 were 8.8% and 11.16% lower than that of WOPOBD. Lower viscosity of modified fuel promotes the evaporation and reduces CO emission (Joy et al. 2017; Devarajan et al. 2017b; Rathinam et al. 2018; Radhakrishnan et al. 2018). Fuel with lower viscosity aids a better evaporation of fuel with air and results in improved combustion and lower CO emissions (Yuvarajan et al. 2016; Devarajan et al. 2017b).
3.2 Unburned hydrocarbon (HC)
Figure 4 shows the variation of hydrocarbon emissions for all test fuels. HC emission for the base and modified fuels is lower than that of diesel at the same brake power. Surplus oxygen present in biofuels and water blends facilitates the combustion and reduce HC emissions (Devarajan et al. 2017c; Joy et al. 2017). Addition of water into orange peel oil biodiesel (WOPOBDE1 and WOPOBDE2) has reduced the unburned hydrocarbon emissions. This is due to the heat sink effect of water in modified fuels (Vellaiyan et al. 2017; Rathinam et al. 2018; Radhakrishnan et al. 2018). In addition, the water in the WOPOBDE1 and WOPOBDE2 increase the evaporation tendency and lower HC emissions (Devarajan and Munuswamy 2016; Vellaiyan et al. 2017; Rathinam et al. 2018; Radhakrishnan et al. 2018).
3.3 Nitrogen oxide (NOx)
Figure 5 shows the variation of NOx emissions for all test fuels. NOx emissions depend on oxygen content and mass of fuel burned. NOx emissions from biofuels are higher than that of diesel at all brake powers. The inbuilt oxygen present in biofuels is the major cause for higher NOx emissions (Devarajan et al. 2017c). NOx emission was significantly lower for WOPOBDE1 and WOPOBDE2 than that of WOPOBD at all conditions. This is due to the occurrence of a water particle in WOPOBDE1 and WOPOBDE2. The water present in modified fuel drastically reduces the peak ignition temperature (Vellaiyan et al. 2017; Rathinam et al. 2018; Radhakrishnan et al. 2018). In addition, the water absorbs the maximum heat energy generated during the combustion. This absorption is owing to its latent heat of vaporization and hence lower NOx generation (Vellaiyan et al. 2017).
3.4 Smoke opacity
Figure 6 portrays the variation of smoke emission with brake power (BP) for all test fuels. The smoke opacity increases with an increase in the brake power. This is due to the increase in the higher quantity of fuel supply to maintain the constant engine power (Devarajan et al. 2018). Smoke value for WOPOBDE1, WOPOBDE2, and WOPOBD was lower than that of diesel. This is owing to the higher oxygen present in biofuel, which enhances the oxidation process (Joy et al. 2017; Devarajan et al. 2017c, 2018). Further, the smoke emissions of WOPOBDE1 and WOPOBDE2 are lower than WOPOBD at all conditions. The water present in modified fuel creates the micro-explosion during the course of combustion. This explosion enhances the atomization process and results in the improved air–fuel mixture, lower ignition delay, improved combustion and lower smoke emissions (Vellaiyan et al. 2017; Rathinam et al. 2018; Radhakrishnan et al. 2018).
3.5 Brake thermal efficiency
Variations in brake thermal efficiency (BTE) are presented in Fig. 7. BTE of all test fuels increases with an increase in brake power. At higher brake power, the frictional and other unaccounted losses are lower which in turn contributed to the increasing trend of BTE, by minimizing exergy destruction (Devarajan et al. 2017c, 2018). The brake thermal efficiency of diesel was higher than other test fuels (WOPOBDE1, WOPOBDE2, and WOPOBD). This is due to the higher calorific value of diesel (Deep et al. 2013; Karthikeyan et al. 2015; Devarajan et al. 2018). BTE for WOPOBDE1 and WOPOBDE2 was higher than neat WOPOBD. The water present in WOPOBDE1 and WOPOBDE2 converts into superheated steam and produces more power that in turn increases the fuel efficiency at all engine brake power (Nguyen et al. 2015; Vellaiyan et al. 2017a). Further, the micro-explosion of water droplets present in WOPOBDE1 and WOPOBDE2 provides enhanced atomization between air and fuel and result in higher efficiency (Rathinam et al. 2018; Radhakrishnan et al. 2018).
3.6 Brake specific fuel consumption (BSFC)
Variations in BSFC with brake power for test fuels are shown in Fig. 8. BSFC reduces with the brake power for all tested fuels. BSFC of diesel was lower than other test fuels (WOPOBDE1, WOPOBDE2 and WOPOBD). This is due to the higher calorific value of diesel (Kishore Pandian et al. 2017; Devarajan et al. 2018). BSFC for WOPOBDE1 and WOPOBDE2 was lower than neat WOPOBD. The water content in the WOPOBDE1 and WOPOBDE2 is converted into superheated steam during the combustion and produces more power which in turn reduces the fuel consumption rate (Nguyen et al. 2015; Vellaiyan and Amirthagadeswaran 2016). Fuel with lower viscosity (WOPOBDE1 and WOPOBDE2) assists the combustion process as it combines the fuel with air and produces lower BSFC (Rathinam et al. 2018; Radhakrishnan et al. 2018).
