Abstract
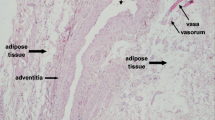
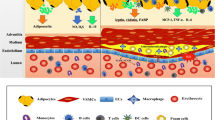
Perivascular adipose tissue is a visceral fat depot with an anatomical and functional contiguity to the vasculature system. Recent evidence suggests that perivascular adipose tissue could mechanically and functionally affect the vasculature, thereby possibly playing a role in adiposity-related atherosclerosis. Experimental and clinical observations suggest both favorable and unfavorable effects of perivascular fat. This review focuses on the emerging physiological and pathophysiological aspects of the perivascular fat and its role in vascular disease, insulin resistance and diabetes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as • Of importance •• Of major importance
Grundy SM et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–8.
Carr DB et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–94.
Kershaw EE et al. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56.
Sharma AM. Adipose tissue: a mediator of cardiovascular risk. Int J Obes Relat Metab Disord. 2004;26 Suppl 4:S5–7.
Dusserre E et al. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500:88–96.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;2:697–738.
Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7.
Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43:1651–4.
Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomical, biomolecular and clinical relation to the heart. Nat Cardiovasc Clin Pract Med. 2005;2:536–43.
Sharma AM. Mediastinal fat, insulin resistance, and hypertension. Hypertension. 2004;44:117–8.
Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des. 2007;13:2185–92.
Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58:591–610.
Takemori K, Gao YJ, Ding L, Lu C, Su LY, An WS, et al. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007;49:365–72.
Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–63.
Sahin AS, Bariskaner H. The mechanisms of vasorelaxant effect of leptin on isolated rabbit aorta. Fundam Clin Pharmacol. 2007;21:595–600.
Beltowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Ex Pharmacol Physiol. 2012;39:168–78. An overview of leptin and its regulation of endothelial function.
Gil-Ortega M, Stucchi P, Guzmán-Ruiz R, Cano V, Arribas S, González MC, et al. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151:3299–306.
Fésus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft F, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–27.
Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7). Hypertension. 1997;30:535–41.
Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–9.
Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol. 1996;271:H1262–6.
Gao YJ, Hirota S, Zhang D, Janssen LJ, Lee RMKW. Mechanisms of hydrogen peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol. 2003;138:1085–92.
Yudkin JS, Eringa E, Stehouwer CDA. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–20.
Maenhaut N, Van de Voorde J. Regulation of vascular tone by adipocytes. BMC Med. 2011;9:25.
Houben AJ, Eringa EC, Jonk AM, Serne EH, Smulders YM, Stehouwer CD. Perivascular fat and microcirculation: relevance to insulin resistance, diabetes, and cardiovascular disease. Curr Cardiovasc Risk Rep. 2012;6:80–90. An update review of perivascular fat and microcirculation.
Chaldakov GN. Cardiovascular adipobiology: a novel heart-associated adipose tissue in cardiovascular disease. Ser J Exp Clin Res. 2008;9:81–8.
Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–6.
Cooney MT, Dubina AL, Graham IN. Value and limitations of existing scores for the assessment of cardiovascular risk. J Am CollCardiol. 2009;54:1209–27.
Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22.
Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–9.
Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–73.
Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–26.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424.
Gustafsson F, Holstein-Rathlou NH. Angiotensin II modulates conducted vasoconstriction to norepinephrine and local electrical stimulation in rat mesenteric arterioles. Cardiovasc Res. 1999;44:176–84.
Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II-induces hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60.
Umemura M, Kawabe T, Shudo K, Kidoya H, Fukui M, Asano M, et al. Involvement of IL-17 in Fas ligand-induced inflammation. Int Immunol. 2004;16:1099–108.
Ishizaka N, Aizawa T, Ohno M, Usui Si S, Mori I, Tang SS, et al. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension. 2002;39:122–8.
Leng X, Zhan R, Wang Y, Liu X, Gong J, Gao X, et al. Anti-heat shock protein 70 autoantibody epitope changes and BD091 promotes atherosclerosis in rats. Cell Stress Chaperones. 2010;15:947–58.
Ghayour-Mobarhan M, Lamb DJ, Tavallaie S, Ferns GA. Relationship between plasma cholesterol, von Willebrand factor concentrations, extent of atherosclerosis and antibody titres to heat shock proteins-60, -65 and −70 in cholesterol-fed rabbits. Int J Exp Pathol. 2007;88:249–55.
Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and type II diabetes. Clin Sci. 2005;109:243–56.
Chen C, Jiang J, Lu JM, Chai H, Wang X, Lin PH, et al. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2010;299:H193–201.
Shyu KG, Lien LM, Wang BW, Kuan P, Chang H. Resistin contributes to neointimal formation via oxidative stress after vascular injury. Clin Sci. 2011;120:121–9.
Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, et al. Adipokine resistin is a key player to modulate mobocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99–109. It provides insights on the role of resistin in the atherosclerosis.
Choi HY, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, et al. Association of adiponectin, resistin, and vascular inflammation: analysis with 18 F-fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol. 2011;31:944–9. It explains the relation of adiponectin, resistin, and vascular inflammation.
Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–53.
Fukuhara A, Matsuda M, Mishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Sci. 2005;307:426–30.
Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–7.
Yamawaki H, Hara N, Okada M, Hara Y. Visfatin causes endothelium-dependent relaxation in isolated blood vessels. Biochem Biophys Res Commun. 2009;383:503–8.
Vallejo S, Romacho T, Angulo J, Villalobos LA, Cercas E, Leivas A, et al. Visfatin impairs endothelium-dependent relaxation in rat and human mesenteric microvessels through nicotinamide phosphoribosyltransferase activity. PLoS ONE. 2011;6:e27299.
Terra X, Auguet T, Quesada I, Aguilar C, Luna AM, Hernández M, et al. Increased levels of adipose tissue expression of visfatin inmorbidly obese women. The relationship with pro-inflammatory cytokines. Clin Endocrinol. 2011. doi:10.1111/j.1365-2265.2011.04327.
Friebe D, Neef M, Kratzsch J, Erbs S, Dittrich K, Garten A, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54:1200–11.
Tune JD, Considine RV. Effect of leptin on cardiovascular physiology. J Am Soc Hypertens. 2007;1:231–41.
Busch HJ, Schirmer SH, Jost M, van Stijn S, Peters SL, Piek JJ, et al. Leptin augments cerebral hemodynamic reserve after three-vessel occlusion: distinct effects on cerebrovascular tone and proliferation in a nonlethal model of hypoperfused rat brain. J Cereb Blood Flow Metab. 2011;31:1085–92.
Biasucci LM, Graziani F, Rizzello V, Liuzzo G, Guidone C, De Caterina AR, et al. Paradoxical preservation of vascular function in severe obesity. Am J Med. 2010;123:727–34.
Leung YM, Kwan CY. Dual vascular effects of leptin via endothelium: hypothesis and perspective. Chin J Physiol. 2008;51:1–6.
Hideyuki Y. Vascular effects of novel adipocytokines: focus on vascular contractility and inflammatory responses. Biol Pharm Bull. 2011;34:307–10.
Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–85.
Gao X, Mi S, Zhang F, Gong F, Lai Y, Gao F, et al. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:87.
Yoo HJ, Choi HY, Yang SJ, Kim HY, Seo JA, Kim SG, et al. Circulating chemerin level is independently correlated with arterial stiffness. J Atheroscler Thromb. 2012;19:59–68.
Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern Med. 2011;50:1093–7.
Ernst MC, Haidl ID, Zúñiga LA, Dranse HJ, Rourke JL, Zabel BA, et al. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology. 2012;153:672–82.
Shin HY, Lee DC, Chu SH, Jeon JY, Lee MK, Im JA, et al. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin Endocrinol. 2011. doi:10.1111/j.1365-2265.2011.04217.x.
HIda K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005;102:10610–5.
Jung CH, Lee WJ, Hwang JY, Seol SM, Kim YM, Lee YL, et al. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem Biophys Res Commun. 2011;413:264–9.
Aust G, Richter O, Rohm S, Kerner C, Hauss J, Klöting N, et al. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis. 2009;204:262–6.
Li HL, Peng WH, Cui ST, Lei H, Wei YD, Li WM, et al. Vaspin plasma concentrations and mRNA expressions inpatients with stable and unstable angina pectoris. Clin Chem Lab Med. 2011;49:1547–54.
Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents TNF-α-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-κB and PKCθ activation in cultured rat vascular smooth muscle cells. Pharmacol Res. 2011;64:493–500.
Könczöl K, Pintér O, Ferenczi S, Varga J, Kovács K, Palkovits M, et al. Nesfatin-1 exerts long-term effect on food intake and body temperature. Int J Obes. 2012. doi:10.1038/ijo.2012.2.
Riva M, Nitert MD, Voss U, Sathanoori R, Lindgvist A, Ling C, et al. Nesfatin-1 stimulates glucagon and insulin secretion and beta cell NUCB2 is reduced in human type 2 diabetic subjects. Cell Tissue Res. 2011;346:393–405.
Yamawaki H, Takahashi M, Mudohda M, Morita T, Okada M, Hara Y. A novel adipocytokine, nesfatin-1 modulates peripheral arterial contractility and blood pressure in rats. Biochem Biophys Res Commun. 2012;418:676–81.
Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 2012;165:643–58. It nicely describes the role of perivascular adipose tissue in vascular smooth muscle cell growth.
Lamers D, Schlich R, Greulich S, Sasson S, Sell H, Eckel J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signalling in human vascular smooth muscle cells. J Cell Mol Med. 2011;15:1177–88.
Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–13.
Motobayashi Y, Izawa-Ishizawa Y, Ishizawa K, Orino S, Yamaguchi K, Kawazoe K, et al. Adiponectin inhibits insulin-like growth factor-1-induced cell migration by the suppression of extracellular signal-regulated kinase ½ activation, but not Akt in vascular smooth muscle cells. Hypertens Res. 2009;32:188–93.
Nguyen Dinh Cat A, Briones AM, Callera GE, Yogi A, He Y, Montezano AC, et al. Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension. 2011;58:479–88.
Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes Jr DR, Richardson DM, Ritman EL, et al. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–6.
Madonna R, De Caterina R. Atherogenesis and diabetes: focus on insulin resistance and hyperinsulinemia. Rev Esp Cardiol. 2012. doi:10.1016/j.recesp.2011.11.010.
Rojas J, Bermúdez V, Leal E, Cano R, Luti Y, Acosta L, et al. Insulinorresistencia e hiperinsulinemia como factores de riesgo para enfermedad cardiovascular. AVFT. 2008;27:29–39.
Galli-Tsinopoulou A, Kyrgios I, Maggana I, Giannopoulou EZ, Kotanidou EP, Stylianou C, et al. Insulin resistance is associated with at least threefold increased risk for prothrombosic state in severely obese youngsters. Eur J Pediatr. 2011;170:879–86.
Monte SV, Caruana JA, Ghanim H, Sia CL, Korzeniewski K, Schentag JJ, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. 2012;151:587–93.
Bonsignore MR, Esquinas C, Barceló A, Sanchez-de-la-Torre M, Paternó A, Duran-Cantolla J, et al. Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. Eur Respir J. 2011. doi:10.1183/09031936.00151110.
Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–11.
Rittig K, Staib K, Machann J, Böttcher M, Peter A, Schick F, et al. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia. 2009;51:2093–9.
Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, et al. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–25. It helps to understand the difference in the secretosome of perivascular fat cells from subcutaneous and visceral fat.
Cicha I, Wörner A, Urschel K, Beronov K, Goppelt-Struebe M, Verhoeven E, et al. Carotid plaque vulnerability: a positive feedback between hemodynamic and biochemical mechanisms. Stroke. 2011;42:3502–10.
Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and vascular dysfunction. Pathophysiology. 2008;15:79–89.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904.
Jonk AM, Houben AJ, Schaper NC, de Leeuw PW, Serné EH, Smulders YM, et al. Meal-related increases in microvascular vasomotion are impaired in obese individuals: a potential mechanism in the pathogenesis of obesity-related insulin resistance. Diabetes Care. 2011;34 Suppl 2:S342–8.
Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–40.
Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–8.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Chengyu Xu and Gianluca Iacobellis declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Xu, C., Iacobellis, G. Perivascular Fat and its Role in Vascular Disease, Insulin Resistance and Diabetes. Curr Cardiovasc Risk Rep 8, 370 (2014). https://doi.org/10.1007/s12170-013-0370-5
Published:
DOI: https://doi.org/10.1007/s12170-013-0370-5