4 Conclusion
The experimental studies were conducted on a four-stroke, single-cylinder, naturally aspirated, water-cooled, direct injection diesel engine. Modified fuels are prepared by altering the quantity of water and surfactant in biodiesel. Based on the result, the following conclusion was drawn:
-
HC and CO emissions for modified fuels (WOPOBDE1 and WOPOBDE2) are lower than the base fuel (WOPOBD). This is owing to enhancement in evaporation of water during the combustion.
-
Smoke and NOx emission of the WOPOBDE1 and WOPOBDE2 are lower than WOPOBD at all brake power. The water present in the modified fuel lowers the peak gas temperature during the combustion.
-
WOPOBDE1 and WOPOBDE2 exhibited higher brake thermal efficiency than that of WOPOBD. The water present in modified fuels converts into superheated steam and produces more power, which in turn increases the fuel efficiency at all engine brake power. In addition, the BSFC reduced in modified fuels when compared to that of base fuel at all brake power.
References
Anbarasu A, Karthikeyan A, Balaji M. Performance and emission characteristics of diesel engine using alumina nanoparticle blended biodiesel emulsion fuel. J Energy Res Technol. 2015;138(2):022203. https://doi.org/10.1115/1.4031834.
Anbarasu A, Karthikeyan A. Performance and emission characteristics of a diesel engine using cerium oxide nanoparticle blended biodiesel emulsion fuel. J Energy Eng. 2016;142(1):04015009. https://doi.org/10.1061/(asce)ey.1943-7897.0000270.
Annamalai M, Dhinesh B, Nanthagopal K, SivaramaKrishnan P, Lalvani JIJ, Parthasarathy M, et al. An assessment on performance, combustion and emission behavior of a diesel engine powered by ceria nanoparticle blended emulsified biofuel. Energy Convers Manag. 2016;123:372–80.
Appavu P, Venkata Ramanan M. Study of emission characteristics of a diesel engine using cerium oxide nanoparticle blended pongamia methyl ester. Int J Ambient Ener. 2018. https://doi.org/10.1080/01430750.2018.1477063.
Arul Gnana Dhas A, Devarajan Y, Nagappan B. Analysis of emission reduction in ethyne–biodiesel-aspirated diesel engine. Int J Green Ener. 2018;15(7):436–40. https://doi.org/10.1080/15435075.2018.1473774.
Deep A, Singh, A, Vibhanshu V, Khandelwal A, Kumar N. Experimental investigation of orange peel oil methyl ester on single cylinder diesel engine. SAE Technical Paper Series. 2013. https://doi.org/10.4271/2013-24-0171.
Devarajan Y, Munuswamy DB. Analysis on the influence of nanoparticles of Alumina, Copper Oxide, and Zirconium Oxide on the performance of a flat-plate solar water heater. Energy Fuels. 2016;30(11):9908–13. https://doi.org/10.1021/acs.energyfuels.6b02264.
Devarajan Y, Jayabal RK, Ragupathy D, Venu H. Emissions analysis on second generation biodiesel. Front Environ Sci Eng. 2016. https://doi.org/10.1007/s11783-017-0891-0.
Devarajan Y, Madhavan VR. Emission analysis on the influence of ferrofluid on rice bran biodiesel. J Chilean Chem Soc. 2017;62(4):3703–7. https://doi.org/10.4067/s0717-97072017000403703.
Devarajan Y, Munuswamy B, Nagappan B. Emissions analysis on diesel engine fueled with cashew nut shell biodiesel and pentanol blends. Environ Sci Pollut Res. 2017a;24(14):13136–41. https://doi.org/10.1007/s11356-017-8915-7.
Devarajan Y, Munuswamy DB, Mahalingam A, Nagappan B. Performance, combustion, and emission analysis of neat palm oil biodiesel and higher alcohol blends in a diesel engine. Energy Fuels. 2017b;31(12):13796–801. https://doi.org/10.1021/acs.energyfuels.7b02939.
Devarajan Y, Munuswamy DB, Mahalingam A. Performance, combustion and emission analysis on the effect of ferrofluid on neat biodiesel. Process Saf Environ Prot. 2017c;111:283–91. https://doi.org/10.1016/j.psep.2017.07.021.
Devarajan Y, Munuswamy DB, Nagappan B, Pandian AK. Performance, combustion and emission analysis of mustard oil biodiesel and octanol blends in diesel engine. Heat Mass Transf. 2018;54(6):1803–11. https://doi.org/10.1007/s00231-018-2274-x.
Joy N, Devarajan Y, Nagappan B, Anderson A. Exhaust emission study on neat biodiesel and alcohol blends fueled diesel engine. Energy Sour Part A Recov Util Environ Eff. 2017;40(1):115–9.
Karthikeyan A, Venkatesh D, Ramkumar T. Experimental investigation on spark ignition engine using blends of bio-ethanol produced from citrus peel wastes. Int J Ambient Energy. 2015;38(2):112–5. https://doi.org/10.1080/01430750.2015.1048900.
Kishore Pandian A, Munuswamy DB, Radhakrishana S, Bathey Ramakrishnan RB, Nagappan B, Devarajan Y. Influence of an oxygenated additive on emission of an engine fueled with neat biodiesel. Pet Sci. 2017;14(4):791–7. https://doi.org/10.1007/s12182-017-0186-x.
Mahalingam A, Munuswamy DB, Devarajan Y, Radhakrishnan S. Emission and performance analysis on the effect of exhaust gas recirculation in alcohol-biodiesel aspirated research diesel engine. Environ Sci Pollut Res. 2018;25:12641–7. https://doi.org/10.1007/s11356-018-1522-4.
Melo-Espinosa EA, Piloto-Rodríguez R, Goyos-Pérez L, Sierens R, Verhelst S. Emulsification of animal fats and vegetable oils for their use as a diesel engine fuel: an overview. Renew Sustain Energy Rev. 2015;47:623–33.
Nguyen KB, Dan T, Asano I. Effect of double injection on combustion, performance and emissions of Jatropha water emulsion fueled direct-injection diesel engine. Energy. 2015;80:746–55. https://doi.org/10.1016/j.energy.2014.12.033.
Pandian AK, Munuswamy DB, Radhakrishanan S, Devarajan Y, Ramakrishnan RBB, Nagappan B. Emission and performance analysis of a diesel engine burning cashew nut shell oil bio diesel mixed with hexanol. Pet Sci. 2018;15(1):176–84. https://doi.org/10.1007/s12182-017-0208-8.
Pandian AK, Ramakrishnan RBB, Devarajan Y. Emission analysis on the effect of nanoparticles on neat biodiesel in unmodified diesel engine. Environ Sci Pollut Res. 2017;24(29):23273–8.
Purushothaman K, Nagarajan G. Experimental investigation on a CI engine using orange oil and orange oil with DEE. Fuel. 2009;88(9):1732–40.
Radhakrishnan S, Munuswamy DB, Devarajan Y, T A, Mahalingam A. Effect of nanoparticle on emission and performance characteristics of a diesel engine fueled with cashew nut shell biodiesel. Energy Sour Part A Recov Util Environ Eff. 2018;40(20):2485–93. https://doi.org/10.1080/15567036.2018.1502848.
Rathinam S, Justin Abraham Baby S, Devarajan Y, T A. Influence of water on exhaust emissions on unmodified diesel engine propelled with biodiesel. Energy Sour Part A Recov Util Environ Eff. 2018;40(21):2511–17. https://doi.org/10.1080/15567036.2018.1503756.
Ravikumar J, Saravanan S. Performance and emission analysis on blends of diesel, restaurant yellow grease and n-pentanol in direct-injection diesel engine. Environ Sci Pollut Res 2016;24(6):5381–5390. https://doi.org/10.1007/s11356-016-8298-1.
Vellaiyan S, Amirthagadeswaran KS. The role of water-in-diesel emulsion and its additives on diesel engine performance and emission levels: A retrospective review. Alex Eng J 2016;55(3):2463–72. https://doi.org/10.1016/j.aej.2016.07.021.
Vellaiyan S, Amirthagadeswaran KS, Vijayakumar S. Combustion of stable water-in-diesel emulsion fuel and performance assessment. Energy Sour Part A Recov Util Environ Eff. 2017;39(5):505–13.
Vellaiyan S, Amirthagadeswaran K. Multi-response optimization of diesel engine operating parameters running with water-in-diesel emulsion fuel. Therm Sci. 2017a;21(1 Part B):427–39. https://doi.org/10.2298/tsci160404220v.
Vellaiyan S, Amirthagadeswaran KS. Emission characteristics of water-emulsified diesel fuel at optimized engine operation condition. Pet Sci Technol. 2017b;35(13):1355–63.
Venkata Ramanan M, Yuvarajan D. Performance study of preheated mustard oil methyl ester on naturally aspirated CI Engine. Appl Mech Mater. 2015;787:761–5. https://doi.org/10.4028/www.scientific.net/amm.787.761.
Venu H, Madhavan V. Effect of diethyl ether and Al2O3 nano additives in diesel-biodiesel-ethanol blends: Performance, combustion and emission characteristics. J Mech Sci Technol. 2017;31(1):409–20. https://doi.org/10.1007/s12206-016-1243-x.
Yuvarajan D, Venkata Ramanan M. Experimental analysis on neat mustard oil methyl ester subjected to ultrasonication and microwave irradiation in four stroke single cylinder Diesel engine. J Mech Sci Technol. 2016;30(1):437–46. https://doi.org/10.1007/s12206-015-1248-x.
Yuvarajan D, Lokesh C, Balaji, P. Analysis on influence of varying compression ratio in Biofuel. Appl Mech Mater. 2016;852:734–8. https://doi.org/10.4028/www.scientific.net/amm.852.734.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Xiu-Qin Zhu
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Siva, R., Munuswamy, D.B. & Devarajan, Y. Emission and performance study emulsified orange peel oil biodiesel in an aspirated research engine. Pet. Sci. 16, 180–186 (2019). https://doi.org/10.1007/s12182-018-0288-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-018-0288-0